Abstract
Background
The aim of this review was to identify relevant randomized controlled trials (RCTs) and non-RCTs to evaluate the existing knowledge on the effect of antibiotic treatment for infants with necrotizing enterocolitis (NEC).
Objective
Identifying 1) the best antibiotic regimen to avoid disease progression as assessed by surgery or death, 2) the best antibiotic regimen for infants operated for NEC as assessed by re-operation or death.
Methods
Embase, MEDLINE and Cochrane were searched systematically for human studies using antibiotics for patients with NEC, Bell’s stage II and III.
Results
Five studies were included, with a total of 375 infants. There were 2 RCT and 3 cohort studies. Four main antibiotic regimens appeared. Three with a combination of ampicillin + gentamycin (or similar) with an addition of 1) clindamycin 2) metronidazole or 3) enteral administration of gentamycin. One studied investigated cefotaxime + vancomycin. None of the included studies had a specific regimen for infants undergoing surgery.
Conclusions
No sufficient evidence was found for any recommendation on the choice of antibiotics, the route of administration or the duration in infants treated for NEC with Bell’s stage II and III.
Similar content being viewed by others
Backgound
Estimated 1–3 of every 1000 newborns are affected by Necrotizing Enterocolitis (NEC) [1]. The majority (90%) of cases are seen in very low and extremely low birthweight (BW) infants, with a gestational age (GA) < 32 week [2]. Among the infants with the lowest BW and GA the incidence may be as high as 22% [2, 3]. The medical treatment consists of antibiotics and cessation of enteral feeding. The rationale is immaturity of the gastrointestinal tract and the innate immune system. In severe cases (Bell’s stage III) surgery may be needed [4] and the indication for postoperative antibiotic treatment is to prevent surgical site infections and recurrence of NEC.
The antibiotic treatment of NEC empirically covers anaerobic and Gram-negative bacteria. The most common regimen reported is intravenous administration of ampicillin and gentamycin combined with metronidazole for a period of 10–14 days [5, 6]. A Cochrane review from 2012 included randomized and quasi randomized studies and could not provide sufficient evidence for a general recommendation for a specific regimen of antibiotics in the treatment of NEC [7]. Several studies have investigated the effects of prophylactic antibiotics to prevent NEC in infants of risk but without any evidence for the effectiveness [8, 9]. Broad spectrum antibiotics covering both aerobic and anaerobic bacteria are recommended [10], because no single organism has proven to consistently cause the disease [11]. Cultures from blood/feces/operative specimen or vascular access devices in NEC-patients treated with a combination of ampicillin, gentamicin and metronidazole showed complete sensitivity to all strains in only 4 out of the 12 included patients [6].
Several reviews have tried to recapitulate the most common treatment combinations used but without a comparison of effectiveness [5, 7, 12]. The rarity and heterogenicity of the disease make it difficult or even impossible to perform clinically randomized trials (RCT) to achieve robust results,
The primary objective of the present study was to clarify which antibiotic regimen is the most effective for children with NEC to avoid progression of disease as assessed by operation or death.
The secondary objective was to identify the effect of antibiotic regimens in children, who have undergone surgery as assessed by the need for reoperation or death.
Methods
This systematic review was performed in accordance with the PRISMA guidelines [13].
Study registration
The study was registered in the PROSPERO register (CRD42020162193) January 15th, 2020.
Eligibility criteria
Studies with documented NEC according to Bell’s classification stage II or III, treatment with at least one type of antibiotic and with reported progression of the disease defined as surgery and/or death as an outcome were included. Exclusion criteria were 1) inability to obtain the full text and insufficient data on primary outcomes, 2) studies with overlapping data, 3) case reports 4) non-original articles such as reviews 5) articles not available in English or Scandinavian languages (Danish, Norwegian or Swedish).
Information sources and search strategy
The following databases were searched: Embase, Medline (both through Ovid) and Cochrane. Clinicaltrials.gov and Prospero were searched for any relevant ongoing trials or reviews. Opengrey.eu was searched to locate relevant grey literature within the area.
