Abstract
Background
Ultra-hypofractionated image-guided stereotactic body radiotherapy (SBRT) is increasingly used for definitive treatment of localized prostate cancer. Magnetic resonance imaging-guided radiotherapy (MRgRT) facilitates improved visualization, real-time tracking of targets and/or organs-at-risk (OAR), and capacity for adaptive planning which may translate to improved targeting and reduced toxicity to surrounding tissues. Given promising results from NRG-GU003 comparing conventional and moderate hypofractionation in the post-operative setting, there is growing interest in exploring ultra-hypofractionated post-operative regimens. It remains unclear whether this can be done safely and whether MRgRT may help mitigate potential toxicity. SHORTER (NCT04422132) is a phase II randomized trial prospectively evaluating whether salvage MRgRT delivered in 5 fractions versus 20 fractions is non-inferior with respect to gastrointestinal (GI) and genitourinary (GU) toxicities at 2-years post-treatment.
Methods
A total of 136 patients will be randomized in a 1:1 ratio to salvage MRgRT in 5 fractions or 20 fractions using permuted block randomization. Patients will be stratified according to baseline Expanded Prostate Cancer Index Composite (EPIC) bowel and urinary domain scores as well as nodal treatment and androgen deprivation therapy (ADT). Patients undergoing 5 fractions will receive a total of 32.5 Gy over 2 weeks and patients undergoing 20 fractions will receive a total of 55 Gy over 4 weeks, with or without nodal coverage (25.5 Gy over 2 weeks and 42 Gy over 4 weeks) and ADT as per the investigator’s discretion. The co-primary endpoints are change scores in the bowel and the urinary domains of the EPIC. The change scores will reflect the 2-year score minus the pre-treatment (baseline) score. The secondary endpoints include safety endpoints, including change in GI and GU symptoms at 3, 6, 12 and 60 months from completion of treatment, and efficacy endpoints, including time to progression, prostate cancer specific survival and overall survival.
Discussion
The SHORTER trial is the first randomized phase II trial comparing toxicity of ultra-hypofractionated and hypofractionated MRgRT in the salvage setting. The primary hypothesis is that salvage MRgRT delivered in 5 fractions will not significantly increase GI and GU toxicities when compared to salvage MRgRT delivered in 20 fractions.
Trial registration
ClinicalTrials.gov Identifier: NCT04422132. Date of registration: June 9, 2020.
Similar content being viewed by others
Background
Prostate cancer is the most common non-cutaneous cancer and the second leading cause of cancer death in men [1]. It is predicted that the number of prostate cancer cases will almost double by the year 2030 [2]. For men with localized prostate cancer, radical prostatectomy is a common definitive treatment modality [3]. After radical prostatectomy, approximately one-third to one-half of men with high-risk features will develop biochemical recurrence (BCR) [4,5,6,7].
Post-operative radiotherapy is the current standard of care for men who develop BCR or are at risk of BCR after radical prostatectomy. Post-operative radiotherapy is a potentially curative treatment after prostatectomy for men with BCR and may avoid or delay the need for chronic, non-curative treatment, such as long-term androgen suppression. Given the increase in both prostate cancer diagnosis and the proportion of men with high-risk disease undergoing radical prostatectomy, the number of men requiring post-operative radiotherapy is likely to increase [8]. As such, optimizing radiotherapy approaches for post-operative management is an important and unanswered question. Minimizing genitourinary (GU) and gastrointestinal (GI) toxicity and maximizing quality of life are critical for men with a high chance of cure and long life-expectancy following salvage treatment [9].
Adjuvant radiotherapy, the administration of immediate post-operative radiotherapy based on adverse pathological features, has been demonstrated to improve biochemical progression free survival (PFS) across multiple prospective randomized trials [10,11,12]. More recently, early salvage post-operative radiotherapy has become an accepted paradigm. In this approach, men delay post-operative intervention until the time of BCR or if there is a persistently elevated serum prostate-specific antigen (PSA) level after prostatectomy. In support of this paradigm shift, three recent randomized controlled trials and the harmonized ARTISTIC meta-analysis have demonstrated that adjuvant radiotherapy, as compared with salvage radiotherapy, does not improve event-free survival [4, 5, 7, 13]. Additionally, the salvage approach may also avoid overtreatment in the subset of men that despite high-risk features do not develop BCR. The aforementioned studies have helped clarify the timing of post-operative intervention, however, integration of genomic classifiers, optimal target volumes (including pelvic nodal coverage), and the use and duration of androgen suppression therapy remain controversial. Additional studies are needed to further refine patient selection and improve post-operative management.
