Abstract
Background
Currently, the value of oral selective estrogen receptor degraders (SERDs) for hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (aBC) after progression on ≥ 1 line of endocrine therapy (ET) remains controversial. We conducted a meta-analysis to evaluate progression-free survival (PFS) and safety benefits in several clinical trials.
Materials and methods
Cochrane Library, Embase, PubMed, and conference proceedings (SABCS, ASCO, ESMO, and ESMO Breast) were searched systematically and comprehensively. Random effects models or fixed effects models were used to assess pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for treatment with oral SERDs versus standard of care.
Results
A total of four studies involving 1,290 patients were included in our analysis. The hazard ratio (HR) of PFS showed that the oral SERD regimen was better than standard of care in patients with HR+/HER2- aBC after progression on ≥ 1 line of ET (HR: 0.75, 95% CI: 0.62-0.91, p = 0.004). In patients with ESR1 mutations, the oral SERD regimen provided better PFS than standard of care (HR: 0.58, 95% CI: 0.47-0.71, p < 0.00001). Regarding patients with disease progression following previous use of CDK4/6 inhibitors, PFS benefit was observed in oral SERD-treatment arms compared to standard of care (HR: 0.75, 95% CI: 0.64-0.87, p = 0.0002).
Conclusions
The oral SERD regimen provides a significant PFS benefit compared to standard-of-care ET in patients with HR+/HER2- aBC after progression on ≥ 1 line of ET. In particular, we recommend oral SERDs as a preferred choice for those patients with ESR1m, and it could be a potential replacement for fulvestrant. The oral SERD regimen is also beneficial after progression on CDK4/6 inhibitors combined with endocrine therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the United States, approximately 60-70% of women with advanced breast cancer (aBC) are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) [1,2,3]. Resistance to treatment, acquisition of novel mutations, and altered gene expression are the major challenges in the management of aBC [4, 5]. There are established guidelines for first-line treatment of these patients, but a consensus has not yet been reached regarding the choice of second-line treatment [6].
Endocrine therapy (ET), with either fulvestrant (Fulv) or aromatase inhibitors (AIs), plus a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) is the recommended first-line standard of care for patients with HR+/HER2- advanced breast cancer [7]. Compared with endocrine monotherapy, the combination can obtain a higher response rate and progression-free survival benefit [8,9,10]. However, the development of resistance to the treatment of aBC is frequent, and its treatment is primarily palliative [11] In general, there are three main strategies after the failure of CDK4/6i treatment: diversion to chemotherapy, endocrine therapy alone, or combined targeted therapy [12,13,14]. Currently, there are no recommended guidelines for the optimal ranking of these options. In any case, ET is still an important treatment strategy.
Estrogen receptor 1 mutations (ESR1m) are one of the common mechanisms of endocrine resistance, accounting for up to 36% of metastatic breast cancers [15, 16]. Selective estrogen receptor degraders (SERDs) can bind to estrogen receptors and induce their degradation [17, 18] and are considered one of the main ways to address endocrine resistance. Fulvestrant, as an intramuscular SERD, is not only the first-line or second-line treatment option for HR+/HER2- aBC [19, 20] but is also a choice for patients with ESR1m, who are still sensitive to it [15, 21, 22]. In recent years, oral SERDs, with their higher bioavailability and pharmacokinetics, have been continuously developed to address the limitations of fulvestrant intramuscular formulations [23]. However, the value of oral SERDs in patients with HR+/HER2- advanced breast cancer remains controversial. EMERALD [24] and SERENA-2 [25] showed positive results, while the other two clinical trials, AMEERA-3 [26] and acelERA [27], failed the study endpoints.
In the present meta-analysis, we aimed to assess the value of oral SERDs in patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of endocrine therapy.
