Abstract
Background
The nature and magnitude of adverse drug events (ADEs) among hospitalized children in low-income countries is not well described. The aim of this study was thus, to assess the incidence and nature of ADEs in hospitalized children at a teaching hospital in Ethiopia.
Methods
We used prospective observational method to study children that were hospitalized to Jimma University Specialized Hospital between 1 February and 1 May 2011. ADEs were identified using review of treatment charts, interview of patient and care-giver, attendance at ward rounds and/or meetings and voluntary staff reports. Two senior pediatric residents evaluated the severity and preventability of ADEs using preset criteria. Logistic regression analysis was employed to determine predictors of ADEs.
Results
There were 634 admissions with 6182 patient-days of hospital stay. There were 2072 written medication orders accounting for 35,117 medication doses. Fifty eight ADEs were identified with an incidence of 9.2 per 100 admissions, 1.7 per 1000 medication doses and 9.4 per 1000 patient-days. One-third of ADEs were preventable; 47 % of these were due to errors in the administration stage of medication use process. Regarding the severity of ADEs, 91 % caused temporary harms and 9 % resulted in permanent harm/death. Anti-infective drugs were the most common medications associated with ADEs. The occurrence of ADEs increased with age, length of hospital stay, and use of CNS, endocrine and antihistamine medicines.
Conclusion
ADEs are common in hospitalized children in low-income settings; however, one-third deemed preventable. A strategy to prevent the occurrence and consequences of ADEs including education of nurses/physicians is of paramount importance.
Similar content being viewed by others
Background
Adverse events, injuries that occur during medical management, have received attention after the Harvard Medical Practice Study [1, 2]. Brennan et al. [1] estimated that 3.7 % of all hospitalized patients experienced an adverse event. In 1999, the Institute of Medicine of USA [3] reported that preventable medication related events alone could result in 7000 deaths annually.
Despite the extensive literature on adverse events due to medications (also known as adverse drug events) in adult populations, there is scarcity of published study on pediatric-specific adverse drug events (ADEs) [4, 5]. But, there are suggestions that harm to medication use might be higher in children than in adults [6]. The rate of pediatric ADEs rates can range from 6.6 to 15.7 events per 1000 patient-days [4, 7] and 1.2 per 1000 medication doses [7], with a potential ADE rate of 10 per 100 admissions [8]. However, studies that utilized adverse drug reaction (ADR) as an outcome reported a higher incidence of ADRs in pediatric inpatients, 10 to 17 % [9–14]. Moreover, in another meta-analysis of prospective studies the overall incidence of ADRs was found to be 9.5 %; severe reactions accounted for 12.3 % of the total [15].
The incidences of adverse drug events may vary depend on study definitions and detection methods. The available methods of detection include voluntary reporting [16–18], patient interviews and chart reviews [16], trigger tools [19] and computerized monitoring systems [20–23]. While spontaneous reporting underestimates the real incidence of ADEs [24], the use of computerized monitoring system generates the best results [22, 23]. Since there is no single best method [20], the use of multiple strategies maximizes the incidence of ADE quantification [23].
ADEs are a major burden on healthcare; the consequences can vary from temporary/permanent harms [25] to costs associated with ADE management [3, 9, 26–28] and prolonged hospital stay [29, 30]. Studies have shown that 2 % of hospital admissions are due to ADEs in the pediatrics [12, 31], but a higher admission is reported in patients with off-label use of medications, 11 % [32]. A Canadian study in the pediatric patients described that 8 % of emergency department visits were attributed to medication-related events, of which two-third were deemed preventable [33]. However, there is paucity of information with regard to the magnitude of ADEs in hospitalized children in developing countries including Africa. Thus, the aim of this study was to assess the incidence and nature of ADEs in hospitalized children in the pediatric ward of a teaching hospital in Ethiopia.
Methods
Study setting and design
This prospective observational study was conducted in the pediatric ward of Jimma University Specialized Hospital (JUSH), Ethiopia, which had a bed capacity of 503. The hospital was providing both outpatient and inpatient pediatric services for children less than 14 years. During the study period, the inpatient department had four units i.e., critical care, neonatal (for neonates ≤ 14 days of age), nutritional rehabilitation and general. There were 4 pediatricians, 5 senior residents, 12 junior residents and 20 nurses during the study period.