Increasingly, there has been a trend toward delivering higher doses of radiation over fewer treatment sessions, termed hypofractionation, as opposed to longer courses of treatment with conventional fractionation (1.8–2 Gy/fraction) [14,15,16]. Further, as prostate cancer is believed to have a low α/β ratio relative to other tumor types, the therapeutic ratio may favor hypofractionation over conventional regimens [17,18,19,20]. The preliminary report of the phase III randomized NRG-GU003 study demonstrated that moderately hypofractionated post-operative radiotherapy (62.5 Gy in 25 fractions) does not increase patient-reported GU or GI toxicity over conventional post-operative radiotherapy (66.6 Gy in 37 fractions). At a median of 24 months, change in mean GU and GI scores in the moderate hypofractionation arm and conventional fractionation arm were neither clinically nor statistically significant (mean GU = -5.2 vs mean GU = -0.3, p= 0.81; mean GI = -2.2 vs mean GI = -1.5, p = 0.12). Therefore, moderate hypofractionation is non-inferior to conventional fractionation for post-operative RT in measures of late toxicity [21]. This finding is significant, as hypofractionation provides patients with shorter and more accessible courses of radiotherapy, thereby reducing treatment burden.
The advent of magnetic resonance imaging (MRI)-guided radiotherapy (MRgRT) – enabled through the integration of MRI and linear accelerator (MR-LINAC) technology may facilitate safe delivery of hypofractionated and ultra-hypofractionated regimens. Multi-parametric MR imaging on the MR-LINAC improves target delineation and this feature combined with real-time MR image-guidance and tracking, allows for reduction in planning margins and accounts for inter-fractional changes in anatomy during treatment thereby decreasing dose to adjacent structures, including the bladder and rectum [22]. In addition, the MR-LINAC technology can facilitate online adaptive planning as needed. Taken together, MRgRT may safely allow larger radiation doses to be delivered in fewer fractions in the intact and post-operative setting. The MIRAGE phase III study comparing MRI-guidance versus standard computed tomography (CT)-guidance with stereotactic body radiotherapy (SBRT) for intact prostate cancer demonstrated that acute grade ≥ 2 GU toxicity was significantly reduced among men receiving MRgRT (43.4.1% vs. 24.4%, p = 0.01). Acute grade ≥ 2 GI toxicity was also significantly reduced in men receiving MRgRT (10.5% vs. 0%, p = 0.003) [23]. Similarly, the SCIMITAR phase II study evaluated the safety profile of post-prostatectomy SBRT and incorporated a pre-planned, non-randomized exploratory analysis of toxicity and patient-reported quality-of-life (QoL) outcomes between MRI-guidance versus CT-guidance. Compared with CT-guided post-operative SBRT, MRgRT was associated with significantly lower rates of any-grade acute GI toxicity (41.9% vs. 72.5%, p = 0.0056) per CTCAE version 4.0 criteria, corresponding to an estimated absolute reduction of 30.5%. In terms of late effects, MRgRT was associated with numerically lower any-grade late GI toxicity, however, these differences were not significantly different (37.7% vs. 29%, p = 0.4) when compared with CT-guidance. There were no differences in acute or late GU toxicity in the comparison of patients treated with CT versus MRI-guided SBRT. Overall, three patients experienced grade 3 toxicity (GU, n = 1; GI n = 2), notably no patients treated with MRgRT experienced any grade 3 toxicity or grade ≥ 2 GI toxicity. While additional randomized studies are needed, these findings demonstrate the potential of MRgRT to improve the precision and safety of post-operative radiation delivery [24].
The SHORTER trial (NCT04422132) is designed to evaluate if MR-guided ultra-hypofractionated post-operative radiotherapy (5 fractions in two weeks) has a non-inferior GU and GI toxicity profile as compared with MR-guided moderately-hypofractionated post-operative radiotherapy (20 fractions in four weeks) among prostate cancer patients undergoing salvage radiotherapy. This randomized phase II study lays the foundation to potentially redefine the standard of care for post-prostatectomy patients with BCR to include MRgRT and decrease treatment burden.