Materials and methods
Search strategy and data extraction
The systematic review of literature and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28]. The corresponding PRISMA checklist is shown in Supplement 2. A systematic and comprehensive literature search was conducted using Embase, PubMed, and Cochrane Library. Conference proceedings from major oncology meetings (ASCO, SABCS, ESMO, and ESMO Breast) from 2020 up to November 2023 were also carefully reviewed. The following search string was used: “(breast OR mammary) AND (cancer OR carcinoma OR malignant OR neoplasm OR tumour) AND (hormone receptor-positive OR HR-positive OR HR OR estrogen receptor-positive OR ER OR ER-positive) AND (HER-2- OR HER2- OR ERBB2- OR HER-2 negative OR HER2-negative OR ERBB2 negative OR human epidermal growth factor receptor 2-negative) AND (metastasis OR metastases OR metastatic OR advanced OR recurrent OR stage IV) AND (oral selective estrogen receptor degrader OR SERD OR Giredestrant OR Camizestrant OR Imlunestrant OR Elacestrant OR Amcenestrant).” Records from the included studies were screened independently by two investigators. In cases of disagreement, the third investigator was consulted to reach a consensus.
Details about the title, publication date, study design, and trial name were extracted. All relevant randomized controlled trials were identified as the recommendations of the Cochrane Collaboration [29]. When duplicate publications were identified, only the latest data were extracted in our study. Other details about the first author, country, sample size, menopausal status, oral SERDs used, dose of oral SERDs, treatment regimens used in the control arm, previous treatment regimen, ESR1m status, hazard ratio (HR), progression-free survival (PFS), median progression-free survival (mPFS) and side effects for each arm were extracted. The primary outcome was progression-free survival, which was defined as the time from randomization to death or disease progression, whichever occurred first. The proportion of patients who achieved an overall response according to the Response Evaluation Criteria in Solid Tumours (RECIST) was selected as a secondary outcome [30]. An exploratory analysis was conducted based on the Common Terminology Criteria for Adverse Events, version 4, reporting the proportion of patients with grade 3-5 adverse events [31]. All data included in the study were extracted independently by two investigators.
Study selection
Studies had to satisfy the following inclusion and exclusion criteria: (I) phase II or III randomized clinical trials (RCTs) including patients with HR+/HER2- aBC after progression on ≥ 1 line of ET; (II) comparison of oral SERD-treated patients and patients treated with standard-of-care ET; and (III) the publication provided PFS and HR for the experimental and control arms. Systemic reviews, case reports, single-arm studies, exploratory studies, and retrospective studies were excluded. If multiple publications were associated with the same clinical trial, only the latest and complete randomized controlled trial was included.
Objectives
The primary objective of the study was to compare the efficacy of oral SERDs with standard-of-care ET in patients with HR+/HER2- aBC after progression on ≥ 1 line of ET. The secondary objective was to analyse the subgroup of patients in the population that might benefit from oral SERDs. We planned the subgroup analysis for the following subgroups: patients with disease progression following previous use of CDK4/6 inhibitors or Fulv; patients with ESR1m; patients with visceral metastasis; comparing oral SERDs with fulvestrant; and comparing oral SERDs with fulvestrant in patients with ESR1m.
Statistical analysis
Global PFS was calculated using a random-effects model or fixed-effects model and reported as pooled hazard ratios (HRS) with 95% confidence intervals (CIs). If the 95% CI did not include 1.0 and the two-sided threshold was P < 0.05, the pooled HR was considered statistically significant. The I2 value was employed for the heterogeneity of included studies. When I2 > 50%, significant heterogeneity was considered established, and the random-effects model was adopted; otherwise, the fixed-effects model was used. When heterogeneity was high in the pooled results, sensitivity analysis was performed after every single study was excluded. All statistical analysis methods were performed using Review Manager (version 5.3). The Cochrane Collaboration’s Risk of Bias tool in Review Manager (version 5.3) was employed to assess the risk of bias for each eligible study.