Relevant search terms were identified in Embase and Medline. The following search was conducted in Embase and adjusted to fit Medline and Cochrane format:
(prematurity [Emtree] or extremely low birthweight [Emtree] or very low birth weight [Emtree] or low birthweight [Emtree] or newborn [Emtree] or infant [Emtree] or Infant* or extremely premature infant*or premature infant* or premature neonate or pre-mature infant or pre-term bab* or pre-term child* or pre-term infant* or pre-term neonate or pre-term newborn or premature or premature bab* or premature child* or premature neonate* or premature newborn* or prematuritas or preterm bab* or preterm child* or preterm infant* or preterm neonate* or preterm newborn* or human neonate* or human newborn* or human infant* or Neonate* or Newborn child* or Newborn bab* or Neonatal Prematurity or Prematurity or Extremely Premature Infant* or Extremely Preterm Infant* or Extremely Premature Infant* or extremely low birthweight infant or extremely low birthweight infant or ELBW or very low birthweight infant or very low birth weight infant or VLBW or low birthweight infant or low birth weight infant or LBW) AND (necrotizing enterocolitis [Emtree] or necrotising enterocolitis or necrotizing enterocolis) AND (antiinfective agent [Emtree] or antibiotic agent [Emtree] or antibiotic prophylaxis [Emtree] or amoxycillin or amoxicillin or ampicillin or azithromycin or augmentin or benzylpenicillin or b-lactam or beta-lactam or beta-lactams or clarithromycin or cefuroxime or cotrimoxazole or co-trimoxazole or cefotaxime or ceftriaxone or ceftriaxone or cefditoren or chloramphenicol or cefpodoxime or cephradine or cephalexin or cefetamet or cefaclor or cephalosporin* or erythromycin or gentamicin or gentamycin or levofloxacin or minocycline or metronidazole or moxifloxacin or penicllin* or quinolone* or roxithromycin or sulphamethoxazole or sulfamethoxazole or trimethoprim or Antibiotic* or folate antagonists or combined antibiotic* or antibiotic or antibiotic prophylaxis or anti bacterial agent* or anti infective agent* or antibacterial agent* or antibacterial drug* or antibacterial spectrum or antiinfective* or antimicrobial* or antiseptic* or microbiological agent* or Anti-Bacterial agent* or Antibacterial Agent* or Anti-Bacterial Compound* or Anti Bacterial Compound* or Bacteriocidal Agent* or Bacteriocide* or Anti-Mycobacterial Agent* or Anti Mycobacterial Agent* or Antimycobacterial Agent*)The search was finalized by excluding conference abstracts from the search. A third search was performed by manually checking the references listed in the included studies. All relevant studies were included in the original search.
Study selection
Covidence14 was used for the screening process. The initial screening was carried out by the authors EMG and KJ. Included studies were screening based on title and abstract. If conflicts occurred, reasons for exclusion were discussed until agreement. Full text screening was carried out by the same two authors. In case of conflict, the article was discussed until a mutual agreement among all authors were obtained.
Data collection and data items
Data collection was conducted with a standardized piloted form within Covidence [14] and on an external platform allowing for extraction in duplicate by both authors. All extracted data was reviewed by both authors to ensure consistency in agreement. When information was missing from the included articles the author [15] was contacted.
The list of study items to be extracted was made according to the Cochrane handbook chapter 5 [16] and modified according to relevance for this review. Extracted items included: Author name, title, publication year, study method, number of included infants, type of antibiotics used, doses, frequency, and route of administration. The number of infants who died or underwent surgery was retrieved. For the group of patients that underwent surgery the postoperative use of antibiotics was registered and the number of postoperative deaths or reported reoperation for NEC was retrieved.
Risk of bias in individual studies (methodological quality appraisal)
RCTs were analyzed for bias using the Risk of Bias tool RoB2 [17]. RoB2 evaluates the risk of bias in a study based on 5 domains of bias: 1) the randomization process 2) deviations from the intended intervention 3) missing outcome data 4) measurement of the outcome 5) selection of the reported result. Each domain is graded low, high, some concerns or no information according to a set of signaling questions, and later summed in an algorithm to determine bias level. The n-RCTs included were analyzed using ROBINS-I [18]. ROBINS-I evaluate how well the n-RCT study handles bias spread across 7 domains compared to a well performed RCT. The 7 domains are: 1) confounding 2) Selection of participants 3) Classification of interventions 4) Deviations from intended interventions 5) Missing data 6) Measurement of outcomes 7) Selection of reported results. A n-RCT might therefore have been without major risks of bias compared to other studies of the same type, but compared to a RCT have issues, e.g., arising from randomization. All analyses were conducted at study level.
