Abstract
Background
Although papillary thyroid cancer (PTC) patients are known to have an excellent prognosis, up to 30% of patients experience disease recurrence after initial treatment. Accurately predicting disease prognosis remains a challenge given that the predictive value of several predictors remains controversial. Thus, we investigated whether machine learning (ML) approaches based on comprehensive predictors can predict the risk of structural recurrence for PTC patients.
Methods
A total of 2244 patients treated with thyroid surgery and radioiodine were included. Twenty-nine perioperative variables consisting of four dimensions (demographic characteristics and comorbidities, tumor-related variables, lymph node (LN)-related variables, and metabolic and inflammatory markers) were analyzed. We applied five ML algorithms—logistic regression (LR), support vector machine (SVM), extreme gradient boosting (XGBoost), random forest (RF), and neural network (NN)—to develop the models. The area under the receiver operating characteristic (AUC-ROC) curve, calibration curve, and variable importance were used to evaluate the models’ performance.
Results
During a median follow-up of 45.5 months, 179 patients (8.0%) experienced structural recurrence. The non-stimulated thyroglobulin, LN dissection, number of LNs dissected, lymph node metastasis ratio, N stage, comorbidity of hypertension, comorbidity of diabetes, body mass index, and low-density lipoprotein were used to develop the models. All models showed a greater AUC (AUC = 0.738 to 0.767) than did the ATA risk stratification (AUC = 0.620, DeLong test: P < 0.01). The SVM, XGBoost, and RF model showed greater sensitivity (0.568, 0.595, 0.676), specificity (0.903, 0.857, 0.784), accuracy (0.875, 0.835, 0.775), positive predictive value (PPV) (0.344, 0.272, 0.219), negative predictive value (NPV) (0.959, 0.959, 0.964), and F1 score (0.429, 0.373, 0.331) than did the ATA risk stratification (sensitivity = 0.432, specificity = 0.770, accuracy = 0.742, PPV = 0.144, NPV = 0.938, F1 score = 0.216). The RF model had generally consistent calibration compared with the other models. The Tg and the LNR were the top 2 important variables in all the models, the N stage was the top 5 important variables in all the models.
Conclusions
The RF model achieved the expected prediction performance with generally good discrimination, calibration and interpretability in this study. This study sheds light on the potential of ML approaches for improving the accuracy of risk stratification for PTC patients.
Trial registration
Retrospectively registered at www.chictr.org.cn (trial registration number: ChiCTR2300075574, date of registration: 2023-09-08).
Similar content being viewed by others
Background
Papillary thyroid cancer (PTC) is one of the most common types of differentiated thyroid cancer (DTC), accounting for more than 90% of DTC. Although the mortality rate for PTC patients is low, 10–30% of PTC patients will still experience recurrence or metastasis after initial treatment [1], which is the main cause of death in PTC patients. Therefore, accurate risk stratification and individualized treatment and follow-up strategies are essential for detecting recurrent disease early and improving the prognosis of PTC patients.
The 2009 American Thyroid Association (ATA) guidelines proposed a three-category system to estimate the likelihood of DTC patients developing structural recurrence during postoperative follow-up [2], and a revised ATA risk stratification system was proposed in 2015 [3]; this system has been widely used and validated in clinical practice. Although the ATA system is flexible and can easily estimate risk based only on surgical/histological findings, several prognostic factors in the system do not specify the cutoff values for risk stratification, especially considering heterogeneity among PTC patients; for example, thyroglobulin (Tg) is a specific product of thyroid follicular cells, and it has been demonstrated that a high level of postoperative thyroid stimulating hormone (TSH)-suppressed Tg is associated with a high risk of recurrent disease and mortality, with a wide range of suppressed Tg cutoff values [3]. Moreover, the prognostic value of several prognostic factors remains controversial in PTC, such as metastatic lymph node (LN) features (i.e., the LN metastasis ratio and extranodal extension) [4,5,6], inflammation-based markers (i.e., the neutrophil-to-lymphocyte ratio [NLR], the platelet-to-lymphocyte ratio [PLR], the lymphocyte-to-monocyte ratio [LMR], and the prognostic nutritional index [PNI]) [7,8,9], and metabolic-related markers (i.e., obesity and dyslipidemia) [10, 11], which therefore need to be confirmed.
