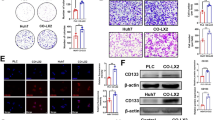
Abstract
Background
Due to the high drug resistance of hepatocellular carcinoma (HCC), sorafenib has limited efficacy in the treatment of advanced HCC. Cancer-associated fibroblasts (CAFs) play an important regulatory role in the induction of chemoresistance. This study aimed to clarify the mechanism underlying CAF-mediated resistance to sorafenib in HCC.
Methods
Immunohistochemistry and immunofluorescence showed that the activation of CAFs was enhanced in HCC tissues. CAFs and paracancerous normal fibroblasts (NFs) were isolated from the cancer and paracancerous tissues of HCC, respectively. Cell cloning assays, ELISAs, and flow cytometry were used to detect whether CAFs induced sorafenib resistance in HCC cells via CXCL12. Western blotting and qPCR showed that CXCL12 induces sorafenib resistance in HCC cells by upregulating FOLR1. We investigated whether FOLR1 was the target molecule of CAFs regulating sorafenib resistance in HCC cells by querying gene expression data for human HCC specimens from the GEO database.
Results
High levels of activated CAFs were present in HCC tissues but not in paracancerous tissues. CAFs decreased the sensitivity of HCC cells to sorafenib. We found that CAFs secrete CXCL12, which upregulates FOLR1 in HCC cells to induce sorafenib resistance.
Conclusions
CAFs induce sorafenib resistance in HCC cells through CXCL12/FOLR1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hepatocellular carcinoma (HCC) is one of the most malignant tumours worldwide, and the incidence is increasing [1]. Studies have shown that more than 50% of HCC patients are initially diagnosed at an advanced stage [2]. Sorafenib is the first Food and Drug Administration (FDA)-approved targeted therapy for advanced HCC [3]. However, since most advanced HCC patients acquire resistance to sorafenib, only 10% to 30% of patients show objective responses to sorafenib [2]. Therefore, the prognosis of advanced HCC patients remains poor, even after sorafenib treatment [4, 5]. Thus, we need to further investigate the mechanisms of sorafenib resistance in HCC. It is important to develop novel therapeutic strategies to overcome sorafenib resistance in patients with advanced HCC.
Cancer-associated fibroblasts (CAFs) represent the main cell population of the tumour microenvironment (TME) [6]. CAFs can stimulate tumour cell drug resistance, proliferation, invasion, and metastasis in various cancers, including HCC [7, 8]. Recent studies have shown that CAFs play a crucial role in the induction of chemoresistance in a variety of cancers, including HCC [9,10,11,12]. However, the mechanism of CAF-mediated sorafenib resistance in HCC remains to be fully elucidated. Clarifying this mechanism is a crucial step towards identifying novel therapeutic targets to overcome chemotherapy resistance and predicting treatment response.
Therefore, we aimed to investigate the mechanism of CAF-induced sorafenib resistance in HCC. This study can help us to identify novel therapeutic targets for overcoming sorafenib resistance. It will be significant to reduce the rate of sorafenib resistance and improve the prognosis of advanced HCC patients.
Methods
Isolation of fibroblasts from cancer tissues and paracancerous tissues in HCC samples
Human HCC tissues were obtained in post-surgical samples, from the department of hepatobiliary surgery, at the Fifth Affiliated Hospital of Sun Yat-sen University. All subjects signed an informed consent form, and the study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University.
Cancer-associated fibroblasts (CAFs) and paracancerous normal fibroblasts (NFs) were isolated from HCC tissues and non-tumor tissues adjacent to the HCC, respectively. Fresh HCC tissues and paracancerous tissues were washed with phosphate buffer (PBS; GenDEPOT, Barker, TX, USA) and mince into small pieces (< 1 mm3). Five minuted small tissues were attached to the cell culture dishes and treated with Dulbecco's modified eagle medium (DMEM medium, Gibco, USA), containing 10% fetal bovine serum (FBS; Invitrogen, Waltham, MA, USA), 50 U/mol penicillin (Sigma-Aldrich, USA) and 50 mg/ml streptomycin (Sigma-Aldrich, USA). The DMEM medium was changed every two days. The fibroblasts extending from the HCC tissue were then trypsinised and transferred to the dish, followed by incubation in fresh medium to facilitate attachment of the isolated fibroblasts to the dish. Cells were maintained in complete medium at 37° C in a humidified incubator with 5% CO2 and 21% O2.
