Abstract
Background
Late Language Emergence (LLE) in the first two years of life is one of the most common parental concerns about child development and reasons for seeking advice from health professionals. LLE is much more prevalent in twins (38%) than singletons (20%). In studies of language development in twins without overt disability, adverse prenatal and perinatal environments have been reported to play a lesser role in the etiology of LLE than adverse postnatal environments. However, there is a lack of population-level evidence about prenatal and perinatal risk factors for LLE in twins. This study investigated the extent to which prenatal and perinatal risk factors were associated with LLE in a population-level sample of twins at age 2 without overt disability.
Methods
The sample comprised 473 twin pairs drawn from a population sample frame comprising statutory notifications of all births in Western Australia (WA), 2000–2003. Twin pairs in which either twin had a known developmental disorder or exposure to language(s) other than English were excluded. Of the 946 twins, 47.9% were male. There were 313 dizygotic and 160 monozygotic twin pairs. LLE was defined as a score at or below the gender-specific 10th percentile on the MacArthur Communicative Development Inventories: Words and Sentences (CDI-WS) (Words Produced). Bivariate and multivariable logistic regression was used to investigate risk factors associated with LLE.
Results
In the multivariable model, risk factors for LLE in order of decreasing magnitude were: Gestational diabetes had an adjusted odds ratio (aOR) of 19.5 (95% confidence interval (CI) 1.2, 313.1); prolonged TSR (aOR: 13.6 [2.0, 91.1]); multiparity (aOR: 7.6 [1.6, 37.5]), monozygosity (aOR: 6.9 [1.7, 27.9]) and fetal growth restriction (aOR: 4.6 [1.7, 12.7]). Sociodemographic risk factors (e.g., low maternal education, socioeconomic area disadvantage) were not associated with increased odds of LLE.
Conclusions
The results suggest that adverse prenatal and perinatal environments are important in the etiology of LLE in twins at age 2. It is important that health professionals discuss twin pregnancy and birth risks for delayed speech and language milestones with parents and provide ongoing developmental monitoring for all twins, not just twins with overt disability.
Similar content being viewed by others
Background
In the first two years of life, children achieve important milestones in language development that are highly anticipated by parents. Children with normal language emergence (NLE) typically start to produce single words around their first birthday. By their second birthday, children with NLE start to combine 2–3 words in simple sentences, signalling the emergence of grammar [1]. The term ‘Late Language Emergence’ (LLE) is used to describe toddlers who, despite otherwise healthy development, do not meet age expectations for receptive and/or expressive language development at 24 months [2]. Failure to attain these milestones are ‘red flags’ for referral to a developmental paediatrician [3].
LLE is a common condition, with population-level estimates for singletons ranging from 13%, based on receptive and expressive criterion [2], to 19%, based on expressive language criterion [2, 4]. Our recent population-level estimate for twins was 37.8%, much higher than for singletons. The prevalence of LLE was higher still for monozygotic (MZ) twins compared to dizygotic (DZ twins (46.5% vs. 31.0%) [5] and highly heritable, consistent with the UK Twins Early Development Study (TEDS) [6]. Postnatal environmental influences, in the form of poorer quality maternal interactions, have been positively associated with LLE in twins [7,8,9]. A recent study reported genotype-environment correlations between parental language input and twin language development [10].
Population-level studies of twins at age 2 have reported higher mean expressive vocabulary scores for females compared to males [5, 11]. This is consistent with studies of singletons [1, 2, 12] and is attributed to differential neurobiological maturation favouring girls [13]. Because early language development follows a different developmental course in girls and boys, gender-specific norms are used to identify LLE [6].
Twin pregnancies have higher rates of prenatal, perinatal and neonatal mortality and morbidity than singleton pregnancies [14, 15]. Twins’ early mental and motor development, at 6, 12 and 18 months, has been reported to lag behind singletons and to be associated with low birthweight, not family socioeconomic circumstances [16]. Studies have yielded a mixed picture of the relative importance of prenatal and perinatal environment risk factors in the etiology of LLE. Findings have varied across study designs and methods. Studies that have included twins whose birthweight and/or gestational age was in the low range have reported significant associations between prenatal and perinatal risk factors and lower verbal and nonverbal cognitive abilities [17,18,19]. Whereas, studies that have selected or adjusted for birthweight and/or gestational age have reported negligible associations between prenatal and perinatal risk factors and LLE [15, 20, 21].
The aim of the present study was to investigate prenatal and perinatal contributions to LLE in a longitudinal population-representative sample of twins without overt disability.
