Abstract
Background
Novel ADC drugs provide a new therapeutic strategy for gastric cancer.The present study aimed to analyze the clinical efficacy and drug toxicities of disitamab vedotin (RC48) plus immune checkpoint inhibitors(ICIs) and RC48 as third-line therapies and beyond for advanced and metastatic gastric cancer patients.
Methods
This was an observational multicenter real-world study.From August 2021 to January 2022,patients with HER2-positive or HER2-low advanced and metastatic gastric cancer and failed from two or more lines of prior therapy were enrolled and treated with RC48 plus ICIs or RC48. In this study, progression free survival(PFS) was the primary end point. Other evaluation indicators were objective response rate(ORR),disease control rate(DCR),overall survival(OS) and drug toxicities.
Results
45 patients were enrolled,of which 25 patients received RC48 plus ICIs,20 patients received RC48.Patients who received RC48 plus ICIs obtained better ORR (36.0% vs. 10.0%, P = 0.044) and DCR (80.0% vs. 50.0%, P = 0.034) compared with RC48,and simultaneously,the median PFS in RC48 plus ICIs group were superior to RC48 group(6.2 m vs. 3.9 m).The median OS was not reached.No statistically differences were found between HER2-positive and HER2-low group with respect to ORR (27.3% vs. 16.7%, P = 0.464),DCR (66.7% vs. 66.7%, P = 1.000),median PFS(5.7 m vs. 4.3 m, P = 0.299).The most common adverse events (AEs) were decreased white blood count,decreased neutrophil count,fatigue,hypoaesthesia and alopecia.Grade 3–4 AEs occurred in 7(35.0%) patients of RC48 group and 10(40.0%) patients of RC48 plus ICIs group,respectively.
Conclusion
Compared with RC48 monotherapy, ICIs plus RC48 demonstrated superior third-line and beyond therapeutic efficacy for HER2-positive or HER2-low advanced and metastatic gastric cancer patients with manageable safety.
Similar content being viewed by others
Introduction
The global cancer statistics data in 2020 showed that the incidence of gastric cancer presents a trend of increasing year by year [1]. It was reported that the global positive rate of HER-2 in gastric cancer is 7.3–20.2%, and the positive rate of HER-2 in Chinese patients is 12–13% [2–3]. Based on the results of phase 3 ToGA study, trastuzumab combined with chemotherapy is the standard first-line treatment manner for HER2-positive advanced and metastatic gastric cancer, and trastuzumab has become the only anti-HER2 targeted drug approved for first-line treatment of advanced and metastatic gastric cancer [4]. However, for patients who have progressed on first-line trastuzumab therapy, there is currently no standard anti-HER-2 regimen.
New antibody-drug conjugate (ADC) drugs, such as T-DXd (DS-8201) and disitamab vedotin (RC48) have shown favorable antitumor efficacy [5,6,7,8]. The clinical trial of DESTINY-Gastric01 demonstrated that for patients with HER-2-positive advanced or metastatic gastric cancer who progressed on second-line therapy, DS8201 increased ORR from 14.3 to 51.3%, and PFS from 3.5 months to 5.6 months compared with physician’s choice of chemotherapy (irinotecan or paclitaxel) [9]. RC48 is an innovative ADC drug conjugated with a microtubule inhibitor (monomethyl auristatin E) via a cleavable linker, which directly and potently kills HER2-expressing tumor cells, and at the same time has a bystander effect on heterogeneous tumor cells [10]. RC48 shows potent and safe clinical advantages in various HER2 positive solid tumors such as advanced breast cancer, urothelial carcinoma, gastric cancer and etc [11,12,13].
There are currently no real-world clinical data on the efficacy of RC48 for gastric cancer. Several clinical studies suggested that immune checkpoint inhibitors (ICIs) may enhanced the antitumor efficacy of RC48. The present study aimed to analyze the clinical efficacy and drug toxicities of RC48 plus immune checkpoint inhibitors(ICIs) and RC48 as third-line therapies and beyond for advanced and metastatic gastric cancer patients in real-world clinical setting.
Materials and methods
Patients
From August 2021 to January 2022, patients with HER2-positive or HER2-low advanced and metastatic gastric cancer and failed from two or more lines of prior therapy in 3 institutions were enrolled in this study. Selection criteria: (1) advanced and metastatic gastric cancer; (2) HER-2 positive or HER2-low expression; (3) failed from at least two lines of prior treatment; (4) based on RECIST v1.1, presence of at least one measurable lesion.