Risk of bias across studies
Risk of bias across studies was analyzed using Robvis plot diagram [19] . Risk ratios (RR) were calculated to investigate trends.
Results
Study selection
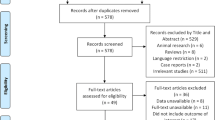
The search resulted in 115 references from Cochrane library, 2063 from Embase, 534 from Medline and 13 from Cochrane trials. A total of 2715 references. No relevant ongoing studies were identified on clinicaltrials.gov nor was any material found on Opengrey.eu at the time of search (updated February 24th, 2021).
EndNote initially removed 379 duplicates. The remaining 2336 references were uploaded to Covidence for screening (Fig. 1). During the initial screening all titles were available in English. A total of 5 studies with 375 infants were included, 2 RCTs and 3 cohort studies (Table 1). The two RCTs have previously been included in a Cochrane review [7],
On September 12th, 2020, the search was repeated. A total of 264 new references was added (Endnote initially removed 2 duplicates). The new references were screened by the author EMG, and none fulfilled the inclusion criteria.
Reasons for exclusion of studies
Several references were excluded due to population or study design not matching the inclusion criteria (tagged as wrong population in Covidence; etc. as it appears from Fig. 1); either because they included infants without confirmed NEC (studies investigating prophylaxis) or investigated the risk of developing NEC by maternal administration of antibiotics during pregnancy. The studies excluded for wrong study design was reviews or animal studies. Wrong comparator/intervention was used when studies switched between mixed treatment as this could be a possible confounder for the primary outcome [4].
Included studies
None of the studies investigated a specific postoperative antibiotic regimen in children undergoing surgery. All infants continued the study specific treatment they had received before surgery.
Results of bias in individual studies
Both of the included RCTs [20, 21]) raised some concern for bias in domain 5 (bias due to selection of reported result) as shown in Fig. 2. No pre-specified plan for study analysis was available and outcome measures were not presented in advance according to the criteria stated in Rob2 [17]. For the study by Hansen et al. [20] domain 4 gave rise to some concern for bias because the outcome assessors role in the study was unclear. No meta-analysis of the two RCTs was carried out because of too different treatment regimens to obtain reliable data.
As for the cohort studies, one study [23] was performed in a methodological manner comparable to a RCT. Two studies [10, 22] had one or more areas that gave reason to concern for bias.
In the study by Vermeylen et al. [22] information about statistical analysis of differences between the two groups compared were missing (domain 1). Scheifele et al. [10] provided well analyzed information about possible confounders, but according to standards specified by the ROBINS-I [18] tool it cannot be compared to a well performed RCT.
Risk of bias across studies
All the cohort studies [10, 22, 23] had a low risk of bias regarding bias due to deviation from intended intervention (D4), missing data (D5), measurement of outcomes (D6) and selection of reported results (D7). Only the study by Luo et al. [23] could receive a low risk of bias equal to a well performed RCT.
With the limited number of studies and difference in study setup it was decided not to calculate the weight of each individual study. Only one study was judged to be of overall low risk of bias [23], and it was decided there was no substance for a generalization (Fig. 3). No synthesis of studies was conducted due to the clinical heterogeneity of the studies (Table 1).
Summarized bias across studies. Top panel for RCT, lower panel for non-RCT. The plots were created using Robvis [19]
Primary outcomes: death and surgery
For both the RCTs, the mortality rate in the intervention group varied from 10 to 20% and 18–20% in the control group with no significant differences. The values for surgery were 10–30% and 18–40%, respectively, and with no significant difference. In the three cohort studies the mortality rate varied from 0 to 15% in the intervention group and 10–32% in the control group. The values for surgery were 15–71% and 8–33%, respectively. The only significant difference found was in the surgical rate in the study by Vermeylen et al. [22] with the lowest rate in the group who received metronidazole but there was no difference in the mortality rates.