All pediatric inpatients that were hospitalized between 1 February and 1 May 2011 were included. Patients were excluded if the hospital admission was for less than 24 h, and/or if the admission was the result of an intentional (self-administered) overdose. All admitted pediatric patients were followed for the main outcome measure (occurrence of actual ADEs) from admission to discharge/transfer/death. In addition, the preventability and severity of each ADE episode was evaluated.
Data collection and ADE case evaluation
ADE was defined as any incident resulting in injury from any stage of the medication use process (ordering, transcribing, dispensing, administrating and monitoring) [34]. A preventable ADE was an injury due to an error at any stage in the medication use - for instance, hypoglycemia due to insulin overdose. Non-preventable ADE was an injury not related to error in the medication process. An allergic reaction in a patient not previously known to be allergic to the medication is an example of non-preventable ADE.
A combination of methods was employed to identify ADEs. From the patient medical record, one nurse & two pharmacists collected the following demographic and clinical data from medical records of participants using a structured format: age, gender, history of previous medication and drug allergy, admission diagnoses, current drug dosage and regimen and length of hospital stay. Charts were reviewed daily until discharge/transfer/death of the child. Moreover, changes in medication regimens including discontinuation or initiation of new medications and abnormal laboratory values were recorded. A list of pediatric trigger tools, these are drugs or clues that have links to potential ADEs because either they are antidotes or given to reverse the action of a drug responsible for ADE, was adapted from US organizations [35, 36] and modified based on availability of medicines in Ethiopia [37] (Additional file 1).
In case of medication management changes, the responsible physician was contacted for clarification. Evaluation was made whether the change was due to ADE. Besides, we asked the pediatric ward staff to report any actual events or potentially unsafe medication systems that was noticed. A clinical pharmacist was attending clinical rounds/meetings and visited the ward daily to solicit any alerts for ADE. The clinical pharmacist forwarded any suspected ADE cases for further evaluation to a multidisciplinary team comprised of senior attending physician, pediatric resident, nurses and pharmacists. Once decided by the multidisciplinary, the suspected ADE was assessed for temporal relationship between the drug and the event as per WHO-UMC criteria [38].
The response to withdrawal plausibility, if possible was also evaluated. Those in the category of possible, probable/likely and certain were considered. We searched biomedical literatures to establish the strength of published data, if any, on the relationship between the ADEs and the medication. During this evaluation, the expertise of the pediatrics team was used when required for further work-up especially on the exclusion of possible disease condition. Since ADEs were actual patient harms, a specific medical care was given when applicable to prevent further damage.
For categorizing severity of ADEs, the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) scale was employed [39]. Each event was assigned a harm level of E - I. Severity category E, F, G, H and I referred to temporary harm to the patient requiring intervention, temporary harm to the patient requiring initial or prolonged hospitalization, permanent harm to the patient, intervention required to sustain life, e.g. cardiovascular/respiratory support and death of the patient respectively.
Preventability was determined using the explicit criteria developed by Schmumock and Thornton [40]. Categorization of events (severity and preventability) was evaluated by a panel of two senior pediatric residents, who independently classified the events using preset criteria. The reviewers reached consensus through discussion for discordant classification.
Data analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 16. Qualitative variables were described as frequencies (percentages) and quantitative variables as mean ± standard deviation (SD). Each ADE was treated in the analysis as a separate independent ADE. ADE incidence was calculated per 100 admissions, per 1000 patient-days, per 1000 medication doses and per 100 medication orders. Kappa statistics were used to determine inter-rater reliabilities. Covariates for occurrence of ADEs were evaluated using logistic regression analysis. The covariates were number of medications ordered, length of hospital stay, age, medication class and presence of infectious disease. Odds ratio (crude and adjusted) with its p-value and 95 % confidence interval was reported. A p-value <0.05 was considered statistically significant.
Ethical consideration
Ethical approval was obtained from Ethical Review Board of Jimma University. Patient/caregiver/family member gave verbal consent for the information required. Appropriate interventions were recommended to the pediatrics team when serious medical errors were identified.
Results
Study population characteristics
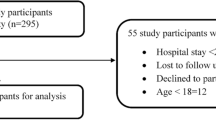
A total of 699 admitted patients were followed. Among these, 65 admissions were excluded (length of stay less than 24 h and/or insufficient data). We included 634 admissions representing 600 patients for analysis.