The detection of HER2 refers to the Guidelines for HER2 detection in gastric cancer(2016) [14]. HER2 positive was defined as immunohistochemistry (IHC) 3 + or IHC 2+/fluorescence in situ hybridization (FISH) positive and HER2-low was defined as IHC 1 + or IHC 2+/FISH negative. For patients who are diagnosed as advanced and metastatic stage at the initial diagnosis, HER2 is tested on initial biopsy confirmed specimens before treatment. For patients who have relapsed after previous adjuvant therapy, HER2 is detected in metastatic lesion specimens. And HER2 was also evaluated via post-progression new biopsy after two or more lines of prior therapy in a very small number of patients. Anyway, the status of HER2 is based on the latest test results before RC48 treatment.
Study design and treatment
This was an observational multicenter real-world study, and the patients received RC48 monotherapy or RC48 plus ICIs therapy as third-line therapies and beyond until progressive disease, death or intolerable toxicity occurs. In RC48 monotherapy group, RC48 was administered intravenously, the dosage was 2.5 mg/kg every two weeks. In the RC48 plus ICIs therapy group, RC48 was given at the same dose as RC48 monotherapy. Tislelizumab was given intravenously, the dosage was 200 mg once every three weeks.
Efficacy and safety assessments
In the RC48 plus ICIs therapy and RC48 monotherapy group, imaging examinations were performed after every 6 weeks. The method of imaging examinations was enhanced computed tomography (CT). According to RECIST version 1.1 response evaluation criteria in solid tumors, the clinical response was divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ratio of CR plus PR was objective response rate (ORR), and the ratio of CR + PR + SD was disease control rate (DCR). The Common Terminology Criteria for Adverse Events (version 4.0) was used to evaluate drug toxicities.
Statistical analysis
Difference between groups were compared by Pearson’s chi squared test or Fisher’s exact test. Progression-free survival (PFS) was calculated from using RC48 or RC48 plus ICIs to progressive disease or death of patient. Overall survival (OS) was calculated from using RC48 or RC48 plus ICIs to death of patient or end of follow-up. Kaplan-Meier method and the log-rank test were used to perform survival and prognostic analysis. The follow-up period ends on May 31, 2022. SPSS 22.0 software (SPSS Inc., IL, US) was used for statistical analysis, and P < 0.05 was defined as statistically significant.
Results
Patient and treatment
45 cases of advanced and metastatic gastric cancer and failed from two or more lines of prior therapy were included. Table 1 summarized the baseline clinicopathological and treatment characteristics of patients. Thirty-three (73.3%) patients in the present study were HER-2 positive, and the other 12 (26.7%) patients had HER2-low expression. All the HER2-positive patients had received trastuzumab targeted therapy in previous first-line therapy. All the patients had received prior therapy, including chemotherapy (45, 100%), antiangiogenic therapy (16, 35.6%) and immunotherapy (26, 57.8%). In the early days, due to the limitation of testing reagents, PD-L1 was not a routine test item in the pathology department of our center. Among the 45 patients in this study, there were 34 patients with PD-L1 expression results, of which 18 were PD-L1 positive and 16 were PD-L1 negative. Twenty five patients received RC48 plus ICIs therapy, and the other 20 patients received RC48 monotherapy. There were no significant differences in the clinicopathological characteristics between RC48 monotherapy and RC48 plus ICIs therapy group.
Treatment response and efficacy
The overall ORR and DCR in this study were 24.4% (11/45) and 66.7% (30/45), respectively (Fig. 1; Table 2). In the RC48 monotherapy group, the overall ORR and DCR were 10.0% (2/20) and 50.0% (10/20), respectively. In the RC48 plus ICIs therapy group, the overall ORR and DCR were 36.0% (9/25) and 80.0% (20/25), respectively. Patients who received RC48 plus ICIs therapy obtained better ORR (36.0% vs. 10.0%, P = 0.044) and DCR (80.0% vs. 50.0%, P = 0.034) compared with RC48 monotherapy. No statistically significant difference was found in ORR (27.3% vs. 16.7%, P = 0.464) and DCR (66.7% vs. 66.7%, P = 1.000) between the HER-2 positive group and HER2-low expression group.