Methods/design
Objectives
The primary objective is to demonstrate that 5 fractions of ultra-hypofractionated MRgRT does not significantly increase patient-reported GI and GU symptoms as compared with 20 days of hypofractionated MRgRT at 2 years after treatment completion.
Primary endpoint
The co-primary endpoints are change scores in the bowel (GI) or urinary (GU) domains of the Expanded Prostate Cancer Index Composite (EPIC). The change scores will reflect the 2-year score minus the pre-treatment (baseline) score.
Secondary endpoints
-
1. Compare patient-reported GI symptoms using the EPIC questionnaire at the end of RT and 3, 6, 12, and 60 months from end of treatment.
-
2. Compare patient-reported GU symptoms using the EPIC questionnaire at the end of RT and 3, 6, 12, and 60 months from end of treatment.
-
3. Compare time to progression (TTP) where progression is defined as the first occurrence of biochemical failure (BF), local failure, regional failure, distant metastasis (DM), institution of new unplanned anticancer treatment, or death from prostate cancer (PCSM).
-
4. Compare freedom from biochemical failure (FFBF) and TTP rates with an alternate PSA ≥ PSA nadir + 2 ng/mL definition of BF.
-
5. Compare local failure, regional failure, salvage therapy (i.e., institution of new unplanned anticancer treatment), DM, PCSM, and overall survival (OS) rates.
Inclusion criteria
-
Men aged ≥ 18 with histologically confirmed prostate cancer after prostatectomy with detectable PSA. Patients with detectable post-prostatectomy PSA whether (1) persistently detectable post-operatively or (2) developing biochemical recurrence after prostatectomy (initially undetectable) are eligible. Patients with early BCR or persistently detectable PSA after prostatectomy must wait a minimum of 6 months post-prostatectomy but can initiate ADT as indicated. PSA does not need to be detectable for men with pathologically node positive disease.
-
KPS ≥ 70
-
Patient with no evidence of distant metastatic disease on positron emission tomography (PET)/CT/MRI or bone scan < 90–180 days prior to enrollment. Patients with positive pelvic lymph nodes are eligible.
-
Ability to receive MRI-guided radiotherapy
-
Equivocal evidence of metastatic disease outside the pelvis on standard imaging requires documented negative biopsy
-
Ability to complete the EPIC questionnaire
Exclusion criteria
-
Prior history of receiving pelvic radiotherapy
-
Patients with inflammatory bowel disease
-
Patients with a prior or concurrent malignancy whose natural history or treatment has the potential to interfere with the safety or efficacy assessment of ultra-hypofractionated radiotherapy
-
History of bladder neck or urethral disease
Evaluation of randomization and blinding
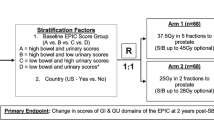
This study will employ a randomized phase II non-inferiority design to compare 5 fractions of ultra-fractionated MRgRT versus 20 fractions of moderately hypofractionated MRgRT in the salvage setting for prostate cancer patients after prostatectomy. Patients will be stratified according to baseline EPIC bowel and urinary domain scores (i.e., high bowel score and high urinary score vs. high bowel score and low urinary score vs low bowel score and high urinary score vs. low bowel score and low urinary score) as well as pelvic radiotherapy and androgen therapy (i.e., yes vs. no), for these factors are expected to be associated with the primary outcome. Within each strata, patients will be randomized 1:1 to receive 5 fractions or 20 fractions using permuted block randomization. The trial schema is displayed in Fig. 1.
