Abstract
Background
Disc hemorrhage (DH) is an important factor often associated with the development and especially progression of glaucoma. In contrast, some studies have reported peripapillary retinoschisis in glaucoma, but it is not recognized as a pathognomonic finding, and opinions on the clinical significance of retinoschisis are not consistent. Here,we present the case of DH following peripapillary retinoschisis in the same area within the same glaucomatous eye.
Case presentation
A 70-year-old man with high intraocular pressure (IOP) was referred to the glaucoma clinic. At the time of the baseline study, the IOP was 30mmHg, and peripapillary retinoschisis was discovered at 7 o’clock on the periphery of the optic nerve with swept-source optical coherence tomography. Accompanying retinal nerve fiber layer defect were manifest in the inferotemporal part with red-free fundus photography. Under the impression of open-angle glaucoma, we prescribe latanoprost ophthalmic solution. Eight months later, the IOP was 17mmHg, and the peripapillary retinoschisis had disappeared. DH was observed in the inferotemporal area in the same direction as that of the previous peripapillary retinoschisis.
Conclusions
The case presented here are the first to report on the relationship between peripapillary retinoschisis and DH. Hopefully future studies will reveal the actual connection between peripapillary retinoschisis and DH.
Similar content being viewed by others
Background
Glaucoma is a progressive optic neuropathy that accompanies structural changes in the optic nerve head and consequent visual field defects. Structural changes in the optic nerve head, such as damage to the neuro-retinal rim, deepening of disc cupping, and peripapillary retinal nerve fiber layer (RNFL) bundle defects, are well known [1]. Disc hemorrhage (DH) is widely known as a worse prognostic factor in glaucoma, and its relationship with the lamina cribrosa (LC) defect has also been reported [2]. In contrast, some studies have reported peripapillary retinoschisis in glaucoma, but it is not recognized as a pathognomonic finding, and opinions on the exact cause or clinical significance of retinoschisis are not consistent. Glaucoma-associated peripapillary retinoschisis often stabilises or spontaneously resolves [3,4,5]; however, some studies have reported that peripapillary retinoschisis is associated with rapid glaucoma progression [6, 7]. There have been no reports of these two findings in the same eye and a relationship between DH and peripapillary retinoschisis. We report a case of peripapillary retinoschisis preceding DH.
Case presentation
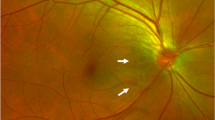
A 70-year-old man with high intraocular pressure (IOP) was referred to the glaucoma clinic. At the time of the baseline study, the IOP was 30 mmHg. and peripapillary retinoschisis was discovered at 7 o’clock at the periphery of the optic nerve with swept-source optical coherence tomography (OCT). Accompanying RNFL defects were observed in the inferotemporal part with red-free fundus photography (Fig. 1, upper row). There was no peripheral anterior synechiae and widely opened angle was observed on gonioscopic examination. Based on the suspicion of open-angle glaucoma, we prescribed a latanoprost ophthalmic solution. The IOP normalized, and the patient was regularly monitored while receiving medications. Eight months later, the IOP was 17 mmHg, and the peripapillary retinoschisis had disappeared. DH was observed in the inferotemporal area in the same direction as the previous peripapillary retinoschisis (Fig. 1, lower row).
Serial red-free fundus photographs (first column), swept-source optical coherence tomography images (second column), and wide-field retinal nerve fiber layer thickness map images (third column) of the right eye of a 70-year-old man with open-angle glaucoma. Peripapillary retinoschisis was observed at the inferotemporal area of the optic disc (upper row). Eight months after the baseline image was taken, a disc hemorrhage was observed in the inferotemporal area of the right eye in the same direction as that of the peripapillary retinoschisis (lower row)
To evaluate the structural changes and blood flow status of the optic nerve head, multimodal imaging, including en-face image and OCT-angiography, was performed (Fig. 2). Morphological changes in the LC, including LC pores and defects, were not detected at baseline or during follow-up. A decrease in the vessel density of the superficial layer along with the RNFL defect was stationary, and the inferotemporal choroidal vascular dropout did not progress. There were no signs of deep vascular structure associated with the subretinal neovascular membrane both on peripapillary and macular OCT-angiography.
Serial disc photographs (first column), en-face images (second column), superficial capillary plexus of OCT-angiography (third column), deep choroidal layer of OCT-angiography (fourth column), and swept-source optical coherence tomography images (fifth column) of the optic disc of a 70-year-old man with open-angle glaucoma. Morphological changes in LC, including LC pore or LC defects, were not detected at baseline (upper row) and 8 months after the baseline (lower row). The decrease in vessel density of the superficial layer along with the RNFL defect is stationary, and the inferotemporal choroidal vascular dropout has not progressed
Discussion and conclusions
With advances in OCT technology, peripapillary retinoschisis has been found in glaucomatous eyes. In the meantime, retinoschisis may affect peripapillary RNFL measurement (peripapillary 3.4 mm circle), and caution is needed when comparing the peripapillary RNFL thickness obtained at different times to evaluate glaucomatous structural progression [4, 8].