Cell lines
HCC cell lines (HepG2, Huh7) were obtained from the Cancer Center, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China. Cancer-associated fibroblasts (CAFs) and normal fibroblasts (NFs) were cultured from postoperative HCC tissues in our center. All cells were cultured in Dulbecco’s modified eagle medium (DMEM medium, Gibco, USA), which was supplemented with 10% fetal bovine serum (FBS), 50 U/mol penicillin (Sigma-Aldrich, USA) and 50 mg/ml streptomycin (Sigma-Aldrich, USA). AMD3100, an inhibitor of CXCL12, was purchased from Selleck (S8030). CXCL12 protein was purchased from MedChemExpress (HY-P7287). The anti-CXCR4 was purchased from Proteintech (60042–1-Ig). All cultured cells were kept in a humidified incubator at the temperature of 37°C, under the concentration of 5% CO2, and 21% O2.
Cell transfection
For the transfection of human hepatic carcinoma cells (Huh7, HepG2), lentivirus with scramble (control) and sh-CXCR4 was used in the experiments, which was constructed and purchased from Genechem (Shanghai, China). Fourty-eight hours after transfection, stable cell lines were screened by DMEM (10% FBS) containing 2ug/ml puromycin. Western blot analyses were performed to verify the success of transfection.
HCC cells were cocultured with CAFs and NFs
CAFs (1 × 106 cells/ml) or NFs (1 × 106 cells /ml) were cultured in Dulbecco’s modified eagle medium (DMEM medium, Gibco, USA), which were supplemented with 10% fetal bovine serum (FBS) for 48 h. Then, their supernatant was collected to treat HCC cells (HepG2 and Huh7) for 48 h.
Western blotting analyses
After all sample proteins were separated by SDS-PAGE gel, they were transferred to the PVDF membrane and then blocked with 5% skimmed milk. Then, they were subsequently incubated with primary and secondary antibodies. The primary antibodies used to detect the target protein were β-actin (abclonal, AC026, 1:1000), FOLR1 (Proteintech, 23355–1-AP, 1:1000), CXCR4 (Proteintech, 60042–1-Ig, 1:1000), Cleaved Caspase-3 (Cell Signaling Technology, 5A1E, 1:500). The targeted bands were analyzed by ImageJ software (v1.8.0; National Institutes of Health, USA). The β-actin was used as the internal control. The relative protein levels were quantified through comparison to β-actin.
Cell viability assays
Cell proliferation was analyzed by the cell counting kit-8 (CCK-8, MedChemExpress, Cat. No. HY-K0301). Cells were seeded at a density of 1 × 104 /well into 96-well microplates. Then, the cells were treated with various concentrations of Sorafenib (0.25, 0.5, 1, 2, 4, 8, 16, and 32 μM). The CCK-8 assay was performed after 48 h of treatment. Treated cells were incubated for 4 h with a culture medium containing the CCK-8 reagent, and absorbance was recorded at 450 nm using the iMark™ Microplate Absorbance Reader (Bio-Rad, iMark, United States). All experiments were repeated three times. The inhibition of cell proliferation was expressed by the absorbance.
Flow cytometry apoptosis assay
Cell apoptosis analysis: cells were implanted into a 6-well plate for apoptosis analysis. Then, the medium was replaced with a fresh medium supplemented with 3 μM Sorafenib. After treatment of 48 h, the cell apoptosis was detected by Apoptosis Detection Kit (eBioscience™ Annexin V Apoptosis Detection Kits, Thermo Fisher Scientific, Lot No. 2106736). All cells were detected by Polychromatic analytical flow cytometry (Beckman, Cytoflex LX, United States).
Colony formation assay
HCC cells (800 cells/well) were implanted into a 6-well plate. Then, they were treated with the supernatant of CAFs or NFs, CXCL12 protein, AMD3100 (20 μM), and sorafenib (3 μM). After treatment of 15 days, cells were fixed with 4% paraformaldehyde and stained with crystal violet (5%). Each experiment was done thrice. Cells colonies formation rate = Number of colonies formed in each treatment group / Number of implanted cells (800 cells) × 100%.