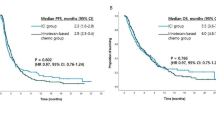
The median follow-up duration was 6.4 m, range from 4.0 to 9.6 m. In the general population, the median PFS was 4.9 months (95% CI = 2.8-7.0) (Fig. 2), and the median OS was not reached. Although the difference was not statistically significant, the median PFS in RC48 plus ICIs therapy group were superior to RC48 monotherapy group (6.2 m vs. 3.9 m, P = 0.140, Fig. 3A). No statistically differences were found between HER2-positive and HER2-low group with respect to the median PFS (5.7 m vs. 4.3 m, P = 0.299, Fig. 3B).
Safety
Most of the adverse events (AEs) were grade 1–2 in severity and grade 3–4 AEs occurred in 7 (35.0%) patients of RC48 group and 10 (40.0%) patients of RC48 plus ICIs group, respectively (Table 3). No patients experienced unanticipated toxicities or treatment-related death after treatment. The treatment interruptions as a result of serious adverse events occurred in 6 (30.0%) and 8 (32.0%) patients, respectively. Treatment discontinuation due to adverse events was not observed. Safety data of RC48 were consistent between the two groups. The most common hematologic AEs in RC48 group and RC48 plus ICIs group were decreased white blood count (55.0% vs. 56.0%), decreased neutrophil count (55.0% vs. 52.0%), increased ALT (45.0% vs. 32.0%) and increased AST (45.0% vs. 32.0%). The most common non-hematologic AEs in RC48 group and RC48 plus ICIs group were fatigue (60.0% vs. 56.0%), hypoaesthesia (55.0% vs. 56.0%) and alopecia (65.0% vs. 60.0%).
Discussion
The present study evaluated the efficacy and safety of RC48 plus ICIs and RC48 as third-line therapies and beyond for patients with HER2-positive or HER2-low advanced and metastatic gastric cancer. In the general population, ORR and DCR reached 24.4% and 66.7%, and the median PFS reached 4.9 months, which confirmed the well clinical efficacy of this novel ADC drug RC48. In RC48 monotherapy group, ORR and DCR reached 10.0% and 50.0%, and median PFS reached 3.9 months. C008 was a single-arm phase II study, which evaluated the efficacy and safety of RC48 in patients with HER2-overexpressing advanced or metastatic gastric cancer. The ORR and DCR in RC48 therapy group were 24.0% and 42.4%, and median PFS was 4.1 months [12]. Based on this study, RC48 was approved by the National Medical Products Administration (NMPA) as third-line therapies and beyond for HER2 overexpressing locally advanced or metastatic gastric cancer. The efficacy of RC48 in this real-world study was consistent with the results of previous C008 clinical trial.
There is still a lack of effective treatment strategies for the third-line therapies and beyond in gastric cancer, especially for patients with HER2-positive tumors who have failed from first-line trastuzumab therapy. Chemotherapy and immunotherapy are currently the main treatment options. Randomized phase III WJOG 4007 clinical trial evaluated the efficacy of irinotecan and paclitaxel in patients with advanced or metastatic gastric cancer after failure of prior fluoropyrimidine plus platinum chemotherapy. The median PFS of paclitaxel and irinotecan was only 3.6 months and 2.3 months [15]. The phase III randomized controlled ATTRACTION-2 study evaluated nivolumab versus placebo in patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma who progressed after two or lines of chemotherapy. In nivolumab therapy group, the ORR was 11.2% and median PFS was 1.61 months [16]. Clinical KEYNOTE-059 trial evaluated the the safety and efficacy of another ICIs pembrolizumab in patients who had previously received at least 2 lines of treatment. The ORR and DCR were 11.6% and 27.0%, and with a median PFS of 2.0 months [17]. Compared with previous chemotherapy and immunotherapy data, RC48 provides a new therapeutic strategy with superior clinical efficacy.
Our present study confirmed that compared with RC48 monotherapy, patients who received RC48 plus ICIs obtained better ORR and DCR, and simultaneously, the median PFS in RC48 plus ICIs group were superior, which suggests that ICIs enhanced the antitumor efficacy of RC48 and novel ADC drugs combined with immunotherapy may become a new drug combination treatment mode. Basic research has shown that ADC drugs can upregulate the expression of PD-L1 and major histocompatibility complex class I (MHC-I) in tumor cells, which play a synergistic mechanism when used in combination with immunotherapy [18]. Meanwhile, ICIs can promote ADC-induced antitumor immunity [19]. Therefore, ADC drugs combined with immunotherapy may help overcome resistance to either single agent [20–21]. Previous studies of trastuzumab deruxtecan (DS-8201,T-DXd) combined with PD-1 inhibitor in HER2-positive mouse tumor models also found that the anti-tumor effect of this combination therapy mode was better than that of either single drug therapy [22]. Especially in this study, 60.0% of patients in RC48 group and 56.0% of patients in the RC48 plus ICIs group had received prior immunotherapy.
