Abstract
Background
Systemic inflammation is crucial for the development and progression of cancers. The advanced lung cancer inflammation index (ALI) is considered to be a better indicator of systemic inflammation than current biomarkers. However, the prognostic value of the ALI in gastrointestinal neoplasms remains unclear. We performed the first meta-analysis to explore the association between ALI and gastrointestinal oncologic outcomes to help physicians better evaluate the prognosis of those patients.
Methods
Eligible articles were retrieved using PubMed, the Cochrane Library, EMBASE, and Google Scholar by December 29, 2022. Clinical outcomes were overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and cancer-specific survival (CSS).
Results
A total of 18 articles with 6898 patients were included in this meta-analysis. The pooled results demonstrated that a low ALI was correlated with poor OS (HR = 1.914, 95% CI: 1.514–2.419, P < 0.001), DFS (HR = 1.631, 95% CI: 1.197–2.224, P = 0.002), and PFS (HR = 1.679, 95% CI: 1.073–2.628, P = 0.023) of patients with gastrointestinal cancers. Subgroup analysis revealed that a low ALI was associated with shorter OS (HR = 2.279, 95% CI: 1.769–2.935, P < 0.001) and DFS (HR = 1.631, 95% CI: 1.197–2.224, P = 0.002), and PFS (HR = 1.911, 95% CI: 1.517–2.408, P = 0.002) of patients with colorectal cancer. However, the ALI was not related to CSS in the patients with gastrointestinal malignancy (HR = 1.121, 95% CI: 0.694–1.812, P = 0.640). Sensitivity analysis supported the stability and dependability of the above results.
Conclusion
The pre-treatment ALI was a useful predictor of prognosis in patients with gastrointestinal cancers.
Similar content being viewed by others
Introduction
Gastrointestinal cancers (GIC) account for over one-quarter of all cancer cases and one-third of cancer-associated deaths worldwide [1]. Although there has been great advancement in the treatment of GIC, the outcome for the majority of GIC patients remains poor [2]. Thus, exploring a reliable prognostic index for patient survival can enable physicians to adopt better therapeutic and preventative measures.
Numerous studies in recent years have confirmed that systemic inflammation is crucial for the development and growth of GIC [3, 4]. A variety of inflammatory cells and proinflammatory cytokines are activated in the early stages of carcinogenesis, which promote the creation of lymphatic ducts and new blood vessels, causing a pro-cancer microenvironment for growth and differentiation [5]. At later stages, cancer-related inflammation can impair immune cell function, creating a conducive environment for metastasis [6]. Thus, inflammatory indicators are anticipated to be important prognostic biomarkers in cancer. For instance, an elevated neutrophil-to-lymphocyte ratio (NLR) is linked to a weak immunological response and a high inflammatory response [7,8,9]. In cancer patients, the nutritional status of the body is also closely associated with tumor development and clinical outcome. Some common nutritional indicators have been shown to have a high prognostic significance in cancer, such as body mass index (BMI) [10] and serum albumin level [11].
Recently, the advanced lung cancer inflammation index (ALI), a new inflammatory marker that is calculated as BMI (kg/m2) × albumin (g/dL)/NLR, was initially found to be a useful prognostic index in lung cancer [12]. ALI is thought to reflect systemic inflammation better than other biomarkers due to combining the indicators of nutrition and inflammation. To date, some retrospective articles have analyzed the association between ALI and prognosis in GIC patients. However, there has not been a systematic evaluation of whether ALI is a reliable predictive factor for GIC patients. Thus, we conducted the first meta-analysis to identify the predictive significance of pre-treatment ALI in GIC patients, which may help to determine prognosis and formulate an effective treatment strategy that will further minimize mortality.
Methods
Literature search strategies
The current meta-analysis accompanied the PRISMA statement [13]. The protocol for this meta-analysis was available in PROSPERO (CRD42022371374). On December 29, 2022, PubMed, EMBASE, and the Cochrane Library were retrieved using the keyword: “advanced lung cancer inflammation index [All Fields]”. We further searched Google Scholar for grey literature. Additionally, we manually retrieved the reference lists of the publications that qualified.
Inclusion and exclusion criteria
If studies met all the following criteria, they were included: patients diagnosed with GIC; research evaluated the prognostic value of ALI; provided at least one of the outcomes [overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and cancer-specific survival (CSS)]. The conference abstracts, case reports, or comments were excluded.
Data extraction and quality assessment
Data extraction mainly focused on the author, year, study region, study design, study period, sample size, the number of male and female patients, cancer types, treatment, follow-up duration, cut-off, and outcomes. The Newcastle–Ottawa Scale (NOS) score was utilized to evaluate the quality of the observational studies. High-quality literature was defined as having a score above six. All of the above steps were double-checked by Lilong Zhang and Kailiang Zhao, and any disparities were addressed by Weixing Wang and Wenhong Deng.