Methods
Study design and twin sample
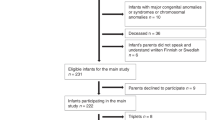
The study design was a prospective cohort study of twins drawn from a total population sample frame comprising statutory notifications of all births in Western Australia (WA) in 2000–2003 [22].
There were 1135 sets of live twins born in this time period; 941 (83%) families were contacted by mail, and 698 (74%) consented to participate in the study, 61% of all twins born in WA in 2000–2003. A comparison with data for all twins born in 2000–2003 showed that the study participants were broadly representative of the total twin population from which they were drawn [5]. Twin pairs with exposure to languages other than English (52 twin pairs) or twin pairs in which at least one twin had hearing impairment, neurological disorders, or developmental disorders (14 twin pairs) were excluded from the twin sample. The exclusionary criteria resulted in 633 twin pairs who were eligible to participate in the prospective longitudinal cohort study. A postal questionnaire was sent to the twins’ parents one month prior to the twins’ second birthday. The response rate to the postal questionnaire was 75%. In this study, questionnaire data were available for 473 eligible twin pairs of approximately 2 years of age (in days, mean age is 755.8, range, 687–899). There were 454 boys (47.9%) and 492 girls (52.1%).
Measures
Outcome variable
An Australian adaptation of the MacArthur Communicative Development Inventories: Words and Sentences (CDI-WS) [6] was administered at age 2 by postal questionnaire. With the permission of the authors, 24 Standard American English vocabulary items were replaced with Standard Australian English vocabulary items (e.g., ‘nappy’ for ‘diaper’; ‘footpath for ‘sidewalk’ [5]. This is consistent with Reilly et al. (2009 [12]. LLE was defined as a gender-specific score at or below the 10th percentile on the CDI-WS (Words Produced). This equated to 119 words or less for girls and 79 words or less for boys [23]. NLE was defined as a gender-specific score above the 10th percentile on the CDI-WS (Words Produced) [6]. This is also the criterion that was used by Reilly et al. (2009) to identify LLE in a population-based sample of Australian children at 24 months. The CDI-WS and its adaptations have robust psychometric properties and are the most well recognized reliable, valid and feasible assessments for toddlers [24, 25].
Predictor variables
Maternal variables
The data source for maternal, pregnancy, labour, delivery and neonatal variables was the Midwives’ Notification System (MNS). These data are collected by statute on all live births, stillbirths, and neonatal deaths in WA [22]. MNS variables included the mother’s age in years, height in centimetres, parity, marital status, ethnic status and residential address. The mother’s residential address at the time of the birth of the twins was linked to the 1996 Population and Housing Census. Three small-area indices (Socioeconomic Indicators for Areas: SEIFA) were available for each twin-pair [26]. Each index summarizes a different aspect of the socio-economic conditions of the Australian population using a combination of variables. The Index of Relative Socio-Economic Disadvantage, which is used here, is derived from variables that reflect or measure relative disadvantage. Variables used to calculate the index of relative socio-economic disadvantage include low income, low educational attainment, high unemployment and people with low skilled occupations. Lower scores are associated with greater disadvantage. Maternal education, country of birth and family income variables were collected by postal questionnaire.
Pregnancy variables
Pregnancy variables included binary variables to indicate the presence or absence of the following circumstances: threatened abortion, pre-eclampsia, placenta praevia, abruption, antepartum haemorrhage (APH), gestational diabetes, fertility treatment, threatened pre-term labour, precipitate delivery, and post-partum haemorrhage (PPH). We also coded a general category for ‘other pregnancy complications’ which occurred in proportions too small to model.
Infant variables
We included several characteristics relevant to the infant’s status at birth. For each infant we included the infant’s gender, twin birth-order and binary indicators for fetal distress, cephalopelvic disproportion, prolapsed cord, 5-min Apgar score, Time to Spontaneous Respiration (TSR), and intubation status.
In addition to these we also included estimated gestational age and a measure of each infant’s proportion of optimal birthweight (POBW). POBW is a measure of the appropriateness of intrauterine growth and is routinely calculated from the birth records of all children born in Western Australia. Because birthweight is the end result of growth over the period of gestation it is therefore determined both by the length of gestation and the rate of intra-uterine growth. Duration of gestation may be curtailed or prolonged, and this is usually the result of pathological factors, hence abnormal duration of gestation may be considered to reflect pathological factors. However, since delivery must follow the period of intrauterine growth, duration of gestation is not a determinant of growth and hence cannot be a pathological determinant of growth, though it is the primary determinant of birthweight.