In the age of precision medicine, there is considerable enthusiasm for estimating prognosis by relying on models that can simultaneously consider many factors and provide an estimate of absolute risk [12]. Machine learning (ML) provides a novel approach to achieve this goal and has advantages in incorporating a larger number of multidimensional variables with more dynamic interactions than traditional prognostic tools [13]. Briefly, ML accounts for partial, nonlinear relationships, or multiple coexistent states between variables and outcomes, and each variable in an ML model can have a variable weight according to the changes in other variables; therefore, it can realize more individual predictions [14].
ML approaches have already proven to be effective predictive tools for various types of tumors [15,16,17]; thus, ML models may also prove useful in PTC risk stratification. However, to the best of our knowledge, only a few studies have developed ML models for predicting death or recurrence in patients with thyroid cancer [18,19,20,21,22,23]. In addition, previous studies were partially limited by the lack of large datasets, single algorithms, inadequate variables, and incomplete model evaluation [18,19,20,21,22,23], which hindered clinicians from better understanding the application of ML in prognosis prediction for PTC. Thus, this study aimed to develop and validate multiple ML models to predict structural recurrence in PTC patients based on a large sample of PTC patients with comprehensive clinical variables.
Methods
Study design and population
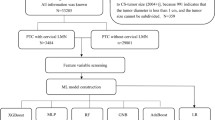
The electronic medical records of patients with thyroid cancer treated at West China Hospital, Sichuan University, were fully screened to retrospectively review all PTC patients treated and followed up. We restricted our analyses to PTC patients who underwent thyroid surgery (with or without lymph node dissection) and radioiodine (131I) therapy at West China Hospital, Sichuan University between January 2009 and December 2018 (n = 6220). We excluded patients with unresected tumors (n = 275), initial distant metastasis (n = 152), or other malignancies combined (n = 46), patients with unavailable information about the 8th edition of the AJCC TNM staging system [24] or the 2015 ATA risk stratification [3] (n = 1081), patients with a positive TgAb (> 40 IU/mL) [25] or missing data on postoperative non-stimulated Tg (TSH < 30 µIU/ml) (n = 1592), and patients with a follow-up period shorter than 1 year (n = 830). Finally, a total of 2244 patients were included in the prediction models (Fig. 1A).
Flowchart of the study (A) and the analytic steps (B). PTC, papillary thyroid cancer; Tg, thyroglobulin; TgAb, anti-thyroglobulin antibody; LASSO, least absolute shrinkage and selection operator; LR, logistic regression; XGBoost, eXtreme gradient boosting; SVM, support vector machine; RF, random forest; NN, neural network; AUCs, area under the receiver operating characteristic curves
This study was conducted following the basic principles of the Declaration of Helsinki and was approved by the West China Hospital Clinical Trials and Biomedical Ethics Committee, Sichuan University (Approval in 2020, No. 678). Written informed consent was waived given the retrospective nature of the study.
Potential input variables
To include as many predictive variables as possible and ensure the availability and representation of variables from the database. We used 29 potential input variables consisting of four dimensions in this study: (1) demographic characteristics and comorbidities, including age, sex, race, smoking status, alcohol drinking status, comorbidity of diabetes status, comorbidity of hypertension status, and comorbidity of Hashimoto’s thyroiditis; (2) tumor-related variables, including histology, tumor diameter, tumor foci, tumor location, external thyroid invasion (ETE), BRAF (V600E) mutation, and postoperative non-stimulated Tg; (3) LN-related variables, including LN dissection, number of LNs dissected, extranodal extension (ENE), lymph node metastasis ratio (LNR), and N stage; and (4) metabolic and inflammatory markers, including body mass index (BMI), triglyceride, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), lymphocyte–monocyte ratio (LMR), and prognostic nutritional index (PNI).
The missing values of potential input variables are represented as a category when the missing value was ≥ 20%, and multiple imputations were used when the missing value was < 20%, as previously reported [26, 27] (Supplementary Data 1). Receiver operating characteristic (ROC) curve analysis was performed, and the Youden index was used to determine the cutoff value for continuous variables [28]. The details of the potential input variables are shown in Supplementary Data 2.
Definition of structural recurrence
The outcome of this study was structural recurrence that occurred during follow-up after initial thyroid surgery. According to the 2015 ATA guidelines [3], patients were considered to have structural recurrent disease if any of the following conditions were met: (1) structural disease confirmed by cytology/histology; (2) highly suspicious lymph nodes or thyroid bed nodules on neck ultrasound; or (3) highly suspicious metastatic disease on whole-body 131I scintigraphy, 18fluorodeoxyglucose positron emission tomography scans, or other cross-sectional imaging. The last follow-up date of this study was July 31, 2021.