Statistical methods
Statistical analysis was conducted by Stata 15.0. The statistical heterogeneity was calculated using the chi-squared test. P < 0.1 and I2 > 50% indicated high heterogeneity, so a random effect model was applied; otherwise, the fixed effect model was used. The tests of Egger’s and Begg’s were employed to evaluate publication bias. If there was significant publication bias, we used the trim-and-fill method to modify the results [14]. Sensitivity analysis was implemented to assess the stability of the results by excluding each study independently.
Results
Characteristics of studies
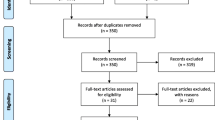
After the initial search, 67 duplicate studies were removed. Then there were 339 articles deleted after carefully reading the titles and abstracts. Later, the full texts of the remaining 46 articles were further assessed. 18 articles involving 6899 patients were ultimately included [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The PRISMA flow diagram is provided in Fig. 1.
The main characteristics of the studies included are shown in Table 1. Of the 18 studies, seven were on colorectal cancer (CRC), three on gastric cancer (GC), two on esophageal cancer (EC), two on pancreatic cancer (PC), one on hepatocellular carcinoma (HCC), one on oral cavity cancer (OCC), and one on cholangiocarcinoma (CC). Besides, the study by Ruan et al. included 270 patients with CRC, 245 patients with GC, 145 patients with EC, and 31 patients with hepatobiliary cancer. Eleven studies were carried out in China and four in Japan, plus one each in Austria, Turkey, and Korea. The cutoff point of the ALI was reported as ranging from 13.2–70.4. The NOS scores for 18 articles ranged from 6–8, which represented a low risk of bias (Table 1).
ALI and overall survival
In total, 16 articles involving 6177 patients explored the association between ALI and OS in cancer patients. The pooled HR was 1.914 (95% CI: 1.514–2.419, P < 0.001), implying that low ALI raised death risk by 91.4% (Fig. 2). Since there was significant heterogeneity, a random effects model was used (I2 = 88.4%, P < 0.001).
We then conducted subgroup analyses based on cancer types. The results showed that patients with low ALI had worse OS than those with high ALI in EC (HR = 1.937, 95% CI: 1.204–3.119, P = 0.006, Fig. 3), GC (HR = 1.451, 95% CI: 1.206–1.746, P < 0.001, Fig. 3), and CRC (HR = 2.279, 95% CI: 1.769–2.935, P < 0.001, Fig. 3). We also found no significant heterogeneity between included studies in the GC (I2 = 0.0%, P = 0.824) and EC (I2 = 0.0%, P = 0.816) subgroups; and lower heterogeneity between included studies in the CRC (I2 = 60.6%, P = 0.013) subgroup, so differences in cancer type were a source of heterogeneity (Fig. 3).
Subgroup analyses based on study region, sample size, treatment, and ALI cutoff were also performed, and the results for each subgroup were consistent with the above findings (Table 2).
ALI and disease-free survival
The relationship between ALI and DFS was also examined using prognostic data from 7 studies involving 3,047 participants. Significant heterogeneity was observed in the included studies (I2 = 86.9%, P < 0.001, Fig. 4), so a random effects model was used. We found that patients with low ALI had a shorter DFS than those with high ALI (HR = 1.631, 95% CI: 1.197–2.224, P = 0.002, Fig. 4).
Subgroup analysis showed that lower ALI was associated with poorer DFS in CRC patients (HR = 1.911, 95% CI: 1.517–2.408, P = 0.002, Fig. 5). No significant heterogeneity was observed in the subgroups (I2 = 0.0%, P = 0.420, Fig. 5), and a fixed effects model was utilized. Therefore, differences in cancer type were the source of heterogeneity.
In addition, subgroup analyses based on study region, sample size, and ALI cutoff were also performed, and the results for each subgroup were generally consistent with the above results (Table 2). Notably, ALI was not found to be associated with worse DFS in subgroups with sample sizes ≤ 300 (HR = 1.385, 95% CI: 0.859–2.233, P = 0.182); the opposite was true in subgroups with sample sizes > 300 (HR = 1.783, 95% CI: 1.369–2.322, P < 0.001).
ALI and progression-free survival and cancer-specific survival
A connection between ALI and PFS in cancer patients was observed in a total of 2 studies involving 803 individuals. As shown in Fig. 6A, patients with low ALI had a worse PFS than those with high ALI (HR = 1.679, 95% CI: 1.073–2.628, P = 0.023). Significant heterogeneity was found in studies (I2 = 71.4%, P = 0.061), and a random effects model was applied to this analysis.
The association between ALI and CSS in cancer patients was explored in two articles with 722 individuals (Fig. 6B). Interestingly, we found no significant correlation between ALI and CSS in cancer patients (HR = 1.121, 95% CI: 0.694–1.812, P = 0.640) using a random effects model (I2 = 78.9%, P = 0.030).