Enzyme-linked immunosorbent assay (Elisa)
The supernatants of CAFs and NFs were carried out with ELISA analyses. The OD value was detected with the enzyme plate analyzer. Meanwhile, the amount of target protein secreted per 100,000 cells was calculated. The Elisa kit was a Human stromal cell-derived factor 1β (CXCL12β/SDF1B) ELISA kit (Cusabio, B04011121).
Immunohistochemistry staining (IHC)
Human HCC tissues and the xenograft tumors of mice were sectioned into 5 μm slices. Hematoxylin and eosin staining was applied to confirm the status of cancer or cancer-free. All tissue sections were baked, dehydrated, hydrated, and antigen-retrieved. Then, they were incubated with primary and secondary antibodies. An N-ACHROPLAN microscope (ZEISS, Germany) was used to photograph the representative areas. Image-Pro Plus v6.0 software (Media Cybernetics Inc., Bethesda, MD, USA) was used to analyze the Information Object Definition (IOD) values of all images. We calculated the relative IOD values based on three parameters (sum of area, average density, and IOD), which were used for further analysis.
IHC was performed according to the kits (Boster, SA1028, SA1027). Primary antibodies were used for IHC staining: α-SMA (Abcam, ab119952, 1:100), Cleaved Caspase-3 (Cell Signaling Technology, 5A1E, 1:100).
Immunohistofluorescence staining (IF)
Human HCC tissue samples were sectioned into 5 μm slices. Hematoxylin and eosin staining was applied to confirm the status of cancer or cancer-free. IF was performed according to the kits (Boster, SA1028, SA1027). Primary antibodies were used for IHC staining: CXCL12 (Boster, BA1389, 1:100), and α-SMA (Abcam, ab119952, 1:100).
Animal xenograft models
The growth of the tumor was observed in vivo. A total of 9 BALB/c mice (4 weeks old; 9 males) were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China) and housed with a 12 h light/dark cycle and fed standard laboratory food and water. We randomly divided the 9 mice into the NC group, CAFs group, and CAFs + AMD3100 group equally, with 3 mice in each group.
1 × 106 HepG2 cells (50 μL) and 1 × 106 NFs cells (50 μL) were mixed and then injected subcutaneously into the left and right flanks of nude mice in the NC group. Meanwhile, 1 × 106 HepG2 cells (50 μL) and 1 × 106 CAFs (50 μL) were mixed and then injected subcutaneously into the left and right flanks of nude mice in the CAFs group, and CAFs + AMD3100 group. Therefore, we would get 6 tumors in each group. The tumor volume (mm3) = (length of tumor × width of tumor2)/2. When tumor volume reached 100 – 150 mm3, the mouse of the NC group and CAFs group were began to receive tail vein injections of an equal volume of normal saline + Sorafenib (30 mg/kg) for 3 weeks. Meanwhile, the mouse of the CAFs + AMD3100 group was began to receive tail vein injections of an equal volume of AMD3100 (2.5 mg/kg) + Sorafenib (30 mg/kg) for 3 weeks.
Statistical analysis
Statistical analysis was performed using GraphPad Prism V.8 software. All data were repeated at least three times. Data were presented as mean ± SEM and analyzed by Student’s t-test and Pearson correlation. One-way analysis of variance (ANOVA) and Brown-Forsythe tests were carried out for multiple group comparisons. Kaplan-Miere analysis and Log-rank test as used to analyze the survival of HCC patients. For each test, values of p < 0.05 were considered statistically significant. *p < 0.01; **p < 0.001; ***p < 0.0001; ****p < 0.00001. N.S, not significance.
Results
CAFs were increasingly activated in HCC tissues, compared with paracancerous tissues
α-SMA is a common marker of CAF activation [13, 14]. The results of IHC and IF revealed that the activation of CAFs was significantly increased in HCC cancer tissues, compared with paratumour tissues (Fig. 1a and b).