Dataset split and resampling
Figure 1B shows the analysis steps of this study. Patients were randomly divided into a training dataset and a test dataset according to a 4 to 1 ratio. The training dataset was used to develop and optimize the models (n = 1795), and the test dataset was used to validate and compare the models (n = 449). To ensure that the models can effectively predict the outcome (minority class), one-sided selection (OSS) under-resampling method was used to establish a balanced training dataset [29] (Supplementary Data 3).
Variable selection
Spearman correlation analysis was used to evaluate the correlation of all variables with each other. Spearman’s correlation coefficients range from -1 to 1, and values of coefficients close to -1 or 1 represent stronger relationships than values closer to zero. Then, the least absolute shrinkage and selection operator (LASSO) method was used to select the input variables. LASSO formulates curve fitting as a quadratic programming problem, where the objective function penalizes the absolute size of the regression coefficients based on the value of a tuning parameter λ involved in the maximum AUC value [30]. Thus, LASSO can perform automatic variable selection by driving the coefficients of irrelevant variables to zero.
Development and optimization of the models
Five-fold cross-validation was used to avoid training overfitting, and the training dataset was divided into 5 equal parts, this process was repeated 5 times. In the first step, the first part was used for validation, and the remaining parts were used for training. Similarly, the second part was used for validation in the second fold, and this process was continued for the rest of the folding. Five popular ML algorithms were applied to develop models based on selected variables, including logistic regression (LR), support vector machine (SVM) [31], eXtreme gradient boosting (XGBoost) [32], random forest (RF) [33], and neural network (NN) [34]. The hyperparameters for the five models were optimized via Bayesian optimization (BO), and the models were trained on a training set for optimization and validated on a validation set for each hyperparameter configuration. The ideal parameter setup provided the highest AUC values [35] (Supplementary Data 4). Finally, the relative importance of the variables of each model was ranked, which can reflect the contribution of each variable when predicting structural recurrence (Supplementary Data 5).
Evaluation and comparison of the models
We evaluated the predictive performance of each model in the test dataset (Fig. 1B). First, we evaluated the discrimination of the models by using the area under the receiver operating characteristic (AUC-ROC) curve [12]. We also compared the AUC values of the ML models and the AUC values of the ATA risk stratification by using the DeLong test [36], and a 2-tailed test with P < 0.05 was considered to indicate statistical significance. We used the Youden index as the threshold to calculate the sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV), and F1 score [37]. Second, we evaluated the calibration of the models by using calibration curves, which represent the accuracy of the absolute recurrent risk estimates of the models [12]. Finally, we analyzed the interpretability of the ML models by using the rank of variable importance. Statistical analyses were performed using python (version 3.6.10, https://www.python.org) and R software (version 4.2.2, https://www.r-project.org/).
Results
Characteristics of the study population
The median age of the 2244 patients was 42.0 years, and 1489 (66.4%) patients were female. During a median follow-up of 45.5 months (range: 12.0 to 142.7 months), 179 patients (8.0%) experienced structural recurrence (Table 1). The median tumor diameters of ≤ 10 mm, 10 to 20 mm, 20 to 40 mm, and > 40 mm were documented in 1093 (48.7%), 777 (34.6%), 322 (14.3%), and 52 (2.3%) patients, respectively. Multifocality was observed in 577 (25.7%) patients. Extensive ETE was observed in 584 (26.0%) patients. The median non-stimulated Tg was 0.24 ng/mL with median TSH was 0.46 µIU/ml. A total of 1537 (68.5%) patients and 698 (31.1%) patients underwent central LN dissection and lateral LN dissection, respectively. A total of 1173 (52.3%) and 564 (25.1%) patients had N1a and N1b disease, respectively. The median LNR was 28.57%. A total of 328 (14.6%), 1334 (59.4%), and 582 (25.9%) patients were classified as low risk, intermediate risk, and high risk, respectively, according to the ATA risk stratification.