Trial Schema. Expanded Prostate Cancer Index Composite (EPIC), EuroQol-5D index (EQ-5D) and International Prostate Symptom Score (IPSS) quality of life (QoL) surveys are collected at baseline, end of salvage radiotherapy, and at 3 month, 6 month, 12 month and 60 month follow up. GI, gastrointestinal; GU, genitourinary; RT, salvage radiotherapy. *EPIC score groups defined as: high bowel score > 96, low bowel score ≤ 96, high urinary score > 84, low urinary score ≤ 84. **Patients with PSA > 0.4 ng/mL, high-risk Decipher genomic classifier scores, or pathologically node positive disease will receive pelvic nodal radiotherapy and androgen deprivation therapy (ADT) as per the clinician’s discretion
Interventions
Radiation treatment planning
After consent and eligibility verification, patients will undergo CT/MRI simulation for radiotherapy planning. Patients will receive delivery of either 32.5 Gy in 5 fractions over 2 weeks or 55 Gy in 20 fractions over 4 weeks to the prostate fossa ± pelvic lymph nodes. Indications for nodal coverage include PSA > 0.4 ng/ml, high Decipher score, or pathologically positive lymph nodes as per the clinician’s discretion [25,26,27]. For patients requiring nodal coverage, those on the 5 fraction arm will receive 25.5 Gy to the pelvic lymph nodes and those on the 20 fraction arm will receive of 42 Gy to the pelvic lymph nodes. Patients randomized to the 5 fraction arm cannot be treated on consecutive days. Patients undergo salvage radiotherapy no earlier than 6 months post-prostatectomy. The radiotherapy prescription doses for the 20 fraction and 5 fraction schedules are provided in Table 1.
Contour
All contouring will be done as per RTOG consensus recommendations for the prostate bed and normal pelvic structures [28]. The planning target volume (PTV) expansion for the CTV will be 2-3 mm depending on physician discretion. The bladder should not be included in the CTV (bladder overlap may be removed from the CTV via Boolean function).
Treatment dose planning parameters for 32.5 Gy in 5 fractions
The planning target volume (PTV) will receive the prescribed dose of 32.5 Gy in 5 fractions. The volume of PTV receiving the prescription dose (VPrescription Dose) of 32.5 Gy should be ≥ 95% and not exceed 110% (hotspot) (see treatment dose planning parameters listed in Additional File 1).
Treatment dose planning parameters for 55 Gy in 20 fractions
The planning target volume (PTV) will receive the prescribed dose of 55 Gy in 20 fractions. The volume of PTV receiving the prescription dose (VPrescription Dose) of 55 Gy should be ≥ 95% and not exceed 115% (hotspot) (see treatment dose planning parameters listed in Additional File 1).
Adaptive planning for 5 fraction arm
Adaptive planning will be permitted for the 5 fraction arm.
-
1.
Prior to treatment, each patient will undergo an MRI scan.
-
2.
2D shifts will be performed to align relevant anatomy (bladder wall, rectum, prostatic fossa).
-
3.
Simulation contours will be rigidly copied to the patient's MRI scan. If delineation changes, the scan will be recontoured.
-
4.
Predicted dose algorithm will determine if treatment dose parameters meet planning dose parameters.
-
5.
Patients will undergo adaptive planning if the treatment dose parameters do not meet the planning parameters and per the protocol planning parameters as outlined (see Additional File 1).
General concomitant medication and supportive care guidelines
Androgen deprivation therapy (ADT) and pelvic nodal irradiation will be administered for patients with PSA > 0.4 ng/ml, high Decipher score (> 0.6), or pathologically positive lymph nodes [25,26,27]. ADT should consist of a luteinizing hormone-releasing hormone (LHRH) agonist or antagonist and be initiated prior to and within 6 months of starting radiotherapy and not exceed 24 months in duration.
Trial Procedures
The following is an outline of the procedures to be performed at each patient visit (see Table 2):
-
1.
Screenings
-
Informed consent
-
Demographics/medical history
-
Physical exam
-
Vital signs, height, weight
-
Post-prostatectomy PSA
-
Pelvic MRI
-
Bone or PET scan
-
Prostate 22-gene test
-
Urodynamic testing (optional)
-
EPIC
-
International Prostate Symptom Score (IPSS)
-
European Quality of Life 5 Dimension (EQ-5D)
-
Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0)
-
-
2.
First Day of RT
-
Whole blood, serum, plasma
-
EPIC
-
IPSS
-
EQ-5D
-
CTCAE v5.0
-
-
3.
Last Day of RT
-
Whole blood, serum, plasma
-
EPIC
-
IPSS
-
EQ-5D
-
CTCAE v5.0
-
-
4.
Follow-up at 3 months
-
Physical exam
-
Post-prostatectomy PSA
-
EPIC
-
IPSS
-
EQ-5D
-
CTCAE v5.0
-
-
5.
Follow-up at Q6 months for 2 years
-
Physical exam
-
Vital signs, height, weight
-
Post-prostatectomy PSA
-
Urodynamic testing (optional)
-
EPIC
-
IPSS
-
EQ-5D
-
CTCAE v5.0
-
-
6.