Results
Study selection
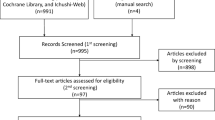
A total of 386 potentially relevant manuscripts and 2 additional abstracts were sorted by using the search string mentioned before. Of these, after reviewing the titles and abstracts, 373 manuscripts were excluded. We then performed a full-text review for the remaining 15 articles, 11 of which were excluded for nonconformity with the present inclusion criteria. Eventually, 4 articles from 4 trials were considered eligible for the meta-analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart is shown in Fig. 1.
Characteristics of studies
Finally, our study involved 4 clinical trials published between February 2022 and November 2023, focusing on different endocrine treatment regimens for HR+/HER2- advanced breast cancer, and included a total of 1,290 patients (Table 1). The oral SERD arms included elacestrant (EMERALD), camizestrant 75 mg/camizestrant 150 mg (SERENA-2), amcenestrant (AMEERA-3), and giredestrant (acelELA). The control arms included fulvestrant, anastrozole, letrozole, exemestane, and tamoxifen. All trials compared oral SERDs to standard-of-care ET in patients with HR+/HER2- aBC after progression on ≥ 1 line of ET.
Progression-free survival
In the whole population, patients with HR+/HER2- advanced breast cancer treated with oral SERDs had significantly improved PFS compared to those treated with standard-of-care ET (HR: 0.75, 95% CI: 0.62-0.91, p = 0.004; I2: 52%, p = 0.08; Fig. 2A). For enrolled patients with disease progression following previous use of CDK4/6 inhibitors, the oral SERD regimen was significantly better than standard-of-care ET (HR: 0.75, 95% CI: 0.64-0.87, p = 0.0002; I2: 48%, p = 0.10; Fig. 2B). In HR+/HER2- ESR1m aBC, the two treatment regimens compared, namely, oral SERDs resulted in a better PFS versus standard-of-care ET (HR: 0.58, 95% CI: 0.47-0.71, p < 0.00001; I2: 42%, p = 0.14; Fig. 2C). Regarding enrolled patients with ESR1 mutations, results in arms of oral SERDs were significantly better than in arms of fulvestrant (HR: 0.47, 95% CI: 0.36-0.62, p < 0.00001; I2: 0%, p = 0.41; Fig. 2D). Regarding patients who had previously failed treatment with fulvestrant, oral SERDs as monotherapy were significantly superior to standard-of-care ET (HR: 0.67, 95% CI: 0.47-0.95, p = 0.02; I2: 0%, p = 0.93; Fig. 3A). In patients with visceral disease, the results in arms of oral SERDs were significantly better than the results in arms of standard-of-care ET (HR: 0.60, 95% CI: 0.48-0.74, p < 0.00001; I2: 33%, p = 0.22; Fig. 3B). The results in arms of oral SERDs were significantly better than those in arms of fulvestrant (HR: 0.65, 95% CI: 0.54-0.78, p < 0.00001; I2: 0%, p = 0.76; Fig. 3C).