In the RC48 plus ICIs therapy group, 14(56.0%) patients received ICIs in previous treatment scheme, we used ICI again based on the following considerations. The efficacy and outcome of treatment beyond progression (TBP) with ICIs in advanced and metastatic gastric cancer have not been clarified. The ATTRACTION-2 study reported the 3-year update and outcome of TBP with nivolumab in in previously nivolumab treated advanced gastric cancer, which showed that long-term efficacy of nivolumab was confirmed at the 3-year follow-up, and a survival benefit of TBP with nivolumab was suggested [23]. A study conducted in our institute evaluated the efficacy of re-administering ICIs, which included a total of 60 patients. The results showed a median PFS of 2.9 months, ORR of 16.7%, and DCR of 55.0% in the re-administering ICIs group, indicating that re-administering ICIs may be a feasible treatment option for advanced and metastatic gastric cancer. Although we still lack more evidence-based medical evidence to support the re-administering ICIs in previous ICIs treated advanced and metastatic gastric cancer patients, re-administering ICIs is a promising research direction.
The emergence of ADC drugs has expanded the indications of anti-HER2 therapy for advanced or metastatic gastric cancer gastric cancer. On the one hand, after the progression of first-line anti-HER2 therapy for advanced or metastatic gastric cancer, there is no effective anti-HER2 therapy at present. The continuous use of trastuzumab beyond progression (TBP) in gastric cancer is controversial [24,25,26,27]. ADC drugs provide an effective back-line anti HER2 treatment strategy. On the other hand, the advent of ADC drugs has redefined the criteria for HER2 classification. Only HER2-positive patients, which was defined as IHC 3 + or IHC 2+/FISH positive, will benefit from traditional anti-HER2 agents. In addition to the efficacy of ADC in this population, it also has promising antitumor activity in patients with HER2 low expression, which was defined as IHC 1 + or IHC 2+/FISH negative [28–29]. Subgroup analysis of the C008 study also showed that both HER2 IHC 3 + and 2 + patients could benefit from RC48 treatment [12]. 26.7% of patients in our present study were HER2 low expression, no statistically differences were found between HER2-positive and HER2-low group with respect to ORR, DCR and median PFS.
The overall safety of RC48 in the present study was consistent with that observed in previous clinical trials [8, 11]. Hematological adverse reactions are the main toxicity of ADC drugs, including decreased white blood count, decreased neutrophil count, anemia and decreased platelet. The mechanism of hematological adverse reactions of ADC drugs has not been fully elucidated. It was generally considered to be related to the drug conjugates, resulting in the bone marrow suppression which was similar to cytotoxic chemotherapy drugs. Due to the lack of clear guidelines or consensus for the management of hematological adverse reactions of ADC drugs, we mostly refer to the guidelines for the management of bone marrow suppression caused by chemotherapy drugs in clinical practice. Compared with RC48 monotherapy, the incidence of adverse reactions in RC48 plus ICIs treatment group did not increase, which means that ICIs will not increase the incidence of RC48 related adverse reactions.
Our study has several strengths and limitations, because it was an observational study with not very large number of patients. Future validation and prospective clinical trials would be needed to confirm the value of ICIs plus RC48 in HER2-positive or HER2-low advanced or metastatic gastric cancer. However, to our knowledge this is the first study exploring novel treatment strategy for advanced or metastatic gastric cancer based on ADC drugs combined with immunotherapy.
Conclusions
In conclusion, compared with RC48 monotherapy, ICIs plus RC48 demonstrated superior third-line and beyond therapeutic efficacy for HER2-positive or HER2-low advanced and metastatic gastric cancer patients with manageable safety.
Data availability
The raw data of this article will be made available by contacting the corresponding author.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 2021/02/05.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9.
Roviello G, Aprile G, D’Angelo A, Iannone LF, Roviello F, Polom K, Mini E, Catalano M. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: where do we stand? Gastric Cancer. 2021;24(4):765–79.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Kotani D, Shitara K. Trastuzumab deruxtecan for the treatment of patients with HER2-positive gastric cancer. Ther Adv Med Oncol. 2021;13:1758835920986518.
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al. Trastuzumab Deruxtecan in previously treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382(7):610–21.