Follow up at Q12 months for 3 years
-
Physical exam
-
Vital signs, height, weight
-
Post-prostatectomy PSA
-
Urodynamic testing (optional)
-
EPIC
-
IPSS
-
EQ-5D
-
CTCAE v5.0
-
Definition of disease assessments
-
Biochemical failure: two definitions of biochemical failure will be assessed:
-
◦ Primary: PSA ≥ 0.4 ng/mL and rising or initiation of salvage hormones
-
◦ Alternate: PSA ≥ PSA nadir + 2 ng/mL where nadir is the lowest post-RT PSA level
-
-
Local failure: development of a new biopsy-proven mass in the prostate bed, after enrollment in the protocol
-
Regional failure: radiographic evidence (CT or MRI) of lymphadenopathy within the pelvis (lymph node size ≥ 1.5 cm in the short axis) in a patient without the diagnosis of a hematologic/lymphomatous disorder associated with adenopathy
-
Distant metastases: radiographic evidence of hematogenous spread (e.g., bone scan, CT, MRI)
-
Progression: first occurrence of biochemical failure, local failure, regional failure, distant metastasis, initiation of salvage ADT
Duration of follow Up
Patients will be followed for 5 years after removal from study or until death, whichever occurs first. Patients removed from study for unacceptable adverse events will be followed until resolution or stabilization of the adverse event.
Statistical analysis
Sample size and accrual
The primary objective of this study is to determine if 5 fractions of MRgRT does not increase GI or GU toxicity over 20 fractions of MRgRT. The primary endpoints are change scores in the bowel (GI) or urinary (GU) domains of the EPIC. The change scores will reflect the 2-year score minus the pre-treatment (baseline) score. It is hypothesized that the EPIC mean change score will be no worse in the 5-fraction arm than it is in the 20-fraction arm for both GI and GU toxicity. The sample size is calculated based on a non-inferiority design. The non-inferiority margins are set to be a change score of 6 points for the GI symptoms and 5 points for the GU symptoms. The standard deviation of the change scores are assumed to be 13.2 for the GI symptoms and 10.5 for the GU symptoms based on estimates in the RTOG 0415 trial [29]. This level of change in scores seems to be clinically meaningful. A sample size of 122 with 61 in each arm will provide 80% power for the GI endpoint and 83% power for the GU endpoint to detect non-inferiority using a one-sided, two-sample t-test with 0.05 level significance. Adjusting for a projected 10% EPIC/non-compliance rate, we will accrue and randomize a total of 136 patients (68 per arm). The primary endpoint analysis will occur approximately 4 years after study activation.
Data analysis
Analysis of primary endpoints
The co-primary endpoints are GI and GU toxicity as measured by the bowel and urinary EPIC domains, respectively. The change scores, calculated as baseline score subtracted from 2-year score, will be analyzed using a non-inferiority t-test based on the prespecified non-inferiority margins with a significance level of 0.05. If the data are determined to be non-normal, a Wilcoxon test may be used instead. All patients with EPIC bowel and urinary domain scores will be included in the primary endpoint analysis. The EPIC scoring manual will be followed which requires ≥ 80% of items in a domain to be completed in order to obtain a score for that domain.
Analysis of secondary endpoints:
-
Secondary safety endpoints: Between treatment arms differences in each safety endpoints measured as change in domain specific EPIC scores at a specific time points (i.e., end of RT, 3, 6, 12, 24, and 60 months) from base line will be evaluated using t-test or Wilcoxon rank sum test whichever is more appropriate. The domain specific EPIC scores measured over time will also be modeled using a linear mixed effects model with fixed effects including time points, treatment arm, Gleason score, baseline PSA, T-stage, age, race, and a random intercept. Between treatment arms differences in GI and GU EPIC scores at 2 years adjusting for baseline scores and other covariates will be assessed using analysis of covariance (ANCOVA). Results from the primary unadjusted analysis and covariates adjusted analysis are expected to lead to similar conclusions. Otherwise, further investigation concerning possible heterogeneous subgroup effects and/or the impacts of missing values will be carried out to ensure that meaningful conclusions can be made.