Patients with recurrent disease were more likely to be older, male, be smokers, be drinkers, have comorbidities of diabetes, hypertension and Hashimoto’s thyroiditis, have larger, multifocal and bilateral tumors, have ETE, have higher levels of Tg, have undergone lateral dissection with a higher number of LN dissected and LNR, have ENE and more advanced N stage, have higher levels of BMI, cholesterol, LDL and NLR, and have lower levels of triglyceride, PLR, LMR and PNI.
Performance of the models
The heatmap of the Spearman correlation analysis showed no significant or weak correlation between the majority of the input variables (Supplementary Data 6). The LASSO method selected nine variables for developing prediction models (Supplementary Data 7), including Tg, LN variables (LN dissection, number of LNs dissected, LNR, and N stage), comorbidities and metabolic-related variables (comorbidity of hypertension, comorbidity of diabetes, BMI, and LDL).
As shown in Table 2; Fig. 2, five models had adequate discrimination in differentiating patients at greater risk of recurrence from those at lower risk, and the AUCs of the five models ranged from 0.738 to 0.767 in the test dataset (LR: AUC = 0.738, 95% CI = 0.636–0.820; SVM: AUC = 0.752, 95% CI = 0.666–0.841; XGBoost: AUC = 0.741, 95% CI = 0.609–0.840; RF: AUC = 0.766, 95% CI = 0.702–0.845; NN: AUC = 0.767, 95% CI = 0.675–0.843). All models showed better discrimination than did the ATA risk stratification (AUC = 0.620, 95% CI = 0.534–0.670; DeLong test: P < 0.01; Supplementary Data 8). The SVM, XGBoost, and RF model showed greater sensitivity (0.568, 0.595, 0.676), specificity (0.903, 0.857, 0.784), accuracy (0.875, 0.835, 0.775), positive predictive value (PPV) (0.344, 0.272, 0.219), negative predictive value (NPV) (0.959, 0.959, 0.964), and F1 score (0.429, 0.373, 0.331) than did the ATA risk stratification (sensitivity = 0.432, specificity = 0.770, accuracy = 0.742, PPV = 0.144, NPV = 0.938, F1 score = 0.216). The calibration curves are shown in Fig. 3. Although all models overestimated the recurrence risk of patients to varying degrees, which may have resulted in a higher false-positive rate if the models were applied in clinical practice, the RF model had generally consistent calibration.
Relative importance of variables in the models
Although slight differences were shown in the importance of variables among those models (Fig. 4), the Tg and the LNR were the top 2 important variables in all the models, the N stage was the top 5 important variables in all the models. The importance of variables in RF model was as follows: Tg, LNR, and N stage, comorbidity of hypertension, LDL, BMI, number of LNs dissected, comorbidity of diabetes, and LN dissection.
Discussion
In this study, based on a large dataset of PTC patients with comprehensive predictive variables (demographic characteristics and comorbidities, tumor-related variables, LN-related variables, and metabolic and inflammatory markers), we developed and validated five ML models to predict structural recurrence in PTC patients. In the test dataset, the SVM, XGBoost, and RF model showed better discrimination than the ATA risk stratification according to the AUC values and corresponding indices, and the RF model generally had consistent calibration compared with the other models. Thus, ML models may aid in treatment decision making and improve postoperative prognosis for PTC patients by accurately estimating the likelihood of structural recurrence and identifying patients at high risk of recurrence. Overall, we suggested that the RF model, which showed overall good performance and interpretability, could be used to predict structural recurrence in patients with PTC.
Nine of 29 variables were selected by the LASSO method and used to develop models in this study, including the Tg, LN variables (LN dissection, number of LNs dissected, LNR, and N stage), comorbidities and metabolic markers (comorbidity of hypertension, comorbidity of diabetes, BMI, and LDL). Further variable importance analysis revealed that the Tg, LNR, and N stage were the three most important variables across all the models. Tg is the most important tumor marker in PTC, and the ATA risk stratification revealed abnormally elevated postoperative suppressed Tg as one of the high-risk predictors; however, it did not specify a cutoff value or include postoperative negative Tg in the postoperative recurrence risk assessment. In this study, a non-stimulated Tg value of 1.08 ng/mL was set as a cutoff by using the ROC curve, and patients with a higher level of non-stimulated Tg had a greater risk of recurrence than those with a lower level of Tg. Consistently, previous studies have used suppressed Tg values > 1 ng/mL to define a biochemical incomplete response to therapy in patients treated with total thyroidectomy and 131I ablation, and approximately 20% of these patients were likely to develop structural disease [3, 38].