The rate of intrauterine growth is determined by many factors both pathological (maternal, fetal or environmental) and non-pathological (genetic endowment, particularly fetal gender, and maternal environment). Thus it is appropriate that fetal growth rate should vary between individuals, since the non-pathological factors determining growth rate varies between individuals. For example, female newborns appropriately weigh less than male newborns of the same gestation; babies of small women weigh less than babies of tall women and a woman’s first birth tends to weigh less than her subsequent births. We define the optimal fetal growth rate for any particular fetus as the median birthweight achieved by fetuses with the same values for the non-pathological determinants of fetal growth and duration of gestation, in the absence of any pathological determinants of fetal growth. This median is expressed as the ‘optimal birthweight’ once the values of the non-pathological determinants of growth have been specified.
The non-pathological determinants considered in our statistical models of POBW were fetal gender, maternal age, height and parity. Exclusion of pathological factors was achieved by limiting the sample from which optimal birthweights were identified to singleton, live births without congenital abnormalities born to non-smoking mothers following pregnancies without any complications known to affect intrauterine growth [27]. The median value of POBW is 100 and values less than this signify infants that are under grown while values greater than this represent growth in excess of optimal growth.
In this study POBW and gestational age were defined as ‘at risk for twins’. For POBW this was defined as the bottom 15% of the study sample (a POBW of ≤ 76.43), and for gestational age this was defined as gestational age of 33 weeks or less.
Zygosity
Twin zygosity was determined by molecular analysis of buccal swab samples. For twin pairs with unknown zygosity, a discriminant analysis of questionnaire items reported by parents was used to assign zygosity. The final twin counts were 313 DZ pairs and 160 MZ pairs, for a total of 473 pairs and 946 individuals [5].
Table 1 indicates that there are a number of candidate predictors with small numbers of children in the ‘at risk’ categories. Although it is important to describe the distribution of these predictors within the twin population, some of these predictors contained so few children they were considered unsuitable for the logistic regression analyses which follow, and were excluded from further consideration. These predictors were: abruption, placenta praevia, precipitate delivery, intubation, cephalopelvic disproportion, prolapsed cord, and 5-min Apgar less than 7.
Statistical analyses
Our outcome measure (i.e., LLE) was a score at or below the gender-specific 10th percentile for Word Produced on the CDI-WS. Because the outcome measure was gender-specific, gender was not included in the models estimated below.
All predictor variables were modelled as risk variables (e.g., POBW <15th percentile of the sample). For each risk variable, the ‘least risk’ category (e.g., normal POBW) was the reference category (see Table 1). To estimate the odds of LLE, a generalised linear mixed model with a logistic link function was used to explicitly account for the paired structure of the data, and estimate the subject-specific risks for LLE. To account for correlation within twin-pairs, twin-pair specific parameters were estimated by incorporating a random effects component for the twin-pair [28]. These analyses were undertaken in PROC GLIMMIX in SAS version 9.4 [29], using maximum likelihood with adaptive quadrature estimation. For the purposes of simplicity, this analysis is referred to as a logistic regression analysis, as we are estimating the odds of LLE for the candidate predictors. This analysis produced subject-specific odds ratios for LLE. Unadjusted odds ratios (ORs), adjusted odds ratios (aORs), and 95% confidence intervals (CIs) were estimated with bivariate and multivariable logistic regression to identify factors associated with LLE in the study sample.
Results
Table 1 shows the adjusted and unadjusted odds of LLE associated with the predictor variables. Of 21 maternal, pregnancy, delivery and neonatal risk factors considered, 5 had statistically significant associations with LLE in the multivariable model. In order of odds ratio, from highest to lowest the risk factors were: Gestational diabetes (aOR: 19.5 [1.2, 313.1]), TSR greater than 2 min (aOR: 13.6 [2.0, 91.1]), parity of 1 (aOR: 7.6 [1.6, 37.5]; parity of 2 or more (aOR:7.9 [1.5, 41.9]), monozygosity (aOR: 6.9 [1.7, 27.9]) and POBW below the 15th percentile of the sample (aOR: 4.6 [1.7, 12.7]). The model included maternal sociodemographic risk factors (e.g., low maternal education, socioeconomic area disadvantage) that were not associated with increased odds of LLE.