CAFs were significantly activated in cancer tissues compared with paracancerous tissues in HCC samples. a Representative images of fibroblasts with the expression of α-SMA in HCC tissues and paracancerous tissues (left). Statistical plot of fibroblasts expressing α-SMA in HCC tissues and paracancerous tissues (right). b Immunofluorescence representative images: the expression of α-SMA in fibroblasts in HCC tissues and paracancerous tissues (left). Statistical plot of fluorescence intensity of fibroblasts expressing α-SMA in HCC tissues and paracancerous tissues (right). The data presented mean ± SEM. ****p < 0.00001
CAFs induced sorafenib resistance in HCC cells
Then, we sorted CAFs from cancer tissues in HCC (Fig. 2a). Meanwhile, we sorted NFs from paracancerous tissues in HCC (Fig. 2a). To explore whether CAFs can induce sorafenib resistance in HCC cells, we cocultured CAFs, NFs and HCC cells. The results showed that CAFs significantly enhanced the resistance of HCC cells to sorafenib (Fig. 2b-d). Therefore, CAFs were significantly activated in cancer tissues of HCC, which suppressed the sensitivity of HCC cells to sorafenib.
Compared with NFs, CAFs significantly promoted sorafenib resistance in HCC cells. a CAFs were isolated from HCC tissues. Meanwhile, paracancerous normal fibroblasts (NFs) were isolated from non-tumor tissues adjacent to HCC (upper). Statistical plot of fluorescence intensity of fibroblasts expressing α-SMA in HCC tissues and paracancerous tissues (below). b Colony forming assays of HCC cells (HepG2 and Huh7), which were treated with the cellular supernatant of CAFs and NFs, and sorafenib. c, d Cell viability assay: compared with NFs, CAFs significantly enhanced sorafenib resistance in HCC cells (HepG2 and Huh7). The data presented mean ± SEM. ***p < 0.0001; ****p < 0.00001
CAFs secrete CXCL12 to induce sorafenib resistance in HCC cells
CXCL12 is a common secreted factor in CAFs [15]. Studies have shown that CXCL12 can induce drug resistance in cancer [16,17,18]. However, there is no report on whether CXCL12 can induce sorafenib resistance in HCC cells. To explore whether CAFs secrete CXCL12 to induce sorafenib resistance in HCC cells, the following experiments were performed. Immunofluorescence assays showed that, CAFs in HCC tissues expressed higher levels of CXCL12 than NFs in paracancerous tissues (Fig. 3a). The ELISA results showed that CAFs secreted higher levels of CXCL12 than NFs (Fig. 3b). Moreover, the results of cell cloning assays, and flow cytometry apoptosis analysis showed that CAFs significantly reduced the sensitivity of HCC cells to sorafenib (Fig. 3c-f). When CXCL12 was inhibited, the sensitivity of HCC cells to sorafenib was increased (Fig. 3c-h). These results suggest that CAFs induce sorafenib resistance in HCC cells through CXCL12.
CAFs induce sorafenib resistance in HCC cells by secreting CXCL12. a The results of immunofluorescence showed that the expression of CXCL12 in CAFs in HCC tissues was significantly higher than that in paracancerous tissues (left). Statistical plot of fluorescence intensity of fibroblasts expressing α-SMA and CXCL12 in HCC tissues and paracancerous tissues (right). b ELISA showed that CAFs secreted higher level of CXCL12 than NFs. c, d Colony forming assays detected the sorafenib resistance of HCC cells (HepG2 and Huh7), after treated with the cellular supernatant of CAFs and NFs, sorafenib, and AMD3100. e, f Flow cytometry apoptosis assay detected the sorafenib resistance of HCC cells (HepG2 and Huh7), after treated with the cellular supernatant of CAFs and NFs, sorafenib, and AMD3100. g, h Western blotting was performed to detect the expression of β-actin, and Cleaved Caspase-3 in HCC cells (HepG2 and Huh7), which were treated with the cellular supernatant of CAFs, sorafenib, and AMD3100. The data presented mean ± SEM. *p < 0.01; **p < 0.001; ***p < 0.0001; ****p < 0.00001
CXCL12 induces sorafenib resistance in HCC cells by upregulating FOLR1
To explore the mechanism by which CXCL12 secreted by CAFs induces sorafenib resistance in HCC cells, we assessed two datasets of cancer cells treated with CXCL12 protein (GSE15893 and GSE40017) in the GEO database. We took the intersection of the differentially expressed genes between these two datasets and combined them with the reported drug resistance genes of HCC [2, 19, 20]. The results showed that FOLR1 was the most significantly upregulated drug resistance-related gene upon CXCL12 treatment (Fig. 4a). Subsequently, we used CXCL12 protein to treat HCC cells. The qPCR results showed that CXCL12 protein significantly upregulated FOLR1 at the mRNA level in HCC cells (Fig. 4b). To further clarify the role of CXCL12 in HCC cells, the CXCR4 knockdown HCC cell lines (HepG2 and Huh7) were constructed (Fig. 4c). The results showed that CXCL12 protein upregulated FOLR1 in HCC cells, and CAFs significantly increased secretion of FOLR1 in HCC cells; both of these phenotypes were associated with sorafenib resistance in HCC cells (Fig. 4d-i). Moreover, when CXCL2 was inhibited by AMD3100, these effects disappeared (Fig. 4d-i).