In the study by Luo et al. [23] 17 infants deteriorated from Bell’s stage II to III in a population of 143 infants. Eleven of these had received metronidazole and 6 had not. There was a lower mortality rate in the group receiving metronidazole, but the difference was not statistically significant.
Two studies specified the number of deaths for Bell’s stage III NEC. Vermeylen et al. [22] reported 5/30 deaths in the intervention group (metronidazole), compared to 4/4 deaths in the control group. In the study by Luo et al. [23] 1/11 infants receiving metronidazole died compared to 2/6 without metronidazole.
For the secondary outcomes no reliable data could be retrieved.
Other effects of intervention
In the study by Faix et al. [21] there was no significant difference in the rate of bowel necrosis (perforation and gangrene) between the two groups (ampicillin+ gentamicin vs. ampicillin+gentamin + clindamycin). Hansen et al. [20] showed no significant differences regarding perforation, strictures, or death between the two groups of regimens (ampicillin+ gentamicin vs ampicillin+ gentamicin + oral gentamicin).
In the study by Scheifele et al. [10] children with a birthweight > 2200 g had better outcomes regardless of treatment. No infants in this group died, but had a similar risk of surgery as the children with a birthweight below 2200 g. For the group of infants with a BW < 2200 g, the group receiving standard treatment (ampicillin and gentamicin) had a significantly higher risk (p = 0.004) for surgery, peritonitis, strictures, but not for re-NEC (4/34 infants, p = 0.07 compared to the intervention with cefotaxime and vancomycin. No infants < 2200 g treated with cefotaxime/vancomycin died compared to 5/38 in the control group (p = 0.048).
Discussion
The 5 included studies reported on 4 different treatment regimes. Although some few statistically significant differences emerged, and two studies [22, 23] showed a trend towards a positive effect from adding metronidazole to the regimen, we were not able to demonstrate consistent results to recommend neither the type of antibiotics, the route of administration or the duration of treatment. The information on the secondary outcome of death and NEC recurrence in those infants who underwent surgery were scarce and insufficient to achieve any conclusions.
A large variation in antibiotics combinations and duration among different institutions has been reported with 14 different combinations of antibiotics. Ampicillin+ gentamicin + metronidazole is reported as the most commonly used [24]. A study including patients with Bell’s type II and III NEC, found no combination of antibiotics to be superior from others and the duration of treatment had no significant implication of the treatment results.
During the literature search, a study from 2019 by Harutynyan et al. [15] was discovered. The study was not included because the number of deaths in Bell’s stage I disease could not be isolated. In addition, the study used antibiotics both as prophylaxis for infants in high risk of developing NEC, and treatment of infants with established NEC. A total of 200 infants were included from 2015 to 2018, where the intervention group (104 infants) were treated with a combination of oral gentamicin sulfate 15 mg/kg/day ev. 12 h + nystatin 10,000 IU/kg/day ev. 6 h and an orally administered symbiotic (Lactobacillus rhamnosus). The control group received the standard treatment of empirical antibiotics (not specified). The overall mortality was 38/96 in the control group compared to 13/104 in the interventional group. For Bell’s stage III infants the numbers were 28/35 and 2/5, respectively. The difference was statistically significant. During the investigation period no infants underwent surgery in either group. It is unknown whether they received peritoneal drainage. A Cochrane review [9] from 2001 supports the finding that enteral antibiotics could have a prophylactic effect on the development of NEC, but raises concerns about unknown harms. Another Cochrane review from 2014 [25] concluded that the use of probiotics has an effect in preventing NEC in preterm infants. Although antibiotics is a core element in the treatment of NEC some studies seem to indicate antibiotics as a risk factor for developing NEC [26].
Ampicillin and gentamicin work synergistically and have created the base for many treatment regimens for NEC [5]. Hansen et al. [20] showed that the intervention group receiving oral gentamicin had significantly higher serum-gentamicin peak values compared to the control group (receiving only IV gentamicin) questioning the safety regarding known side effects of aminoglycosides.