-
Secondary efficacy endpoints: For competing-risk endpoints such as PCSM, local failure (LF), regional failure (RF), TTP, and DM, Gray’s cumulative incidence method will be used with death as a competing risk for LF, RF and DM and death not due to prostate cancer for PCSM and TTP. OS and FFBF will be estimated by the Kaplan–Meier method and compared between arms with the log-rank test. Cox regression will be used to obtain hazard ratios (HRs) for OS and TTP. Fine and Gray’s regression will be used for the endpoints with competing risks. Adjusted HRs and the respective 95% confidence interval will be computed. Baseline PSA, stratification variables (baseline EPIC score and ADT status), age, race, and other covariates (Gleason, T-stage), will be adjusted for as appropriate in this analysis.
Early stopping guidelines
An interim futility analysis will be conducted when one-third of patients have had their 6 months follow-ups. If the upper 95% confidence limit of the mean difference in 6-months change scores between the treatment arms is less than the pre-specified non-inferiority margins (i.e., <-6 for GU and <-5 for GI), then the 5-fraction arm will be deemed inferior to the 20-fraction arm and the study will be halted.
Discussion
With growing acceptance of hypofractionation and the increase of ultra-hypofractionation for definitive radiation treatment of intact prostate cancer, hypofractionated regimens for post-operative management are poised to become a convenient option for patients. Abbreviated radiation schedules may reduce the burden of treatment on patients and treatment centers alike while maintaining clinical efficacy and safety. A primary consideration for patients in need of post-operative radiotherapy is toxicity, manifesting as both acute and late side effects of the bladder and bowel. Such side effects impact quality of life following treatment, highlighting the importance of treatment approaches that minimize toxicity. MRgRT may facilitate higher radiation doses to be delivered in fewer sessions with increased accuracy and precision. The potential advantages of MRgRT include better visualization of the prostatic fossa, the capacity for real-time tracking, and the ability to perform online adaptive radiotherapy that accounts for organ deformation. These factors may decrease the radiation dose received by adjacent structures, thereby minimizing toxicity [23, 24]. The SHORTER trial is the first randomized trial to evaluate whether the theoretical advantages of hypofractionation combined with MRI-guidance translate into the salvage radiotherapy setting from a toxicity standpoint. The SHORTER trial will provide information not only on toxicities, but also well-documented information on time to progression, prostate cancer-specific survival and overall survival. We hope that the data from this trial will elucidate the non-inferiority of ultra-hypofractionated, MRI-guided post-operative radiotherapy.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the ongoing nature of the trial and possible compromise of individual privacy but are available from the corresponding author upon reasonable request.
Abbreviations
- SBRT:
-
Stereotactic Body Radiotherapy
- MRI:
-
Magnetic Resonance Imaging
- OAR:
-
Organs at Risk
- MRgRT:
-
MRI-guided Radiotherapy
- GU:
-
Genitourinary
- GI:
-
Gastrointestinal
- BCR:
-
Biochemical recurrence
- PSA:
-
Prostate Specific Antigen
- MR-LINAC:
-
Magnetic Resonance Linear Accelerator
- CT:
-
Computed Tomography
- EPIC:
-
Expanded Prostate Cancer Index Composite
- TTP:
-
Time to progression
- DM:
-
Distant metastasis
- PCSM:
-
Prostate cancer-specific morbidity
- FFBP:
-
Freedom from biochemical progression
- OS:
-
Overall survival
- PET:
-
Positron Emission Tomography
- PTV:
-
Planning Target Volume
- LHRH:
-
Luteinizing hormone-releasing hormone
- IPSS:
-
International Prostate Symptom Score
- EQ-5D:
-
European Quality of Life 5 Dimension
- CTCAE v5.0:
-
Common Terminology Criteria for Adverse Events version 5.0
- ADT:
-
Androgen Deprivation Therapy
- LF:
-
Local failure
- RF:
-
Regional failure
- HR:
-
Hazard ratio
References
Haas GP, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15(1):3866–71.
Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Hamilton AS, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107(4):576–84.
Kneebone A, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21(10):1331–40.
Parker CC, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet. 2020;396(10260):1413–21.
Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7.
Sargos P, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1341–52.
Agrawal V, et al. Trends in Diagnosis and Disparities in Initial Management of High-Risk Prostate Cancer in the US. JAMA Netw Open. 2020;3(8): e2014674.
Donovan JL, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016;375(15):1425–37.
Bolla M, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–27.