Pediatrics of various ages were included (minimum 7 days, maximum 14 years). The mean age of the patients was 2.9 years, 371 (61.8 %) were males and 215 (33.9 %) infants (Fig. 1). The length of hospital stay was 6182 patient-days. A total of 2072 medication orders were written accounting for 35,117 medication doses and 55.4 medication doses per admission. Of those included in the analysis, 15 (2.5 %) admissions didn’t receive any medication during their hospital stay. The mean ± SD length of hospital stay and medications ordered were 9.8 ± 8.8 days and 3.3 ± 1.9 respectively.
The top 10 admitting diagnosis were severe pneumonia 173 (27.3 %), severe acute malnutrition 120 (18.9 %), early/late onset neonatal sepsis 108 (17.0 %), meningitis 59 (9.3 %), acute gastroenteritis 46 (7.3 %), malaria 39 (6.2 %), anemia’s of different causes 43 (6.8 %), first episode of wheeze 32 (5.1 %), congestive heart failure 27 (4.3 %) and soft tissues abscess 26 (4.1 %). Anti-infective drugs, 1330 (64.2 %) were the leading class of medications prescribed followed by drugs acting on the central nervous system (CNS), 206 (9.9 %) (Table 1).
Incidence, preventability and severity of ADEs
A total of 58 ADEs were identified in 46 patients. The incidences of ADEs were found to be 9.2 per 100 admissions (crude rate), 1.7 per 1000 medication doses, 9.4 per 1000 patient days and 2.8 per 100 medication orders. The majority of ADEs occurred in the general pediatric ward, 33 (56.9 %) followed by the critical unit, 21 (36.2 %). Twelve patients had more than 1 ADEs during hospitalization. 4 of the 58 (6.9 %) ADEs were the primary reasons for hospitalization i.e. these ADEs were a cause of hospital admissions. For example, a child with known type I diabetes was admitted as result of severe hypoglycemia following insulin injection.
Thirteen (22.4 %) of the ADEs were injection site phlebitis (pain, swelling and redness) and 12 (20.7 %) were maculopapular skin rash with or without urticaria (Table 2). Of the 58 ADEs, the reviewers classified 39 (67.2 %) as non-preventable and 19 (32.8 %) as preventable. Among the preventable ADEs, 9 (47 %) were following errors in the administration stage of medication use process, 8 (42 %) due to improper dosage and 2 (11 %) were attributed to monitoring errors. Examples of preventable ADEs are listed in Table 3.
Majority of the ADEs were of temporary harms i.e. 39 (67 %) category E, and 14 (24 %) category F. Only 5 (9 %) of the ADEs resulted in permanent harm/death. Three out of four permanent harms were due to wrong administration of drugs. There was one death associated with monitoring error; it was most likely due to a failure to use appropriate clinical or laboratory data to monitor response to crystalline penicillin prescribed for severe pneumonia. The level of agreement between reviewers for severity rating of ADE was “good” (Kappa = 0.65) whereas it was “moderate” (Kappa = 0.46) for preventability.
Anti-infective was the most common medication class responsible for the ADEs (Table 4). Injection site phlebitis, skin rash with/without urticaria and antibiotic associated colitis were the common ADEs associated with anti-infectives. Of 58 ADEs, 21 (36.2 %) were detected in infants and 14 (24.1 %) in school-age children. Thirty nine (67.2 %) of all the ADEs occurred with IV route of administration and 16 (27.6 %) followed oral route. Drugs were used to manage 30 (51.7 %) ADEs while the offending drugs were discontinued in 16 (27.6 %) cases. Fifteen (25.8 %) ADEs required increased monitoring of vital signs and/or laboratory values (e.g. serial of random blood glucose level determinations) and 4 (6.9 %) ADEs required dose reduction. Other interventions included change of the IV injection site, saline flushing of the IV line, daily wound care and abscess drainage.
In the multivariable analysis, length of stay greater than 23 days (AOR 8, 95 % CI: 2.93, 22.04), presence of infectious disease (AOR 3.43, 95 % CI: 1.19, 9.91), use of anti-histamines and anti-allergic (AOR 32.5, 95 % CI: 6.0, 176.45), CNS (AOR 2.09, 95 % CI: 1.01, 4.32) and endocrine (AOR 3.38, 95 % CI: 1.40, 8.15) medicines were associated with occurrence of ADEs (Table 5).