The Forrest plot of PFS for patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of endocrine treatment. A PFS pooled result for overall patients; B PFS pooled result for patients with previous use of CDK4/6 inhibitors; C PFS pooled result for patients with ESR1m; D PFS pooled result for comparing oral SERDS with fulvestrant in patients with ESR1m subgroup. Note: PFS, progression-free survival; CI, confidence interval; HR, hazard ratio; HR+/HER2-, hormone receptor-positive and human epidermal growth factor receptor 2-negative; SERDs, selective estrogen receptor degraders; ESR1m, estrogen receptor 1 mutations
The Forrest plot for global PFS for patients with (A) previous use of fulvestrant; (B) visceral metastasis; (C) Forrest plot for global PFS comparing oral SERDS with fulvestrant. Note: PFS, progression-free survival; CI, confidence interval; HR, hazard ratio; HR+/HER2-, hormone receptor-positive and human epidermal growth factor receptor 2-negative; SERDs, selective oestrogen receptor degraders
Safety
Adverse events (AEs) of grade 3 or higher were more frequent in the oral SERD regimen than in standard-of-care ET (HR: 1.40, 95% CI: 1.03-1.90, p = 0.03; I2: 0%, p = 0.99; Fig. 4). The proportion of treatment-emergent adverse events (TEAEs) leading to discontinuation was 6.3% (Elacestrant) vs. 4.4% (SOC) in EMERALD's two treatment arms. The most common adverse event was nausea. The proportion of drug discontinuation caused by treatment related AEs (TRAEs) in the three treatment groups of SERENA-2 was 2.7% (camizestrant 75 mg), 0% (camizestrant 150 mg), and 0% (fulvestrant as standard-of-care ET), respectively; common adverse events were photopsia and sinus bradycardia. In AMEERA-3, the proportion of TRAEs ≥ Grade 3 was 4.9% in the experimental arm and 0.7% in the control arm. The most common adverse event was nausea. In acelELA, the incidence of AE ≥ Grade 3 was 12% (giredestrant) vs. 8.6% (physician’s choice of endocrine monotherapy); the most common adverse event was hepatotoxicity.
The Forrest plot for AE ≥ Grade 3 for patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of ET. Note: AE, adverse event; progression-free survival; CI, confidence interval; HR, hazard ratio; HR+/HER2-, hormone receptor-positive and human epidermal growth factor receptor 2-negative; SERDs, selective estrogen receptor degrader
Bias assessment
In all trials included, the overall risk of bias was low (Supplement 1 Fig. 1). Since these trials were conducted with an open-label design, performance bias that did not affect the results may exist. There was no obvious publication bias (Supplement 1 Figs. 2 and 3).
Discussion
Our study showed that the oral SERD regimen was superior to standard-of-care ET in patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of ET. However, the characteristics of these patients were complex, so it is crucial to select the characteristics of those patients who are likely to have sustained benefits.
Patients with ESR1m develop resistance to ET and exhibit worse overall survival [32,33,34]. Our meta-analysis showed that for patients with ESR1 mutations, outcomes in the arms of oral SERDs were significantly better than those in the arms of standard-of-care ET. Surprisingly, in these four clinical trials, oral SERDs were able to provide PFS benefits in ESR1m patients. In addition, patients with ESR1m showed a trend of OS improvement in Elacestrant (HR = 0.59; p = 0.03). AIs not only enhance the acquisition of ESR1 mutations in aBC, but patients with ESR1 mutations also showed a worse prognosis in AI treatment [35]. However, patients with ESR1 mutations remained sensitive to fulvestrant [15, 21, 22]. As an intramuscular SERD, fulvestrant binds to estrogen receptors and induces their degradation, [17, 18] so it still plays a role in patients with ESR1 mutations. A pooled analysis of patients with ESR1 mutations in the EFECT and SoFEA trials (115/383) found no significant difference in PFS in the Fulv group (3.9 months versus 4.1 months) [36,37,38]. However, the clinical utilization of Fulv is limited by its intramuscular formation. In the Elacestrant and SERENA-2 trials, the arms of oral SERDs were significantly better than the arms of fulvestrant (HR: 0.47, p < 0.00001). In addition, its better bioavailability and patient preference for oral medication may lead to better compliance. Patient tolerability of the drug also needs to be considered. The overall toxicity of oral SERDs was found to be greater in our analysis. However, considering that a proportion of patients in the control arms were on AI and tamoxifen regimens, the toxicity of AIs and tamoxifen was lower than that of Fulv [39,40,41]. Therefore, this does not mean that oral SERDs are more toxic than Fulv. Moreover, treatment resistance to Fulv leading to disease progression remains a major concern for HR+/HER2- aBC. Therefore, both additional endocrine therapy and effective combination therapy are clinically necessary [15, 16]. Data from the Elacestrant and acelELA trials also support oral SERD regimens for patients who failed Fulv therapy. Thus, oral SERDs are recommended in HR+/HER2- ESR1m aBC after ET ≥ 1 line progression, and oral SERDs could be a potential replacement for Fulv.