Thompson IM Jr, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–35.
Wiegel T, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96–02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30.
Vale CL, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396(10260):1422–31.
Mahase S, Nagar H. Hypofractionated postoperative radiotherapy for prostate cancer: is the field ready yet? Eur Urol Open Sci. 2020;22:9–16.
Mahase SS, et al. Trends in the use of stereotactic body radiotherapy for treatment of prostate cancer in the United States. JAMA Netw Open. 2020;3(2):e1920471.
Nagar H, Spratt DE. Challenging the Norm: What Level of Evidence Is Necessary to Adopt Postprostatectomy Hypofractionated Radiation Therapy? Int J Radiat Oncol Biol Phys. 2020;107(2):297–8.
Vogelius IR, Bentzen SM. Dose response and fractionation sensitivity of prostate cancer after external beam radiation therapy: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2018;100(4):858–65.
Widmark A, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–95.
Brand DH, et al. Estimates of Alpha/Beta (alpha/beta) Ratios for Individual Late Rectal Toxicity Endpoints: An Analysis of the CHHiP Trial. Int J Radiat Oncol Biol Phys. 2021;110(2):596–608.
Brand DH, et al. Genitourinary alpha/beta ratios in the CHHiP trial the fraction size sensitivity of late genitourinary toxicity: analysis of Alpha/Beta (alpha/beta) ratios in the CHHiP Trial. Int J Radiat Oncol Biol Phys. 2022. PMID: 35985457.
Buyyounouski MK, et al. Primary Endpoint Analysis of a Randomized Phase III Trial of Hypofractionated vs. Conventional Post-Prostatectomy Radiotherapy: NRG Oncology GU003. Int J Radiati Oncol *Biol*Phys. 2021;111(3, Supplement):S2-3.
Yuan J, et al. A narrative review of MRI acquisition for MR-guided-radiotherapy in prostate cancer. Quant Imaging Med Surg. 2022;12(2):1585–607.
Kishan AU, et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023;9(3):365–73.
Ma TM, et al. Quality-of-life outcomes and toxicity profile among patients with localized prostate cancer after radical prostatectomy treated with stereotactic body radiation: the SCIMITAR multi-center phase 2 trial. Int J Radiat Oncol Biol Phys. 2022. PMID: 36007724.
Pollack A, et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022;399(10338):1886–901.
Pra AD, et al. Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy - an ancillary study of the SAKK 09/10 randomized clinical trial. Ann Oncol. 2022. PMID: 3563662.
Feng FY, et al. Validation of a 22-Gene Genomic Classifier in Patients With Recurrent Prostate Cancer: An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial. JAMA Oncol. 2021;7(4):544–52.
Michalski JM, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–8.
Lee WR, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34(20):2325–32.
Acknowledgements
We would like to acknowledge the patients who will enroll on this study to further the cause of medical science.
Funding
Fund #: 56581093.
Sponsor: ViewRay Inc.
Author information
Authors and Affiliations
Contributions
Study conception: HN, STT, DMN, JCH, CEB, DSS; Initial study design: HN, XKZ, CEB, JCH, AMM, DMN, DSS, CNS, STT; Revision of study design and protocol: HN, AEM, XKZ, SCF, DMN, STT, AMM, JTN, CNS; Study coordination and acquisition of data: HN, AEM, XKZ; Drafting the manuscript: AEM, SW, XKZ, HN, JTN, DMN, CNS; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by the Institutional Review Board (IRB) of Weill Cornell Medicine (IRB #: 20–03021572 – SHORTER Trial). The IRB-approved protocol and study data are reviewed by the Weill Cornell Medicine Data and Safety Monitoring Board (DSMB). All methods in this study are carried out in accordance with relevant guidelines and regulations, including the Weill Cornell Medicine Data and Safety Monitoring Plan (DSMP) and the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians. Patients’ eligibility on the trial were reviewed and signed off by the treating physicians. Consented and eligibility verified patients were enrolled on the Weill Cornell Medicine Clinical Trial Management System (CTMS).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marciscano, A.E., Wolfe, S., Zhou, X.K. et al. Randomized phase II trial of MRI-guided salvage radiotherapy for prostate cancer in 4 weeks versus 2 weeks (SHORTER). BMC Cancer 23, 781 (2023). https://doi.org/10.1186/s12885-023-11278-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11278-3