One study investigating the effect of clindamycin was terminated prior to completion due to an increase in the frequency of intestinal stenosis [21]. A study by Autmizguine et al. [27] analyzing data from 2780 infants with NEC who were treated with or without antibiotics for anaerobic bacteria (clindamycin or metronidazole) showed that infants treated with the anaerobic antimicrobials developed more strictures (OR 1.73; 95% CI, 1.11–2,72). However, this was not significant when analyzed for clindamycin alone. Other studies [21, 23, 27] showed no effect of anaerobe antimicrobials in preventing progression of stage II NEC.
Piperacillin/tazobactam might be an alternative treatment in NEC. In a small study [28] with 27 very low birth weight infants there was a trend towards preventing progression of NEC. and no adverse events were reported.
The limitations of this review are studies of older date where methodology and reporting were of a different, less strict, and structured format. As a result, it was difficult to obtain equivalent information from all the included studies.
With the rising concerns about antibiotic resistance, efforts to find a treatment regimen with maximal effect is warranted, as is more knowledge on antibiotic treatment in the prevention and progression of NEC in addition to postoperative treatment. Another problem is the dose needed for a sufficient penetration of the antibiotic to the peritoneal cavity. The approved method with intraperitoneal microdialysis to measure antibiotic concentrations could be an option [29].
Conclusion
No sufficient evidence was found for any recommendation on the choice of antibiotics, the route of administration or the duration in infants treated for Bell’s stage II and III NEC.
Availability of data and materials
The search string for this study can be found at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=162193. For data on analysis of risk of bias in the included studies, this can be available by request to the author EMG at ester.m.gill@gmail.com.
Abbreviations
- NEC:
-
Necrotizing enterocolitis
- BW:
-
Birth weight.
- GA:
-
Gestational age
- IM:
-
Intramuscular
- IV:
-
Intravenous
- PS:
-
Propensity score
- RCT:
-
Randomized controlled trial
- N-RCT:
-
Non-randomized controlled trial
- GI:
-
Gastrointestinal
References
Ahle M, Drott P, Andersson R. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics. 2013;132:e443–51. https://doi.org/10.1542/peds.2012-3847.
Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103:F182–9. https://doi.org/10.1136/archdischild-2017-313880.
Hein-Nielsen A, Petersen S, Greisen G. Unchanged incidence of necrotising enterocolitis in a tertiary neonatal department. Dan Med J. 2015;62:A5091.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. https://doi.org/10.1097/00000658-197801000-00001.
Tickell D, Duke T. Evidence behind the WHO guidelines: Hospital Care for Children: for young infants with suspected necrotizing enterocolitis (NEC), what is the effectiveness of different parenteral antibiotic regimens in preventing progression and sequelae? J Trop Pediatr. 2010;56:373–8. https://doi.org/10.1093/tropej/fmq110.
Jardine LA, Cartwright D, Inglis GT, Davies MW. Antimicrobial susceptibility of Bacteria isolated from newborns with suspected or confirmed Necrotising enterocolitis. Maced J Med Sci. 2009;2:301–4. https://doi.org/10.3889/MJMS.1857-5773.2009.0071.
Shah D, Sinn JKH. Antibiotic regimens for the empirical treatment of newborn infants with necrotising enterocolitis. Cochrane Database Syst Rev. 2012:CD007448. https://doi.org/10.1002/14651858.CD007448.pub2.
Greenwood C, Morrow A, Lagomarcino A, Altaye M, Taft D, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of enterobacter. J Pediatr. 2014;165:23–9. https://doi.org/10.1016/j.jpeds.2014.01.010.
Bury R, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2001. https://doi.org/10.1002/14651858.CD000405.
Scheifele D, Ginter G, Olsen E. Comparison of two antibiotic regimens for neonatal necrotizing enterocolitis. J Antimicrob Chemother. 1987;20:421–9. https://doi.org/10.1093/jac/20.3.421.
Sharma R, Hudak M. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40:27–51. https://doi.org/10.1016/j.clp.2012.12.012.