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, Nagasaka M, Bazhenova L, Saltos AN, Felip E, et al. Trastuzumab Deruxtecan in HER2-Mutant non-small-cell Lung Cancer. N Engl J Med. 2022;386(3):241–51.
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, Shi B, Liu J, He Z, Yu G, et al. Open-label, Multicenter, Phase II study of RC48-ADC, a HER2-Targeting antibody-drug Conjugate, in patients with locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27(1):43–51.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al. Trastuzumab Deruxtecan in previously treated HER2-Positive gastric Cancer. N Engl J Med. 2020;382(25):2419–30.
Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29(1):1335–44.
Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, Mao C, Fan Q, Bai Y, Ba Y, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25.
Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, Zhang X, Fan N, Luo S, Li Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41(11):1173–82.
Yu J, Fang T, Yun C, Liu X, Cai X. Antibody-drug conjugates targeting the Human Epidermal Growth Factor Receptor Family in cancers. Front Mol Biosci. 2022;9:847835.
Guideline Recommendations for HERDiGCG. [Guidelines for HER2 detection in gastric cancer(2016)]. Zhonghua Bing Li Xue Za Zhi. 2016;45(8):528–32.
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal Metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–44.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and Efficacy of Pembrolizumab Monotherapy in patients with previously treated Advanced gastric and gastroesophageal Junction Cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013.
Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, Kiermaier A, Swain SM, Baselga J, Michiels S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive Breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62.
Saini KS, Punie K, Twelves C, Bortini S, de Azambuja E, Anderson S, Criscitiello C, Awada A, Loi S. Antibody-drug conjugates, immune-checkpoint inhibitors, and their combination in Breast cancer therapeutics. Expert Opin Biol Ther. 2021;21(7):945–62.
Shitara K, Bang Y, Iwasa S, et al. Exploratory Biomarker Analysis of Trastuzumab Deruxtecan in DESTINY-Gastric01, a Randomized, phase 2, Multicenter, open-label study in patients with HER2-positive or -low Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. ESMO World Congress on Gastrointestinal Cancer; 2021.
Matsumoto T, Yamamura S, Ikoma T, et al. Real-World Data of Trastuzumab Deruxtecan for Advanced Gastric Cancer: a multi-institutional Retrospective Study. J Clin Med. 2022;11(8):2247.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-Targeting antibody-drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), enhances Antitumor Immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–503.
Boku N, Satoh T, Ryu MH, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer. 2021;24(4):946–58.
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced Breast cancer: a German breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006.
Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, Tsuji A, Moriwaki T, Tanioka H, Shinozaki K, et al. Randomized, phase II study of Trastuzumab Beyond Progression in patients with HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT study). J Clin Oncol. 2020;38(17):1919–27.
Ter Veer E, van den Ende T, Creemers A, de Waal L, van Oijen MGH, van Laarhoven HWM. Continuation of trastuzumab beyond progression in HER2-positive advanced esophagogastric cancer: a meta-analysis. Acta Oncol. 2018;57(12):1599–604.
Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y, Wang Y, Cui Y, Li W, Yu S, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–65.
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in patients with HER2-Low-expressing advanced Breast Cancer: results from a phase ib study. J Clin Oncol. 2020;38(17):1887–96.
Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K, Tsurutani J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141(8):1682–9.
Acknowledgements
We gratefully acknowledge the follow-up team for their contribution to this study.
Funding
This work was supported by Medical Science and Technique Foundation of Henan Province (No. 212102310623), 1000 Talents Program of Central plains (No. 204200510023), Young and Middle-aged Health and Technology Innovation Leading Talent Project of Henan Province (No. YXKC2020008), the Sate Key Laboratory of Esophageal Cancer Prevention & Treatment (No. Z2020000X), Medical Science and Technique Foundation of Henan Province (No. LHGJ20210172) and Science and Technique Foundation of Henan Province (No. 222102310424).
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript. Caiyun Nie and Xiaobing Chen designed the study, and drafted the paper. Caiyun Nie, Yanwei Guo, Xiaohui Gao, Weifeng Xu, Huifang Lv and Beibei Chen collected and analyzed the data. Caiyun Nie, Jianzheng Wang, Yingjun Liu, Jing Zhao, Saiqi Wang and Yunduan He performed statistical analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University(KY-0192). Written informed consent was obtained from all patients for the use of the medical records for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nie, C., Xu, W., Guo, Y. et al. Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: a multicenter real-world study. BMC Cancer 23, 1239 (2023). https://doi.org/10.1186/s12885-023-11735-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11735-z