CXCL12 induces sorafenib resistance in HCC cells by up-regulating the expression of FOLR1. a We found two datasets of cancer cells treated with CXCL12 protein (GSE15893 and GSE40017) in the GEO database. We took the intersection of the differentially expressed genes between these two datasets and combined them with the reported drug-resistant genes of HCC to obtain two genes. FOLR1 was the most significantly upregulated drug resistance-related gene upon CXCL12 treatment. b The qPCR was performed to detect the level of FOLR1 in Huh7 and HepG2, which treated with CXCL12 protein and AMD3100. c Western blotting was performed to detect the expression of β-actin and CXCR4 in CXCR4 knockdown HCC cells (Huh7 and HepG2). d, e Western blotting was performed to detect the expression of β-actin, CXCR4, FOLR1, and Cleaved Caspase-3 in Huh7 and HepG2, after treated with sorafenib, CXCL12 protein, and AMD3100. f, g Western blotting was performed to detect the expression of β-actin, CXCR4, FOLR1, and Cleaved Caspase-3 in Huh7 and HepG2, after treated with sorafenib, AMD3100, the supernatant of CAFs, and NFs. h, i Colony forming assay detected the sorafenib resistance of HCC cells (HepG2 and Huh7), after treated with sorafenib, CXCL12 protein, the supernatant of CAFs, and AMD3100. j, k Western blotting was performed to detect the expression of β-actin, FOLR1, CXCR4, and Cleaved Caspase-3 in Huh7 and HepG2, after treated with sorafenib, anti-CXCR4, CXCL12 protein, and the supernatant of CAFs. The data presented mean ± SEM. **p < 0.001; ***p < 0.0001; ****p < 0.00001
Studies have revealed that the upregulation of FOLR1 is associated with sorafenib resistance in cancer cells [19, 21]. Additionally, CXCR4 has been identified as the major cognate receptor for CXCL12. The CXCL12/CXCR4 biological axis plays an important role in the malignant progression of cancer [17, 22, 23]. To investigate whether CXCL12 upregulates FOLR1 to induce sorafenib resistance in HCC cells through CXCR4, we inhibited CXCR4 in HCC cells and subsequently treated them with CXCL12 protein or CAF supernatant. The results demonstrated that when CXCR4 was inhibited, the ability of CXCL12 protein or CAF supernatant to upregulate FOLR1 in HCC cells was inhibited, and the sensitivity of HCC cells to sorafenib was increased (Fig. 4j-k). These findings suggest that CAFs induce sorafenib resistance in HCC cells through the CXCL12/CXCR4/FOLR1 pathway.
CAFs enhance sorafenib resistance in HCC cells through CXCL12 in vivo
Next, to further verify the effects of CAF-induced sorafenib resistance in HCC cells in vivo, a xenograft model in nude mice was established (Fig. 5a). The results showed that CAFs significantly enhanced the resistance of HCC cells to sorafenib, while CXCL12 inhibition reversed this phenomenon (Fig. 5b-c). Furthermore, the IHC results showed that during sorafenib treatment, CAFs reduced the sensitivity of HCC cells to sorafenib, while CXCL12 inhibition promoted the apoptosis of HCC cells (Fig. 5d-e). In conclusion, CAFs could induce sorafenib resistance in HCC cells via CXCL12 in vivo.