LN-related variables were also considered important contributing predictors by the ML models. According to the 2015 ATA risk stratification system, the N stage and the size of the metastatic LN were proposed as key predictors for structural recurrence. In this study, we selected the number of dissected LNs, the LNR, and the N stage to develop models. The LNR was among the 2 most important variables, and the N stage was among the 5 most important variables according to all the models. A higher LNR (≥ 22.70%), greater number of LNs dissected (≥ 21), and advanced N stage were strongly associated with a high risk of recurrence. Several studies have reported various optimal cutoff values for LN-related variables [4,5,6]. For instance, in a recent study in which five ML models were constructed to predict recurrence among patients diagnosed with PTC, the LNR (cutoff = 0.24) and LN metastasis were identified as important variables [21]. Another study determined the predictive cutoff values for the number of metastatic LNs (4 and 13) and the LNR (0.28 and 0.58) using the K-means clustering algorithm [39]. The optimal predictive cutoff may depend on the extent of LN dissection, the number of LNs dissected, the annual number of surgeries performed by physicians, etc. Thus, more evidence is needed before combining LN-related variables with newly developed risk stratification or staging systems for PTC patients.
The comorbidities and metabolic-related markers (comorbidities of hypertension and diabetes, BMI, and LDL) were included in our models and showed potential predictive value. Although the current risk stratification or staging system for PTC does not include these predictors, a few studies have reported that hypertension [40], diabetes [41], a high level of BMI [11] and LDL [42] were significantly associated with the aggressiveness of PTC. The underlying mechanism between metabolism-related predictors and poor prognosis in PTC patients is less clear. Increasing insulin, insulin-like growth factor or TSH were associated with the aggressiveness of PTC in obese patients [43]. The LDL receptor played an important role in the RAS/RAF/MAPK (MEK)/ERK signaling cascade, and synergy between LDL-mediated receptor uptake and BRAF may lead to a worse prognosis in thyroid cancer patients [42].
Compared with existing studies on prognosis prediction for PTC [18,19,20,21,22], our study has several strengths. First, we developed ML models for predicting structural recurrence based on a large dataset of PTC patients. By using multiple ML algorithms, 5-fold cross-validation and Bayesian optimization, more reliable and robust predictions can be achieved. Second, benefitting from the simultaneous consideration of multiple predictors and the use of LASSO, we identified nine variables that were strongly associated with the risk of recurrence to develop models. Our results provided comprehensive evidence for the interpretation and clinical application of these predictors. Third, to help clinicians making optimal use of the models, we evaluated the discrimination, calibration, and interpretability of the ML models; however, these items were underreported in the published literature addressing PTC prognosis prediction [12, 44].
This study has several limitations. First, the retrospective nature of the study might have resulted in selection bias. Second, the ML models we developed were based on data from a single institution, and more studies covering wider populations are warranted for validation. Third, our models were built on data from patients diagnosed with PTC and treated with thyroidectomy and 131I; thus, they were unlikely to be accurate for patients whose tumor behavior was considerably different, such as children and adolescents with PTC [45] or patients who undergo lobectomy [46]. Fourth, our sample data used for developing the models were unbalanced due to the low incidence of recurrence in PTC patients (only 8.0% of patients experienced recurrence in our study). Unbalanced data may typically affect model training; thus, we performed resampling to minimize this effect. Advanced methods for handling imbalanced data have been proposed recently and need to be applied [47]. Fifth, 61.1% of the BRAF mutations were missing, which was used as a categorical variable and may affect the risk estimating of this variable. Finally, a median follow-up period of 45.5 months might be insufficient for the assessment of outcomes in PTC patients; thus, our models were mainly used to estimate short-term recurrence risk.