For HR+/HER2- aBC patients who progressed after first-line treatment with ET combined with CDK4/6i, the oral SERD regimen also had a statistically significant PFS benefit. In the event of disease progression during the use of CDK4/6is, ET-based regimens remain an appropriate option [12, 13]. Patients' menopausal status, tolerance to drugs, and previous treatment regimens will affect the subsequent selection of endocrine agents [42]. These enrolled patients had previously used one or two ET regimens, so it is still necessary to find new endocrine agents. Camizestrant therapy may be a new option for these patients. The median PFS in the oral SERDs group was 7.2 (75 mg) and 7.7 (150 mg) months, respectively, while that in the Fulv group was only 3.7 months. Even in the subgroup with previous use of CDK4/6i, there was a significant improvement in PFS [median PFS 5.5 (75 mg) and 3.8 (150 mg) months vs. 2.1 months]. However, the absolute benefit in Elacestrant was very small (median PFS 2.8 months vs. 1.9 months). In ESR1m aBC patients previously treated with CDK4/6i for ≥12 months, elacestrant had a median PFS of 8.6 months and SOC of 2.1 months, which was a clinically and statistically significant improvement. This suggests that a possible indication for elacestrant may be the duration of previous CDK4/6i [43]. In addition, in those patients with visceral metastasis, oral SERDs also showed advantages (HR: 0.60, P < 0.00001). Endocrine therapy is the preferred option for HR+ breast cancer patients even in the presence of visceral metastases [44]. Compared with endocrine monotherapy, the combination can obtain a higher response rate and progression-free survival benefit [45]. Chemotherapy is recommended for patients with visceral crisis. However, chemotherapy is more toxic and causes many side effects in patients [46]. In contrast, oral SERDs show better efficacy in patients with visceral metastasis and can also reduce the serious side effects caused by chemotherapy.
EMERALD and SERENA-2 showed positive results in these four randomized controlled trials, while the other two trials, AMEERA-3 and acelERA, failed the study endpoints. Due to the heterogeneity of enrolled patients and differences in control settings, indirect cross-comparisons between different trials should be undertaken with caution. First, prior treatment regimens after disease progression varied across the four trials. In the SERENA-2 trial, 31.3% of patients had previously not received ET in the advanced setting, whereas in the other three trials, patients had previously received at least one or two lines of ET. Studies have shown that monotherapy with Fulv had advantages in PFS compared to aromatase inhibitors or tamoxifen monotherapy [47, 48]. In the control arm of AMEERA-3 and acelERA, the proportion of patients treated with Fulv was higher (89.8% and 75%, respectively), which may have resulted in prolonged mPFS in the control group. In addition, all patients in the SERENA-2 control group received Fulv, but previous Fulv was not permitted for aBC patients. In EMERALD, however, 30.4% of patients had previously been treated with Fulv; in AMEERA-3, the corresponding value was 9.7%, and in acelERA, it was 26.19%.
Our study is the first to evaluate the value of oral SERDs in patients with HR+/HER2- aBC after progression on ≥ 1 line of endocrine therapy. The characteristics of the population that may benefit are also analysed. Especially for patients with ESR1m, oral SERDs are advantageous. Further screening of advantaged oral SERD groups for stratified treatment is the future development trend. The value of SERDs may not be limited to patients in advanced settings. Studies such as CAMBRIA-1 [49] are being conducted to assess the potential of oral SERDs in early-stage breast cancer. In addition, oral dosage forms are more convenient. This can save manpower and material resources to a certain extent, and the compliance of patients will be better. It is believed that it will have good application prospects. There are several limitations to our study. First, this was not a network meta-analysis, and we could not directly compare all drugs or drug combinations with each other. As a result, a certain degree of precision was lost. In addition, we could not evaluate the overall survival (OS) benefit due to the unavailability of data. Although OS is the "gold standard" for efficacy evaluation in cancer clinical research, it has certain limitations in practical application. OS as the primary endpoint requires a large sample size, and clinical development is difficult. It is affected by death from nontumour causes. For tumour types with long survival, the duration of the study is extremely long. Therefore, alternative end points are often used for those patients with long survival, and the FDA currently supports the use of PFS as an end point. However, these limitations are unavoidable at present. At present, there are relatively few studies on oral SERDs, and it is hoped that more clinical trials will follow to confirm our experiments.