CAFs enhance sorafenib resistance of HCC cells through CXCL12 in vivo. a Representative images of tumors in mice of CAFs + AMD3100 group, CAFs group, and NFs group after different treatments. b The tumor volume in different treatment groups. c The tumor proliferation trend in different treatment groups. d Pathological validation of tumors under a microscope (40X), after H&E staining and Immunohistochemistry in tumor tissues. The immunohistochemistry staining to detect the expression of Cleaved Caspase-3 in different treatment groups from the tumor tissues of mice. e The expression level of Cleaved Caspase-3 in different treatment groups from the tumors of mice. The data presented mean ± SEM. **p < 0.001; ***p < 0.0001; ****p < 0.00001
Discussion
Sorafenib is a first-line targeted drug for the treatment of advanced HCC, but increasing evidence has shown that the high drug resistance of HCC cells leads to poor efficacy of sorafenib [2]. Various groups have attempted to investigate the underlying mechanisms of sorafenib resistance in HCC and to identify new therapeutic targets to overcome drug resistance [24, 25]. In this study, we determined that the activation level of CAFs is significantly increased in HCC tissues, which could induce sorafenib resistance in HCC cells. Moreover, CAFs secrete C-X-C motif chemokine 12 (CXCL12) to upregulate FOLR1 expression in HCC cells via the CXCL12/CXCR4 axis. Furthermore, the overexpression of FOLR1 is positively correlated with sorafenib resistance in HCC. Finally, our results revealed that CAFs induce sorafenib resistance in HCC cells through the CXCL12/CXCR4/FOLR1 pathway.
Generally, CAFs promote the malignant progression of cancer cells by secreting cytokines, chemokines, and proangiogenic factors, including CXCL12, TGF-β1, VEGF, PDCF, IL-6 and CXCL16 [26,27,28]. Among them, SDF-1 and TGF-β1 are two of the most-powerful and widely investigated molecules in various solid tumours, including HCC, pancreatic carcinoma, and colorectal cancer [26,27,28]. Our results also suggest that compared with NFs, CAFs secrete higher levels of CXCL12, which specifically binds to CXCR4 in HCC cells.
CXCL12, also known as stromal cell-derived factor-1 (SDF-1), is an extracellular homeostatic chemokine that often binds to CXCR4, which could regulate cancer cell malignant progression [29,30,31]. Relevant studies have shown that the high expression of CXCL12 could promote drug resistance in pancreatic cancer, breast cancer, and acute lymphoblastic leukaemia [16,17,18]. However, the underlying mechanism by which CXCL12 regulates sorafenib resistance in HCC cells remains unclear. In this study, we found that CXCL12, secreted by CAFs, induced sorafenib resistance in HCC cells by upregulating FOLR1.
Folate receptor 1 (FOLR1), a protein receptor for transporting folate into cells, is expressed at a very low level in normal epithelial cells [19, 32,33,34,35], but is abnormally upregulated in HCC, ovarian cancer, and pancreatic cancer [19, 32,33,34,35]. Studies have found that FOLR1 plays a crucial role in various types of malignant cancers [19, 21, 36, 37]. Furthermore, the upregulation of FOLR1 was correlated with the drug resistance of cancer cells [19]. However, the mechanism of FOLR1 upregulation in HCC cells is still unclear, especially in the tumour environment. As reported in our study, we found that FOLR1 is upregulated by CAF-secreted CXCL12. These results suggest that CAFs secrete CXCL12 to induce sorafenib resistance in HCC cells by upregulating FOLR1. However, further research is needed to explore the molecular mechanism of the effect of FOLR1 on cancer cell behaviour.
Sorafenib resistance is a persistent clinical challenge for HCC therapy. CAFs act as a key player in HCC cell evasion of chemotherapy drugs [11, 38]. In this study, we revealed that CAFs secrete CXCL12 to induce sorafenib resistance in HCC cells. CXCL12 has been revealed to participate in the development of chemotherapy resistance in various tumours [18, 22, 39]. However, there is no study on the correlation between CXCL12 and FOLR1 in sorafenib resistant HCC. Our study showed that CAFs secreted CXCL12 to induce sorafenib resistance in HCC cells by upregulating FOLR1.