To the best of our knowledge, and even after a review of the literature, there is no report on retinoschisis and DH coexisting in a glaucomatous eye. Therefore, there are several explanations for their coexistence. First, they may have been observed in the same eye by accident. Both are often found in glaucomatous eyes. Retinoschisis can occur in glaucoma patients, but may also occur without glaucoma. Even an association between retinoschisis and DH may be mere coincidence. Second, mechanical changes in the optic discs, such as LC defects, may share a common pathophysiology with these two phenomena [5, 9] However, no distinct anatomical change of the optic nerve has been found even with the currently available multimodal imaging. Hence, this could be a possible hypothesis.
One of the theories on the pathogenesis of DH is that capillary disruption occurs due to the predispositions to reactive gliosis, such as a maximum tractional force at the RNFL defect margin, the absence of an internal limiting membrane, and loose connective tissue support at the surface of the RNFL [2]. In these patients, retinoschisis occurs as part of the RNFL defect before DH occurs, and we can infer that retinoschisis may develop with these anatomical predispositions. A recent study showed that OCT signs of retinal glia (Müller) cell involvement could be associated with the pathogenesis of glaucoma-associated peripapillary retinoschisis [6]. Biomechanical forces on the optic nerve head are likely contributors to the development of peripapillary retinoschisis in glaucoma through the activation of mechanosensitive glia. Activated glial cells play an important role in the progression of glaucoma [7, 9, 10]. The progressive glaucomatous damage accompanying such glial dysfunction may induce peripapillary retinoschisis as an epiphenomenon of glaucoma progression [7]. From the perspective of this gliosis, DH and peripapillary retinoschisis may play similar roles in glaucoma.
Only one case does not provide sufficient information to provide a definitive explanation for the causal relationship between peripapillary retinoschisis and DH. As we did not continuously observe the changes in retinoschisis during the two follow-up visits, it is difficult to conclude that DH has a direct causal relationship with peripapillary retinoschisis. The case of DH following peripapillary retinoschisis in the same glaucomatous eye may demonstrate the possible pathophysiology and the relationship between these two phenomena in glaucoma.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RNFL:
-
Retinal nerve fiber layer
- DH:
-
Disc hemorrhage
- LC:
-
Lamina cribrosa
- IOP:
-
Intraocular pressure
- OCT:
-
Optical coherence tomography
References
Weinreb RN, Khaw PT. Primary open-angle glaucoma. The Lancet. 2004;363(9422):1711–20.
Lee EJ, Han JC, Kee C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res. 2017;60:20–43.
Dhingra N, Manoharan R, Gill S, Nagar M. Peripapillary schisis in open-angle glaucoma. Eye. 2017;31(3):499–502.
Hwang YH, Kim YY, Kim HK, Sohn YH. Effect of peripapillary retinoschisis on retinal nerve fibre layer thickness measurement in glaucomatous eyes. Br J Ophthalmol. 2014;98(5):669–74.
Roberts JD, Hunter A, Mega J, Cesaro T, Greenberg PB. Case Report: Glaucoma-associated Peripapillary Retinoschisis with Corresponding Lamina Cribrosa Defect. Optometry vision science: official publication of the American Academy of Optometry. 2020;97(2):104–9.
Fortune B, Ma KN, Gardiner SK, Demirel S, Mansberger SL. Peripapillary Retinoschisis in Glaucoma: Association With Progression and OCT Signs of Müller Cell Involvement. Invest Ophthalmol Vis Sci. 2018;59(7):2818–27.
Lee EJ, Kee HJ, Han JC, Kee C. The Progression of Peripapillary Retinoschisis May Indicate the Progression of Glaucoma. Invest Ophthalmol Vis Sci. 2021;62(2):16.
Lee EJ, Kim TW, Kim M, Choi YJ. Peripapillary retinoschisis in glaucomatous eyes. PLoS One. 2014;9(2):e90129.
Fortune B. Pulling and Tugging on the Retina: Mechanical Impact of Glaucoma Beyond the Optic Nerve Head. Invest Ophthalmol Vis Sci. 2019;60(1):26–35.
Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19(3):297–321.
Acknowledgements
Not applicable.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (MSIT) (No. NRF-2019M3E5D1A01069352, to WJL) and (NRF-2019R1A2C1011676, to MS). The role of the funding was in the collection and analysis of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design: WJL and MS; Data collection: WJL and MS; Analysis and interpretation: WJL and MS; writing the article: WJL and MS; Critical revision of the article: WJL and MS; final approval of the article: WJL and MS. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local ethics committee ruled that no formal ethics approval was required in this case report.
The authors declare that they adhered to the CARE guidelines/methodology.
Consent for publication
Written informed consent for publication of potentially identifying information and clinical images was obtained from the patient. The copy of written consent to publish is available for the journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, W.J., Seong, M. Disc hemorrhage following peripapillary retinoschisis in glaucoma: a case report. BMC Ophthalmol 21, 253 (2021). https://doi.org/10.1186/s12886-021-02010-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-021-02010-5