Conclusion
The oral SERD regimen has a significant PFS benefit compared to standard-of-care ET in patients with HR+/HER2- aBC after progression on ≥ 1 line of ET. In particular, we recommend oral SERDs as a preferred choice for those patients with ESR1m, and it could be a potential replacement for fulvestrant. The oral SERD regimen also benefits after progression on CDK4/6 inhibitors combined with endocrine therapy.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Change history
13 February 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12885-024-11932-4
References
Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–15.
Malmgren JA, Mayer M, Atwood MK, et al. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res Treat. 2018;167(2):579–90.
Gong Y, Liu YR, Ji P, et al. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep. 2017;7:45411.
Almendro V, Cheng YK, Randles A, et al. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep. 2014;6(3):514–27.
Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513.
Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395(10226):817–27.
Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250–60.
Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375(18):1738–48.
Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–36.
Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–46.
Hart CD, Migliaccio I, Malorni L, et al. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin Oncol. 2015;12(9):541–52.
Xi J, Ma CX. Sequencing endocrine therapy for metastatic breast cancer: what do we do after disease progression on a CDK4/6 inhibitor? Curr Oncol Rep. 2020;22(6):57.
Huang J, Zheng L, Sun Z, et al. CDK4/6 inhibitor resistance mechanisms and treatment strategies (Review). Int J Mol Med. 2022;50(4):128.
Basile D, Gerratana L, Corvaja C, et al. First- and second-line treatment strategies for hormone-receptor (HR)-positive HER2-negative metastatic breast cancer: a real-world study. Breast. 2021;57:104–12.
Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):182r–313r.
Herzog SK, Fuqua S. ESR1 mutations and therapeutic resistance in metastatic breast cancer: progress and remaining challenges. Br J Cancer. 2022;126(2):174–86.
Lee CI, Goodwin A, Wilcken N. Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev. 2017;1(1):D11093.
McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10(6):620–8.
Li J, Wang Z, Shao Z. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: a review. Cancer Med. 2019;8(5):1943–57.
Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–5.
Fribbens C, Garcia MI, Beaney M, et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann Oncol. 2018;29(1):145–53.
Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446–51.
Pagliuca M, Donato M, D’Amato AL, et al. New steps on an old path: Novel estrogen receptor inhibitors in breast cancer. Crit Rev Oncol Hematol. 2022;180:103861.
Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022;40(28):3246–56.
Oliveira M, Pominchuk D, Nowecki Z, et al. Camizestrant, a next-generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2- negative breast cancer: Results of the randomized, multi-dose Phase 2 SERENA-2 trial. Cancer Res. 2023;83(suppl 5):GS3–02.
Tolaney SM, Chan A, Petrakova K, et al. AMEERA-3: Randomized Phase II Study of Amcenestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Monotherapy in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. J Clin Oncol. 2023;41(24):4014–24.
Jimenez MM, Lim E, Mac Gregor MC, et al. Giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2– locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol. 2022;33(suppl 7):S633–4.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from: www.cochrane-handbook.org.
Eisenhauer E A, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009,45(2):228-247.
National Cancer Institute. Common terminology criteria for adverse events. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 25 June 2018.
Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2016;34(25):2961–8.
Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 Mutations in Cell-Free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016;2(10):1310–5.
Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34(3):427–38.
Zundelevich A, Dadiani M, Kahana-Edwin S, et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res. 2020;22(1):16.
Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–70.
Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–98.
Turner NC, Swift C, Kilburn L, et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor-Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin Cancer Res. 2020;26(19):5172–7.
Robertson J, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005.
Blackburn SA, Parks RM, Cheung KL. Fulvestrant for the treatment of advanced breast cancer. Expert Rev Anticancer Ther. 2018;18(7):619–28.
Boer K. Fulvestrant in advanced breast cancer: evidence to date and place in therapy. Ther Adv Med Oncol. 2017;9(7):465–79.
Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28(1):16–33.
Lipsyc-Sharf M, Tolaney SM. Elacestrant: who are optimal candidates for the first oral SERD?. Ann Oncol. 2023;34(5):449–51.
Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49.
Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–9.
Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2–Negative (or unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307–29.
Shimoi T, Sagara Y, Hara F, et al. First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer. 2020;27(3):340–6.
Zhang J, Huang Y, Wang C, et al. Efficacy and safety of endocrine monotherapy as first-line treatment for hormone-sensitive advanced breast cancer: a network meta-analysis. Medicine (Baltimore). 2017;96(33):e7846.
Hamilton E, Loibl S, Niikura N, et al. A phase III randomised open-label study of extended adjuvant therapy with camizestrant vs standard endocrine therapy (ET) in patients with ER+/HER2e early breast cancer (BC) and an intermediate or high risk of recurrence (CAMBRIA-1). Ann Oncol. 2023;34(suppl 2):S323–4.
Acknowledgements
We thank Yuan Su, MD, PhD from Fujian Medical University for statistics consultation.
Funding
The authors state that no funds, grants or other lines of support were received in this manuscript preparation process.
Author information
Authors and Affiliations
Contributions
Xiewei Huang, Yushuai Yu, Shiping Luo, and Wenfen Fu performed the study design, article search, and data collection. Yushuai Yu performed the analysis. Xiewei Huang and Yushuai Yu wrote the first draft of the manuscript. Jie Zhang and Chuangui Song reviewed the article. All authors participated in commenting on the manuscript and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
As the data used in this study were from previously published literature, ethical approval and informed consent were not needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Text in the safety sub-section of the results section has been corrected.
Supplementary Information
Additional file 1:
Supplementary Figure 1. Quality assessment for the bias items of RCTs. (a) Risk of the bias summary. (b) Risk of the bias graph. Supplementary Figure 2. The funnel plot PFS for patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of endocrine treatment: (A) The funnel plot PFS for overall patients; (B) The funnel plot PFS for patients with previous use of CDK4/6 inhibitors; (C) The funnel plot PFS for patients with ESR1m; (D) The funnel plot PFS for comparing oral SERDS with fulvestrant in patients with ESR1m subgroup. Note: PFS, progression-free survival; CI, confifidence interval; HR, hazard ratio; HR+/HER2-, hormone receptor-positive and human epidermal growth factor receptor 2-negative; SERDS, selective estrogen receptor degrader; ESR1m, estrogen receptor 1 mutations. Supplementary Figure 3. The funnel plot PFS for patients with (A) previous use of fulvestrant; (B) visceral metastasis; (C) funnel plot for PFS comparing oral SERDS with fulvestrant. (D) The funnel plot for AE ≥ Grade 3 for patients with HR+/HER2- advanced breast cancer after progression on ≥ 1 line of ET. Note: PFS, progression-free survival; CI, confifidence interval; HR, hazard ratio; HR+/HER2-, hormone receptor-positive and human epidermal growth factor receptor 2-negative; SERDS, selective estrogen receptor degrader; AE, adverse event.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, X., Yu, Y., Luo, S. et al. The value of oral selective estrogen receptor degraders in patients with HR-positive, HER2-negative advanced breast cancer after progression on ≥ 1 line of endocrine therapy: systematic review and meta-analysis. BMC Cancer 24, 21 (2024). https://doi.org/10.1186/s12885-023-11722-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11722-4