Conclusion
In summary, we demonstrated that the activation level of CAFs is increased in HCC tissues, which significantly enhances sorafenib resistance in HCC cells. CAFs secrete CXCL12 to induce sorafenib resistance in HCC cells by upregulating FOLR1. Our study provides the first evidence that the CXCL12/CXCR4/FOLR1 axis is associated with sorafenib resistance in HCC, suggesting a potential new target for improving the efficacy of sorafenib and the prognosis of HCC patients.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and are also available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- CAFs:
-
Cancer-associated fibroblasts
- α-SMA:
-
α-Smooth muscle actin
- CXCL12:
-
Stromal cell-derived factor-1 (SDF-1)
- CXCR4:
-
CXC receptor 4
- FOLR1:
-
Folate receptor 1
- JAK:
-
Janus kinase
- STAT3:
-
Signal transducer and activator of transcription 3
- ERK1/2:
-
Extracellular signal-related kinases 1 and 2
- BAFF:
-
B cell activating factor
- NF-κB:
-
Nuclear factor-κB
- IL6:
-
Interleukin-6
- IL17A:
-
Interluekin-17A
- IGF1:
-
Insulin-like growth factor 1
- ChIP-qPCR:
-
Chromatin immunoprecipitation quantitative PCR assays
- SHH:
-
Sonic Hedgehog
- FAP:
-
Fibroblast activation protein
- SPP1:
-
Plasma-secreted phosphoprotein 1
- IKKβ:
-
Inhibitor of nuclear factor kappa-B kinase subunit beta
- SULF2:
-
Sulfatase 2
- GEO:
-
Gene Expression Omnibus
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (Lond). 2023;43:455–79.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Zhu GQ, Tang Z, Huang R, Qu WF, Fang Y, Yang R, et al. CD36(+) cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 2023;9:25.
Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–18.
Ying F, Chan MSM, Lee TKW. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma. Cell Mol Gastroenterol Hepatol. 2023;15:985–99.
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70.
Liu J, Li P, Wang L, Li M, Ge Z, Noordam L, et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell Mol Gastroenterol Hepatol. 2021;11:407–31.
Song M, He J, Pan QZ, Yang J, Zhao J, Zhang YJ, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73:1717–35.
Zaki MYW, Alhasan SF, Shukla R, McCain M, Laszczewska M, Geh D, et al. Sulfatase-2 from cancer associated fibroblasts: an environmental target for hepatocellular carcinoma? Liver Cancer. 2022;11:540–57.
Geng X, Chen H, Zhao L, Hu J, Yang W, Li G, et al. Cancer-Associated Fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol. 2021;9:655152.
Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp Clin Cancer Res. 2020;39:112.
Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31.
Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018;9:1065.
Li J, Shu X, Xu J, Su SM, Chan UI, Mo L, et al. S100A9-CXCL12 activation in BRCA1-mutant breast cancer promotes an immunosuppressive microenvironment associated with resistance to immunotherapy. Nat Commun. 2022;13:1481.
Hong Z, Wei Z, Xie T, Fu L, Sun J, Zhou F, et al. Targeting chemokines for acute lymphoblastic leukemia therapy. J Hematol Oncol. 2021;14:48.
Chu H, Wu C, Zhao Q, Sun R, Yang K, Zhao B, et al. Quantitative proteomics identifies FOLR1 to drive sorafenib resistance via activating autophagy in hepatocellular carcinoma cells. Carcinogenesis. 2021;42:753–61.
Ladd AD, Duarte S, Sahin I, Zarrinpar A. Mechanisms of drug resistance in HCC. Hepatology. 2023.
Nawaz FZ, Kipreos ET. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol Metab. 2022;33:159–74.
Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 Axis in Colorectal cancer: therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22:7371.
Zheng X, Rui S, Wang XF, Zou XH, Gong YP, Li ZH. circPVT1 regulates medullary thyroid cancer growth and metastasis by targeting miR-455-5p to activate CXCL12/CXCR4 signaling. J Exp Clin Cancer Res. 2021;40:157.
Wei L, Lee D, Law CT, Zhang MS, Shen J, Chin DW, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun. 2019;10:4681.
Sueangoen N, Tantiwetrueangdet A, Panvichian R. HCC-derived EGFR mutants are functioning, EGF-dependent, and erlotinib-resistant. Cell Biosci. 2020;10:41.
Lotti F, Jarrar AM, Pai RK, Hitomi M, Lathia J, Mace A, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851–72.
McAndrews KM, Chen Y, Darpolor JK, Zheng X, Yang S, Carstens JL, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-Mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:1580–97.