Conclusion
This study demonstrated that the RF model achieved the expected prediction performance with generally good discrimination, calibration and interpretability. It is likely that ML approaches could improve the accuracy of the existing risk stratification for PTC as well as assist physicians in better understanding how ML approaches can be applied to optimize treatment and follow-up decisions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- PTC:
-
Papillary thyroid cancer
- DTC:
-
Differentiated thyroid cancer
- ML:
-
Machine learning
- ATA:
-
American Thyroid Association
- 131I:
-
Radioiodine
- BMI:
-
Body mass index
- ETE:
-
Extrathyroid extension
- ENE:
-
Extranodal extension
- LNR:
-
Lymph node metastasis ratio
- PTC:
-
Papillary thyroid cancer
- FV-PTC:
-
Follicular variant of papillary thyroid carcinoma
- Tg:
-
Thyroglobulin
- TSH:
-
Thyrotropin
- TgAb:
-
Anti-thyroglobulin antibody
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- NLR:
-
Neutrophil–lymphocyte ratio
- PLR:
-
Platelet–lymphocyte ratio
- LMR:
-
Lymphocyte–monocyte ratio
- PNI:
-
Prognostic nutritional index
- OSS:
-
One-sided selection
- LASSO:
-
Least absolute shrinkage and selection operator
- LR:
-
Logistic regression
- SVM:
-
Support vector machine
- XGBoost:
-
EXtreme gradient boosting
- RF:
-
Random forest
- NN:
-
Neural network
- BO:
-
Bayesian optimization
- AUC-ROC:
-
The area under the receiver operating characteristic curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Chen DW, et al. Disparities in risk perception of thyroid cancer recurrence and death. Cancer. 2020;126(7):1512–21.
Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214.
Haugen BR et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid, 2016. 26(1): p. 1-133.
Parvathareddy SK et al. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr Connect, 2022. 11(2).
Ito Y, et al. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36(6):1274–8.
Lee J, et al. Clinical Value of Lymph Node Ratio Integration with the 8(th) Edition of the UICC TNM classification and 2015 ATA risk Stratification systems for recurrence prediction in papillary thyroid Cancer. Sci Rep. 2019;9(1):13361.
Zhou C, Duan D, Liu S. Predictive value of a Prognostic Model based on lymphocyte-to-monocyte ratio before Radioiodine Therapy for recurrence of papillary thyroid carcinoma. Technol Cancer Res Treat. 2021;20:15330338211027910.
Offi C, et al. Clinical significance of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio and prognostic nutritional index in low-risk differentiated thyroid carcinoma. Acta Otorhinolaryngol Ital. 2021;41(1):31–8.
Chen W, et al. Association of the preoperative inflammation-based scores with TNM Stage and recurrence in patients with papillary thyroid carcinoma: a Retrospective, Multicenter Analysis. Cancer Manag Res. 2020;12:1809–18.
Rahman ST, et al. Obesity is Associated with BRAF(V600E)-Mutated thyroid Cancer. Thyroid. 2020;30(10):1518–27.
Li CL, et al. Influence of body mass index on the clinicopathological features of 13,995 papillary thyroid tumors. J Endocrinol Invest. 2020;43(9):1283–99.
Alba AC, et al. Discrimination and calibration of clinical prediction models: users’ guides to the Medical Literature. JAMA. 2017;318(14):1377–84.
Kong G, Lin K, Hu Y. Using machine learning methods to predict in-hospital mortality of sepsis patients in the ICU. BMC Med Inf Decis Mak. 2020;20(1):251.
Chen JH, Asch SM. Machine learning and prediction in Medicine - Beyond the peak of inflated expectations. N Engl J Med. 2017;376(26):2507–9.
Tong J, et al. Machine learning can predict total death after Radiofrequency ablation in Liver Cancer patients. Clin Med Insights Oncol. 2021;15:11795549211000017.
Liu WC, et al. Machine learning for the prediction of bone metastasis in patients with newly diagnosed thyroid cancer. Cancer Med. 2021;10(8):2802–11.
Montazeri M, et al. Machine learning models in breast cancer survival prediction. Technol Health Care. 2016;24(1):31–42.
Jajroudi M, et al. Prediction of survival in thyroid cancer using data mining technique. Technol Cancer Res Treat. 2014;13(4):353–9.
Mourad M, et al. Thyroid Cancer Prognosis Sci Rep. 2020;10(1):5176. Machine Learning and Feature Selection Applied to SEER Data to Reliably Assess.
Banerjee M, Reyes-Gastelum D, Haymart MR. Treatment-free survival in patients with differentiated thyroid Cancer. J Clin Endocrinol Metab. 2018;103(7):2720–7.
Park YM, Lee B. Machine learning-based prediction model using clinico-pathologic factors for papillary thyroid carcinoma recurrence. Sci Rep, 2021. 11(1).
Kim SY, et al. New approach of prediction of recurrence in thyroid cancer patients using machine learning. Medicine. 2021;100(42):e27493.
Borzooei S, et al. Machine learning for risk stratification of thyroid cancer patients: a 15-year cohort study. European Archives of Oto-Rhino-Laryngology; 2023.
Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for differentiated and anaplastic thyroid Cancer (Eighth Edition): what changed and why? Thyroid. 2017;27(6):751–6.
Tian T, et al. Prognostic implications of Preablation stimulated tg: a retrospective analysis of 2500 thyroid Cancer patients. J Clin Endocrinol Metabolism. 2021;106(11):e4688–97.
Gravesteijn BY, et al. Missing Data in Prediction Research: a five-step Approach for multiple imputation, Illustrated in the CENTER-TBI Study. J Neurotrauma. 2021;38(13):1842–57.
Lee JH, Huber JJ. Evaluation of multiple imputation with large proportions of Missing Data: how much is too much? Iran J Public Health. 2021;50(7):1372–80.
Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75(1):25–36.
Bennin KE, et al. An empirical study on the effectiveness of data resampling approaches for cross-project software defect prediction. IET Software. 2022;16(2):185–99.
LASSO RR. A feature selection technique in predictive modeling for machine learning. in 2016 IEEE International Conference on Advances in Computer Applications (ICACA). 2016.
Shmilovici A, Maimon O, Rokach L, Maimon O, Rokach^Editors L. 2005, Springer US: Boston, MA. 257–76.
Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, 2016.
Liu Y, Wang Y, Zhang J. New Machine Learning Algorithm: Random Forest. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012.
Svozil D, Kvasnicka V, Pospichal J. Introduction to multi-layer feed-forward neural networks. Chemometr Intell Lab Syst. 1997;39(1):43–62.
C., H., et al. Automatic Parameter Tuning using Bayesian Optimization Method. in 2019 IEEE Congress on Evolutionary Computation (CEC). 2019.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Barrett BJ, Fardy JM, Parfrey PS, Barrett BJ, Parfrey PS, Barrett^Editors BJ. 2021, Springer US: New York, NY. 319–33.
Vaisman F, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012;77(1):132–8.
Jang S, et al. Recurrence risk evaluation in patients with papillary thyroid carcinoma: Multicenter Machine Learning evaluation of lymph node variables. Cancers. 2023;15(2):550.
Li LR, et al. Hypertension was Associated with higher tumor stages in papillary thyroid Cancer: a large sample single-center study. Metab Syndr Relat Disord. 2022;20(8):466–72.
Shi P, et al. Clinicopathological features and prognosis of papillary thyroid cancer patients with type 2 diabetes mellitus. Gland Surg. 2022;11(2):358–68.
Revilla G, et al. Low-density lipoprotein receptor is a key driver of aggressiveness in thyroid tumor cells. Int J Mol Sci. 2023;24(13):11153.
Tresallet C, et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery. 2014;156(5):1145–52.
Wessler BS, et al. Clinical prediction models for Cardiovascular Disease: Tufts Predictive Analytics and comparative effectiveness clinical prediction Model Database. Circ Cardiovasc Qual Outcomes. 2015;8(4):368–75.
Francis GL, et al. Management guidelines for children with thyroid nodules and differentiated thyroid Cancer. Thyroid. 2015;25(7):716–59.
Ritter A et al. Detecting recurrence following lobectomy for thyroid Cancer: role of Thyroglobulin and Thyroglobulin antibodies. J Clin Endocrinol Metab, 2020. 105(6).
Morris C, Yang JJ. Effectiveness of resampling methods in coping with imbalanced crash data: Crash type analysis and predictive modeling. Accid Anal Prev. 2021;159:106240.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant 81971653) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant ZYJC21063).
Author information
Authors and Affiliations
Contributions
All authors reviewed the results and approved the final version of the manuscript. HX Wang: data collection, statistical analysis, manuscript preparation. C Zhang: statistical analysis, data collection. QR Li: data collection, manuscript revision. T Tian: data collection. R Huang: manuscript revision. JJ Qiu: statistical analysis, manuscript revision. R Tian: study conception and design, manuscript revision.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Research Ethics Committee of West China Hospital of Sichuan University (# 2020678), and the requirement for written informed consent was waived by Institutional Research Ethics Committee of West China Hospital of Sichuan University (# 2020678). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Zhang, C., Li, Q. et al. Development and validation of prediction models for papillary thyroid cancer structural recurrence using machine learning approaches. BMC Cancer 24, 427 (2024). https://doi.org/10.1186/s12885-024-12146-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12146-4