Discussion
Late language emergence has long been regarded as the hallmark individual difference between twins and singletons. Large-scale population-level studies have drawn attention to the neurobiological etiology of LLE in singletons [2, 12] and twins at age 2 [5, 11]. Recent population-level behavior genetics studies have drawn attention to the important role of genetic factors in the etiology of LLE in twins [5, 11]. This study has drawn attention to five risk factors for LLE that can be detected and treated by clinicians in the prenatal, perinatal and neonatal periods in twins without frank disability. The benefits of early intervention should translate to reduced risk for LLE at age 2. The current study selected on twins without frank disability but did not select on or control for birthweight and/or gestational age variation. This meant that the independent risk conferred by birthweight, gestational age and fetal growth restriction was quantified in a model that included pregnancy and birth risks as well as sociodemographic risks. Necessarily, studies of twin-singleton differences [19, 20, 30] have selected on or controlled for birthweight and/or gestational age variation between twins and singletons to elucidate mediators and moderators of twinning effects on LLE [21].
The results of this study have drawn attention to the role of gestational diabetes, prolonged TSR, fetal growth restriction in the etiology of LLE. These risks are all well-known complications of twin pregnancy [15, 31] and risk factors for LLE in singletons. This study has shown the pervasive adverse influence of these risks on twins’ neurodevelopment in the second year of life. Prenatal life is a critical phase of brain development, during which even subtle differences in fetal growth have been associated with differences in postnatal brain maturation and cognitive abilities in twins [32].
Multivariate analysis yielded the following significant predictors of LLE in twins, in order of odds ratio from highest to lowest: Gestational diabetes; TSR > 2 min; multiparity; monozygosity and POBW below the 15th percentile of the twin sample. The only risk factor unique to twin pregnancies was monozygosity. This risk factor retained statistical significance in a model that multivariately adjusted for the effects of other risk factors. This suggests that the biological mechanisms underlying MZ twinning itself may contribute to the elevated prevalence of LLE in MZ twins, compared to DZ twins [5], that cannot be attributed to a shared postnatal environment, which all twins share, irrespective of zygosity [7, 10, 33]. The only family environment risk factor was multiparity (i.e., ≥ 1 biological sibling). It was striking to see that the presence of one or more siblings was a risk exposure for LLE in twins, entirely consistent with birth order effects for LLE in singletons [2, 12, 34].
POBW is a population-based estimate of fetal growth that is a more differentiated measure of fetal growth than absolute birthweight. POBW is an important index of the child’s developmental status [2, 35]. The advantage of this measure of appropriateness of growth, over birthweight, is that it is individualised and takes into account the duration of gestation. The advantage over the commonly used percentile measures (sometimes termed ‘small for gestational age’) is that it is more accurate and generalizable at the extremes, and being a parametric ratio quantity, is more amenable to statistical manipulation. The results of this study support the view that where POBW can be calculated, it is generally preferable to more traditional measures such as gestational age and birthweight [36].
Strengths and limitations
Strengths of the study include the population-based prospective cohort design; use of a reference-group based definition of LLE; use of maternal, pregnancy, labour, delivery and neonatal variables collected prospectively by statute; use of a population-based estimate of fetal growth; and exclusion of twins with developmental disorders. The main limitation of the study was the relatively low prevalence of some of the risk factors, leading to wide CIs for some of the estimates. Another limitation is that the MNS does not include data on pregnancy complications that are unique to twin pregnancies (e.g., twin reversed arterial perfusion and twin-twin transfusion syndrome).
Follow-up investigations are needed to find out if complications in the fetal and neonatal periods play a role in the course of twins’ language development over time.
Conclusions
The results provided evidence for the role of complications in the fetal and neonatal periods, and monozygotic twinning in the etiology of LLE in twins with otherwise healthy development at age 2. The results draw attention to the importance of optimising prenatal life for twins to counter adverse neurodevelopmental outcomes in the postnatal period.
Abbreviations
- aOR:
-
Adjusted Odds Ratio
- APH:
-
Antepartum Haemorrhage
- CDI-WS:
-
MacArthur Communicative Development Inventories: Words and Sentences
- CIs:
-
Confidence Intervals
- DZ:
-
Dizygotic
- LLE:
-
Late Language Emergence
- MNS:
-
Midwives’ Notification System
- MZ:
-
Monozygotic
- NLE:
-
Normal Language Emergence
- OR:
-
Unadjusted Odds Ratio
- POBW:
-
Proportion of Optimal Birthweight
- PPH:
-
Post-Partum Haemorrhage
- SEIFA:
-
Socioeconomic Indicators for Areas
- TEDS:
-
Twins Early Development Study
- TSR:
-
Time to Spontaneous Respiration
- WA:
-
Western Australia
References
Fenson L, DP S, Reznick JS, Bates E, Thal D, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev. 1994;59(5):1–173.