Yi Y, Zeng S, Wang Z, Wu M, Ma Y, Ye X, et al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:793–803.
Yang J, Zhang L, Jiang Z, Ge C, Zhao F, Jiang J, et al. TCF12 promotes the tumorigenesis and metastasis of hepatocellular carcinoma via upregulation of CXCR4 expression. Theranostics. 2019;9:5810–27.
Vila-Coro AJ, Rodríguez-Frade JM, Martín De Ana A, Moreno-Ortíz MC, Martínez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb j. 1999;13:1699–710.
Gao D, Tang T, Zhu J, Tang Y, Sun H, Li S. CXCL12 has therapeutic value in facial nerve injury and promotes Schwann cells autophagy and migration via PI3K-AKT-mTOR signal pathway. Int J Biol Macromol. 2019;124:460–8.
Köbel M, Madore J, Ramus SJ, Clarke BA, Pharoah PD, Deen S, et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An ovarian tumour tissue analysis consortium study. Br J Cancer. 2014;111:2297–307.
Liang Z, Dong J, Yang N, Li SD, Yang ZY, Huang R, et al. Tandem CAR-T cells targeting FOLR1 and MSLN enhance the antitumor effects in ovarian cancer. Int J Biol Sci. 2021;17:4365–76.
Cheung A, Opzoomer J, Ilieva KM, Gazinska P, Hoffmann RM, Mirza H, et al. Anti-folate receptor alpha-directed antibody therapies restrict the growth of triple-negative breast cancer. Clin Cancer Res. 2018;24:5098–111.
Jia L, Li J, Li P, Liu D, Li J, Shen J, et al. Site-specific glycoproteomic analysis revealing increased core-fucosylation on FOLR1 enhances folate uptake capacity of HCC cells to promote EMT. Theranostics. 2021;11:6905–21.
Li W, Tan G, Ma Y, Li H, He G. Inhibition of α folate receptor resulting in a reversal of taxol resistance in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2012;146:250–8.
Sakai H, Kawakami H, Teramura T, Onodera Y, Somers E, Furuuchi K, et al. Folate receptor α increases chemotherapy resistance through stabilizing MDM2 in cooperation with PHB2 that is overcome by MORAb-202 in gastric cancer. Clin Transl Med. 2021;11:e454.
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–97.
Lin YN, Schmidt MO, Sharif GM, Vietsch EE, Kiliti AJ, Barefoot ME, et al. Impaired CXCL12 signaling contributes to resistance of pancreatic cancer subpopulations to T cell-mediated cytotoxicity. Oncoimmunology. 2022;11:2027136.
Acknowledgements
We thank the Department of Hepatobiliary Surgery, Fifth Affiliated Hospital of Sun Yat-sen University for providing clinical samples.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81971773, 82272105), Guangdong Basic and Applied Basic Research Foundation (grant number 2018A0303130292, 2022A1515011244, 2023A1515011521, 2023A1515010475), Medical Scientific Research Foundation of Guangdong Province of China (grant number A2019099, A2017421).
Author information
Authors and Affiliations
Contributions
Jiali Zhao designed the research, did most of the experiments, analyzed the data, interpreted the results, and wrote the original draft. En Lin, Zirui Bai, Yingbin Jia, and Bo Wang analyzed the data and interpreted the results. Yihua Dai, Wenfeng Zhuo, and Guifang Zeng took part in the sample collection. Xialei Liu provided funding support and took part in sample collection. Jian Li, Baojia Zou, Peiping Li, and Chaonong Cai provided funding support and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (NO. 00337). Human HCC tissues were obtained from patients undergoing hepatectomy at the Department of Hepatobiliary Surgery, the Fifth Affiliated Hospital of Sun Yat-sen University. All HCC patients agreed to provide HCC tissue specimens for this study. All subjects signed an informed consent form. Meanwhile, animal experiment in this study was approved by the ethics committee of the Fifth Affiliated Hospital of Sun Yat-sen University (NO. 00337). The study was carried out in accordance with the ARRIVE guidelines. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, J., Lin, E., Bai, Z. et al. Cancer-associated fibroblasts induce sorafenib resistance of hepatocellular carcinoma cells through CXCL12/FOLR1. BMC Cancer 23, 1198 (2023). https://doi.org/10.1186/s12885-023-11613-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11613-8