Discussion
In countries with better resources, medication safety programs are well integrated with the health care system [7, 9, 10]. In low income countries like Ethiopia, however, healthcare coverage is prioritized to medication safety. Moreover, the medication use system is not evidence based. To our knowledge, this is the first study from Ethiopia assessing the extent of ADEs in children and hence, this study will provide baseline data for patient safety advocates.
Estimation of the incidence of ADEs significantly depends on the trigger to which the event was searched, the methodology and definition used. The incidence rates of ADEs in our study were higher when compared with two studies in the USA; 6 per 100 admissions and 7.5 per 1000 patient-days [41] and 2.3 per 100 admissions and 6.6 per 1000 patient-days [4]. But the rates were lower than a finding from New Zealand study (12.9 per 100 admissions and 22.1 per 1000 patient-days) [9]. Another study from USA [7], conducted through a retrospective focused chart review and pediatric trigger tools, reported an ADEs incidence of 11.1 per 100 admissions, 15.7 per 1000 patient-days and 1.23 per 1000 medication doses. A similar study definition was employed in Takata et al. study [7], but a little bit higher ADE incidence was reported. It shouldn’t be mistakenly noticed that our finding is lower than that of the USA because of the methodology used is different (retrospective, focused chart review). But, when we compared with Kaushal et al. [4] and Holdsworth et al. [41] studies that used similar methods, we found a higher rate of ADEs.
Comparing the preventability of ADEs in our study with previous studies, different authors reported different figures: Holdsworth et al. [41] 61 %, Kaushal et al. [4] 19.2 %, Kunnac et al. [9] 57 % and Takata et al. [7] 29 %. In this study, the most common medication errors responsible for preventable ADEs were errors occurred during administration and improper dose (dose too low/high). According to Takata et al. [7], most of preventable ADEs occurred during monitoring stage; defined as a failure to use appropriate clinical or laboratory data for adequate assessment of patient response to prescribed therapy.
In this study, the severity rating for observed ADEs showed that around 91 % resulted in temporary harm (either category E or G). Takata et al. [7], reported that all of the ADEs they detected caused temporary harm. Other than the NCC MERP scale, other previous studies also reported serious to life-threatening events in 24–34 % of ADEs [4, 41], and permanent harms in 5 % of ADEs [41]. Thus, comparatively the severity of ADEs in our study (9 % resulted in permanent harm/death) was very high. In this study, 3 of the 4 events that resulted in permanent harm were due to inadvertent route of administration of medication, and were classified as preventable. The incidence of ADEs was comparable with most previous reports, but we can still appreciate that hospitalized children in our setting were facing a considerable amount of preventable medication-related permanent harm.
In our study, injection site phlebitis was the commonest ADEs detected. Kaushal et al. [4] reported, two third of non-preventable ADEs were related to antibiotic associated Clostriduim difficile infections, rashes, allergic reactions and yeast infection. Again similar findings were reported by Takata et al. [7] where pruritus was the most common ADE.
In our study, the most common medication class responsible for the ADEs was anti-infectives. This medication class was also the most commonly used medication classes among all study participants. In other studies, the most commonly cited medication classes associated with ADEs were analgesics/opioids followed by antibiotics [7, 41]. Opioids are mentioned frequently as a cause of ADEs in the literature but they were not available in JUSH during the study period.
Additional interventions were required to manage ADEs. ADE management was mostly done through prescriptions of additional medications followed by discontinuation of the offending agent. These additional interventions can predict the impact of ADE on this hospital as well as to the patient. In developing countries like Ethiopia, these interventions are associated with immense cost imposition to the health system.
Presence of infectious disease, use of antihistamines and anti-allergic, CNS and endocrine medicines was associated with occurrence of ADEs. The use of anti-histamine and anti-allergic medications were strongly associated with ADEs. This interpretation should be in caution, however. None of the antihistamine and anti-allergic drugs were implicated in causing ADEs, but 6 of the 10 prescriptions were written for reversing the causality. This correlates well to the notion that during monitoring for occurrence of ADEs, the use of anti-histamines and anti-allergic medications give a clue for further evaluation of ADEs.