Zubrick S, Taylor C, Rice M, Slegers D. Late language emergence at 24 months: an epidemiological study of prevalence, predictors and covariates. J Speech, Language, and Hearing Res. 2007;50:1562–92.
Bellman M, Byrne O, Sege R. Developmental assessment of children. BMJ. 2013;346. https://doi.org/10.1136/bmj.e8687.
Reilly S, Bavin EL, Bretherton L, Conway L, Eadie P, Cini E, Prior M, Ukoumunne OC, Wake M. The early language in Victoria study (ELVS): a prospective, longitudinal study of communication skills and expressive vocabulary development at 8, 12 and 24 months. Int J Speech-Language Pathol. 2009;11(5):344–57.
Rice M, Zubrick S, Taylor C, Gayan J. Late language emergence in 24 month twins: heritable and increased risk for twins. J Speech, Language and Hearing Res. 2014;57(3):917–28.
Fenson L, Marchman V, Thal D, Dale P, Reznick J, Bates E. MacArthur-bates communicative development inventories, User’s guide and technical manual. Baltimore: Brookes; 2007.
Thorpe K, Rutter M, Greenwood R. Twins as a natural experiment to study the causes of mild language delay: II: family interaction risk factors. J Child Psychol & Psychiat. 2003;44(3):342–55.
Lytton H, Conway D, Sauve R. The impact of twinship on parent-child interaction. J Pers Soc Psychol. 1977;35(2):97–107.
Beer C, Israel C, Johnson S, Marlow N, Whitelaw A, Glazebrook C. Twin birth: an additional risk factor for poorer quality maternal interactions with very preterm infants? Early Hum Dev. 2013;89(8):555–9.
Dale P, Tosto M, Hayiou-Thomas M, Plomin R. Why does parental language input style predict child language development? A twin study of gene–environment correlation. J Commun Disord. 2015;57:106–17.
Dale PS, Simonoff E, Bishop DVM, Eley TC, Oliver B, Price TS, Purcell S, Stevenson J, Plomin R. Genetic influence on language delay in two-year-old children. Nat Neurosci. 1998;1(4):324–8.
Reilly S, Wake M, Bavin EL, Prior M, Williams J, Bretherton L, Eadie P, Barrett Y, Okoumunne OC. Predicting language at 2 years of age: a prospective community study. Pediatrics. 2007;120:e1441–9.
Galsworthy M, Dionne G, Dale P, Plomin R. Sex differences in early verbal and non-verbal cognitive development. Dev Sci. 2000;3:206–15.
Alexander G, Wingate M, Salihu H, Kirby R. Fetal and neonatal mortality risks of multiple births. Obstet Gynecol Clin N Am. 2005;32:1–16.
Cooke R. Does neonatal and infant neurodevelopmental morbidity of multiples and singletons differ? Semin Fetal Neonatal Med. 2010;15(6):362–6.
Riese M. Risk and early development: Findings from the Louisville Twin Study. In: Blickstein I, Keith L, editors. In: Multiple pregnancy: Epidemiology, gestation and perinatal outcomes. 2ed ed. Abbington, Oxon: Taylor & Francis; 2005. p. 797–806.
Lytton H, Watts D, Dunn B. Twin-singleton differences in verbal ability: where do they stem from? Intelligence. 1987;11:359–69.
Kyriakidou M, Karagianni P, Iliodromiti Z, Exadaktilou S, Nikolaidis N. Comparison of 24 months neurodevelopmental outcomes in twins and singletons ≤ 34 weeks gestation at birth. J Pediatric and Neonatal Individualized Med. 2013;2(1):48–54.
Ronalds GA, De Stavola BL, Leon DA. The cognitive cost of being a twin: evidence from comparisons within families in the Aberdeen children of the 1950s cohort study. BMJ: British Med J. 2005;331(7528):1306.
Rutter M, Thorpe K, Greenwood R, Northstone K, Golding J. Twins as a natural experiment to study the causes of mild language delay: I: design; twin-singleton differences in language, and obstetric risks. J Child Psychol Psychiatry. 2003;44(3):326–41.
Lung FW, Shu BC, Chiang TL, Lin SJ. Twin–singleton influence on infant development: a national birth cohort study. Child Care Health Dev. 2009;35(3):409–18.