Knell J, Han S, Jaksic T, Modi B. Current status of necrotizing enterocolitis. Curr Probl Surg. 2019;56:11–38. https://doi.org/10.1067/j.cpsurg.2018.11.005.
Moher D, Liberati A, Tetzlaff J, Altman D, Group TP. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Harutyunyan A, Muradyan A, Hovhannisyan M, Badalyan A, Lorenc D, K.v N, et al. The transformation of multi-modal 3 component preventive scheme into treatment protocol for necrotizing enterocolitis in newborns. New Armen Med J. 2019;13:89–102.
Li T, Higgins JPT, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355. https://doi.org/10.1136/bmj.i4919.
McGuinness LA. robvis: An R package and web application for visualising risk-of-bias assessments 2019. https://github.com/mcguinlu/robvis.
Hansen T, Ritter D, Speer M, Kenny J, Rudolph A. A randomized, controlled study of oral gentamicin in the treatment of neonatal necrotizing enterocolitis. J Pediatr. 1980;97. https://doi.org/10.1016/s0022-3476(80)80283-6.
Faix R, Polley T, Grasela T. A randomized, controlled trial of parenteral clindamycin in neonatal necrotizing enterocolitis. J Pediatr. 1988;112:271–7. https://doi.org/10.1016/S0022-3476(88)80069-6.
Vermeylen D, De Laet M, Pardou A, Govaerts M, Bouton J. Neonatal necrotizing enterocolitis: from reduction of mortality to reduction of morbidity. Acta Anaesthesiol Belg. 1985;36:153–9.
Luo L-J, Li X, Yang K-D, Lu J-Y, Li L-Q. Broad-spectrum antibiotic plus metronidazole may not prevent the deterioration of necrotizing enterocolitis from stage II to III in full-term and near-term infants a propensity score-matched cohort study. Med U S. 2015;94. https://doi.org/10.1097/MD.0000000000001862.
Murphy C, Nair J, Wrotniak B, Polischuk E, Islam S. Antibiotic treatments and patient outcomes in necrotizing enterocolitis. Am J Perinatol. 2020;37:1250–7. https://doi.org/10.1055/s-0039-1693429.
AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD005496.pub4.
Raba AA, O’Sullivan A, Semberova J, Martin A, Miletin J. Are antibiotics a risk factor for the development of necrotizing enterocolitis-case-control retrospective study. J Pediatr. 2019;178:923–8. https://doi.org/10.1007/s00431-019-03373-0.
Autmizguine J, Hornik C, Benjamin D, Laughon M, Clark R, Cotton C, et al. Anaerobic antimicrobial therapy after necrotizing enterocolitis in VLBW infants. Pediatrics. 2015;135:e117–25. https://doi.org/10.1542/peds.2014-2141.
Berger A, Kretzer V, Apfalter P, Rohrmeister K, Zaknun D, Pollak A. Safety evaluation of piperacillin/tazobactam in very low birth weight infants. J Chemother. 2004;16:166–71. https://doi.org/10.1179/joc.2004.16.2.166.
Marchand S, Chauzy A, Dahyot-Fizelier C, Couet W. Microdialysis as a way to measure antibiotics concentration in tissues. Pharmacol Res. 2016;111:201–7. https://doi.org/10.1016/j.phrs.2016.06.001.
Acknowledgements
SDU Library and the Center for Evidence-Based Medicine Odense (CEBMO).
Funding
The first author of the review received funding from OUH pregraduate scholarship grant. This funding is an institutional and non-commercial. The grant offers were not involved in the work of this study.
Author information
Authors and Affiliations
Contributions
EMG prepared the search, selection and drafted the manuscript. KJ aided in selection and analysis of included studies. NQ and MBE aided in the structure of the search and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An approval from the Committees on Health Research Ethics was not needed for this review. This review is based on published material where consent to participate was obtained in the individual studies.
Consent for publication
Not applicable.
Competing interests
The authors of this review have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gill, E.M., Jung, K., Qvist, N. et al. Antibiotics in the medical and surgical treatment of necrotizing enterocolitis. A systematic review. BMC Pediatr 22, 66 (2022). https://doi.org/10.1186/s12887-022-03120-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03120-9