We found that ADEs increased with the length of hospital stay akin to a finding by Holdsworth et al. [41]. Santos et al. [13] have also reported that children with longer length of stay, greater number of medications had higher ADR incidence. In one study among adults [42], the authors identified that exposure to psychoactive and CV drugs were independent correlates of preventable ADEs. This was similar to our findings which showed CNS and endocrine medicines and presence of infectious disease as strong predictors of ADE.
This study has limitations, however. It is a single center study and therefore might not be generalized to other hospitals in Ethiopia. The incidence of ADEs might have been underestimated as some ADES may not have been recorded in the charts and may thus have not been detected. Any event that has occurred in patients with less than 24 h of hospital stay was not included but it was unlikely that we missed those events as such events required prolonged stay.
Conclusion
The incidence of ADE was high among children hospitalized to JUSH and ADEs were more likely to occur among children with longer length of hospital stay, presence of infectious disease, use of CNS, endocrine and anti-histamine medications. Anti-infectives were the most commonly implicated drugs for development of ADEs. Only one third of ADEs were found to be preventable. Though most of the ADEs were evaluated to cause temporary harm, clinically significant number of children suffered from permanent harm. This calls for a strategy to prevent the occurrence and consequences of ADEs in the pediatric ward of JUSH including education of nurses/physicians.
Abbreviations
- ADE:
-
Adverse drug event
- ADR:
-
Adverse drug reaction
- AOR:
-
Adjusted odds ratio
- CNS:
-
Central nervous system
- CV:
-
Cardiovascular
- JUSH:
-
Jimma University Specialized Hospital
- NCC MERP:
-
National Coordinating Council for Medication Error Reporting and Prevention Scale
- SD:
-
Standard deviation
- WHO-UMC:
-
World Health Organization- Uppsala Monitoring Centre
References
Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients: Results of the Harvard Medical Practice Study I. NEJM. 1991;324:370–6.
Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II. NEJM. 1991;324:377–84.
Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building A Safer Health System. Washington, DC: National Academies Press; 1999. p. 26–68.
Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAM. 2001;285(16):2114–20.
Buck ML. Adverse drug events in children: Recent cases from the medical literature. Pediatr Pharm. 2010;16(9).
Ghaleb MA, Barber N, Franklin BD, Wong IC. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child. 2010;95:113–8.
Takata GS, Mason W, Taketomo C, Logsdon T, Sharek PJ. Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children’s hospitals. Pediatrics. 2008;121(4):e297–35. doi:10.1542/peds.2007-1779.
Long AL, Horvath MM, Cozart H, Eckstrand J, Whitehurst J, Ferranti J. Tailoring adverse drug event surveillance to the paediatric inpatient. Qual Saf Health Care. 2010;19:1–5.
Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of adverse drug events (ADEs) and potential ADEs in hospitalized children in New Zealand. Pediatr Drugs. 2009;11(2):153–60.
Buajordet I, Wesenberg F, Brørs O, Langslet A. Adverse drug events in children during hospitalization and after discharge in a Norwegian University Hospital. Acta Paediatr. 2002;91:88–94.
Rodriguez-Monguio R, Otero M, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21(9):623–50.
Clavenna A, Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–8.
Dos Santos DB, Coelho HL. Adverse drug reactions in hospitalized children in Fortaleza, Brazil. Pharmacoepidemiol Drug Saf. 2006;15:635–40.
Martinez-Mir I, Garcia-Lopez M, Palop V, Ferrer JM, Rubio E, Morales-Olivas FJ. A prospective study of adverse drug reactions in hospitalized children. Br J Clin Pharmacol. 1999;47:681–8.
Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: A systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83.
Silas R, Tibballs J. Adverse events and comparison of systematic and voluntary reporting from a paediatric intensive care unit. Qual Saf Health Care. 2010;19:568–71.
Ferranti JM, Horvath MM, Cozart H. Reevaluating the safety profile of pediatrics: a comparison of computerized adverse drug event surveillance and voluntary reporting in the pediatric environment. Pediatrics. 2008;121(5):1201–7.
Weiss J, Krebs S, Hoffmann C, Werner U, Neubert A, Brune K, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics. 2002;110:254–7.
Sharek PJ, Horbar JD, Mason W. Adverse events in the neonatal intensive care unit: development, testing, and findings of an NICU-Focused Trigger Tool to identify harm in North American Nicus. Pediatrics. 2006;118:1332–40.