Gee V, Green T. Perinatal statistics in Western Australia, 2003. Twenty-first Annual Report of the Western Australian Midwives' Notification System. In: Department of Health. Perth; 2004.
Fenson L, Dale PS, Reznick S, Thal D, Bates E, Hartung J, Pethick S, Reilly J. MacArthur communicative development inventories: users guide and technical manual. Singular: San Diego; 1993.
Law J, Roy P, Law J, Roy P. Parental report of infant language skills: a review of the development and application of the communicative development inventories. Child Adolesc Mental Health. 2008;13(4):198–206.
Heilmann J, Weismer SE, Evans J, Hollar C, Heilmann J, Weismer SE, Evans J, Hollar C. Utility of the MacArthur-Bates Communicative Development Inventory in Identifying Language Abilities of Late-Talking and Typically Developing Toddlers. Am J Speech-Lang Pathol. 2005;14(1):40–51.
Australian Bureau of Statistics: 1996 Census of Population and Housing. Socio-Economic Indexes for Areas (Catalogue no. 2039.0). In. Canberra, Australia: Australian Bureau of Statistics; 1998.
Blair E. Why do Aboriginal neonates weigh less? II. Determinants of birthweight for gestation. J Pediatrics and Child Health. 1996;32:498–503.
Carlin J, Gurrin L, Sterne J, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34(5):1089–99.
SAS Institute Inc.: SAS for Windows Version 9.4. Cary, NC: SAS Institute Inc; 2013.
Reznick JS, Corley R, Robinson J. A longitudinal twin study of intelligence in the second year. Monogr Soc Res Child Dev. 1997;62(1):1–162.
Blickstein I, Keith LG, editors. Multiple pregnancy: epidemiology, gestation and perinatal outcomes. 2edn ed. Abbington, Oxon: Taylor & Francis; 2005.
Raznahan A, Greenstein D, Lee NR, Clasen L, Giedd J. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci. 2012;109(28):11366–71.
Lytton H. Parent-child interaction: the socialization process observed in twin and singleton families. New York: Plenum Press; 1980.
Pine JM. Variation in vocabulary development as a function of birth order. Child Dev. 1995;66:272–81.
Malacova E, Li J, Blair E, Mattes E, de Klerk N, Stanley F. Neighbourhood socioeconomic status and maternal factors at birth as moderators of the association between birth characteristics and school attainment: a population study of children attending government schools in Western Australia. J Epidemiol Community Health. 2009;63(10):842–9.
Blair E, Liu Y, de Klerk N, Lawrence D. Optimal fetal growth for the Caucasian singleton and assessment of appropriateness of fetal growth: analysis of a total population perinatal database. BMC Pediatr. 2005;5(13):1471–2431.
Acknowledgements
We especially thank the children and families who participated in the study and the following members of the research team: Antonietta Grant, Erika Hagemann, Alani Morgan, Virginia Muniandy, Elke Scheepers and Alicia Watkins. We greatly appreciate Dr. David Lawrence’s statistical advice as well as Denise Perpich’s data management and preparation of data summaries. We also wish to thank the staff at the Western Australian Data Linkage Branch and the Maternal and Child Health Unit.
Funding
This work was made possible by grants from the National Institutes of Health (RO1DC05226, P30DC005803, P30HD002528). Catherine Taylor, Stephen Zubrick and Daniel Christensen are supported by the Australian Research Council Centre of Excellence for Children and Families over the Life Course (CE140100027).
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available due to the terms of consent to which the participants agreed. The datasets are available from the corresponding author on reasonable request and approval from the Department of Health Western Australia Human Research Ethics Committee.
Author information
Authors and Affiliations
Contributions
CLT, SRZ and MLR conceived of the paper. CLT, SRZ, DC, EB and MLR contributed to the study design. DC and SRZ undertook the analyses. CLT, MLR, DC, EB and SRZ contributed to the interpretation of the results and writing of the paper. CLT, MLR, DC, EB and SRZ approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval to conduct this study was obtained from the Curtin University of Technology Human Research Ethics Committee (155/2009), the Department of Health Western Australia Human Research Ethics Committee (2010_6), and the University of Kansas Human Research Committee (12582). As the study children were all minors at the time these data were collected, written informed consent was obtained from the primary caregiver on behalf of each of the study children.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Taylor, C.L., Rice, M.L., Christensen, D. et al. Prenatal and perinatal risks for late language emergence in a population-level sample of twins at age 2. BMC Pediatr 18, 41 (2018). https://doi.org/10.1186/s12887-018-1035-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-018-1035-9