Kilbridge PM, Noirot LA, Reichley RM, Berchelmann KM, Schneider C, Heard KM, et al. Computerized surveillance for adverse drug events in a pediatric hospital. J Am Med Inform Assoc. 2009;16(5):607–12.
Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266:2847–51.
Cohen MM, Kimmel NL, Benage MK, Cox MJ, Sanders N, Spence D, et al. Medication safety program reduces adverse drug events in a community hospital. Qual Saf Health Care. 2005;14:169–74.
Field TS, Gurwitz JH, Harrold LR, Rothschild JM, Debellis K, Seger AC, et al. Strategies for detecting adverse drug events among older persons in the ambulatory setting. J Am Med Inform Assoc. 2004;11:492–8.
Gardner RM, Evans RS. Using computer technology to detect, measure, and prevent adverse drug events. J Am Med Inform Assoc. 2004;11:535–6.
Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301–6.
Evans RS, Classen DC, Stevens LE, Pestotnik SL, Gardner RM, Lloyd JF, et al. Using a hospital information system to assess the effects of adverse drug events. Proc Annu Symp Comput Appl Med Care. 1993;161–165.
Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277(4):307–11.
Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics. 2002;110(5), e53.
Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13:306–14. doi:10.1136/qshc.2004.010611.
Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Anna Pharmacother. 2000;34(12):1373–379.
Jonville-Bera AP, Giraudeau B, Blanc P, Salinas FB, Autret-Leca E. Frequency of adverse drug reaction in children: a prospective study. Br J Clin Pharmacol. 2002;53(2):207–10.
Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88:965–8.
Zed PJ, Black KJ, Fitzpatrick EA, Ackroyd-Stolarz S, Murphy NG, Curran JA, et al. Medication-related emergency department visits in pediatrics: a prospective observational study. Pediatrics. 2015;135(3):435–43.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: Implications for Prevention. JAMA. 1995;274(1):29–34.
Institute for Health Care Improvement. Trigger tool for measuring adverse drug events. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/Pediatric+ADE+Patient+Record+Review+Sheet+(IHI + Tool).htm. Accessed: 30/12/2013.
Child Health Corporation of America. Pediatric trigger toolkit: Measuring adverse drug events in the Children’s Hospital. May 2007. Available at: http://www.chca.com/triggers/docs/ADE%20Trigger%20Toolkit%202007.pdf. Accessed 30/12/2013
Food, Medicine and Health care administration and control authority. List of medicines for Ethiopia, 6th edition, Sept 2010, Addis Ababa. Available at: http://www.fmhaca.gov.et/documents/MedicineForEthiopia_NDL.pdf. Accessed 25/03/2015.
The use of the WHO–UMC system for standardized case causality assessment. Available at: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf (Accessed: 30/12/2014).
National Coordinating Council for Medication Error Reporting and Prevention. Taxonomy and index category of medication errors. Available At: www.nccmerp.org. Accessed: 30/12/2013
Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538–9.
Holdsworth MT, Fichtl RE, Behta M, Raisch DW, Mendez-Rico E, Adams A, et al. Incidence and impact of adverse drug events in pediatric inpatients. Arch Pediatr Adolesc Med. 2003;157:60–5.
Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, et al. Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med. 1999;159:2553–60.
Acknowledgments
The authors would like to thank Jimma University and Management Sciences for Health, Ethiopia for the financial support. Our special thanks go to data collectors and physicians. Our appreciation also extends to pediatric ward of JUSH staffs who were involved as supervisors. We are also grateful to Dr. Genet and Dr. Alelign for being part of the review panel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
All authors have made significant contribution to this study. TC and GP were involved in the conception of the study. TC, BM, NM and TG were involved in the design of the research study. TC and AB were responsible for the data management and statistical analyses. All authors contributed in the manuscript write-up and correction. The final version of the submitted manuscript was approved by all authors.
Tesfahun Chanie Eshetie, Bisrat Hailemeskel, Negussu Mekonnen, Getahun Paulos and Tsinuel Girma contributed equally to this work.
Additional file
Additional file 1:
Trigger tools or clues for a focused chart review.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Eshetie, T.C., Hailemeskel, B., Mekonnen, N. et al. Adverse drug events in hospitalized children at Ethiopian University Hospital: a prospective observational study. BMC Pediatr 15, 83 (2015). https://doi.org/10.1186/s12887-015-0401-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-015-0401-0