Sensitivity analysis
We used the leave-one-out method to do a sensitivity analysis to assess how each study might impact the combined results. We found that the pooled HR for OS was not significantly changed after excluding one study at a time, ranging from 1.853 (95% CI: 1.469–2.337, after omitting Deng et al. 2022) to 1.966 (95% CI: 1.523–2.537, after omitting Zhang et al. 2022, Fig. 7A). Similarly, the pooled HR for DFS was not significantly different in the sensitivity analysis (Fig. 7B). The overall HR ranged from 1.513 (95% CI: 1.123–2.039, after omitting Deng et al. 2022) to 1.708 (95% CI: 1.155–2.526, after omitting Zhang et al. 2022). From the above, we can see that our results are stable and reliable.
Publication bias
The publication bias in OS (Egger’s test: P = 0.001, Begg’s test: P = 0.548) and DFS (Egger’s test: P = 0.021, Begg’s test: P = 0.548) was found by Egger's test. Next, the trim and fill method was utilized to calculate the number of missing studies in OS and DFS. By factoring in the missing hypothesis studies, the combined HR of OS and DFS was recalculated but was not substantially different. As a result, the publication bias had little impact, and the outcome was stable.
Discussion
Our goal was to explore the predictive significance of ALI in GIC patients, and the pooled data demonstrated that a lower ALI was remarkably related to shorter OS, DFS, and PFS. Furthermore, these results held steady even after sensitivity analysis and subgroup analysis. This is the first meta-analysis to thoroughly explore the impact of ALI on the prognosis of GIC patients. As an extremely accessible indicator in clinical practice, pre-treatment assessment of patients’ ALI can help physicians more effectively and easily predict clinical outcomes and assist them to adjust treatment in a timely manner, thereby further reducing mortality. However, it is worth noting that our results also found that ALI levels were not associated with CSS in patients with GIC. Considering that this index (including PFS) only integrated the data of two studies, it may lead to instability in the results, which need to be further confirmed by subsequent studies.
Both the systemic inflammatory response and nutritional state are recognized prognostic factors in cancer patients, and mounting research has shown a close relationship between the systemic inflammatory response and nutritional status in various cancers [33]. Furthermore, the latest view is that systemic inflammatory response via host-tumor interaction is now considered to be the 7th hallmark of cancer [34]. Systemic inflammatory response and nutritional status have been assessed using a variety of blood examination-based derivatives up to this point, such as NLR [35], platelet-lymphocyte ratio (PLR) [36, 37], prognostic nutrition index (PNI) [38], BMI [39], and albumin [40], and a number of lines of research have shown that these derivatives have the potential to be employed by patients with malignancies as prognostic markers [35,36,37,38,39,40].
The ALI is a newly defined cancer index, and one of its unique features is as a composite index combining the nutritional state and the inflammatory state [12]. Deng et al. confirmed the predictive ability of the ALI for 5-year OS and 5-year DFS was better than that of the PNI or systemic inflammation index (SII) in CRC patients [20]. Some studies also found that ALI was superior to albumin, NLR, and BMI in predicting complications, 5-year PFS, and 5-year OS in CRC and OCC patients [17, 22]. Interestingly, Wu et al. revealed that ALI outperformed NLR, PLR, monocyte-lymphocyte ratio (MLR), SII, and PNI in predicting OS and DFS in patients with cholangiocarcinoma by using time-dependent ROC analysis [16]. Thus, the ALI may have a higher discriminating value compared to other biomarkers. Taking all the current evidence together, our study found that ALI predicted a poor prognosis in patients with GIC, and the results held true in gastric, oesophageal, and colorectal cancers, according to subgroup analysis.
Surely, this analysis still has some limitations. The absence of ALI dynamics' evaluations, rather than the use of a single time-point value, is a significant limitation. The absence of a correlation between interleukins, chemokines, and ALI prevents us from elucidating the mechanistic relationship between ALI values and clinical outcomes. The use of various salvage maneuvers may, by chance, have altered the results in favor of one group depending on the opportunities at the treatment center. The vast majority of articles were retrospective cohort studies, which possibly limited their statistical power. There is a lack of uniformity in the cut-off values for ALI across studies, and aggregated survival results may deviate from the actual values. Thus, in order to confirm and update our conclusion, more high-quality studies with sizable sample sizes, particularly multicentre RCTs, were urgently required. At the same time, these studies should also include patients of different races and explore the optimal cut-off values to guide the clinic more precisely for the benefit of patients.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159(1):335-349.e315.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.
Hong T, Shen D, Chen X, Cai D, Wu X, Hua D. A novel systematic inflammation related index is prognostic in curatively resected non-metastatic colorectal cancer. Am J Surg. 2018;216(3):450–7.
Wu LX, Wang XY, Xu KQ, Lin YL, Zhu WY, Han L, Shao YT, Zhou HY, Jiang H, Hang JJ, et al. A Systematic Inflammation-based Model in Advanced Pancreatic Ductal Adenocarcinoma. J Cancer. 2019;10(26):6673–80.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, Song Y. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res. 2019;8(3):214–26.
Moon G, Noh H, Cho IJ, Lee JI, Han A. Prediction of late recurrence in patients with breast cancer: elevated neutrophil to lymphocyte ratio (NLR) at 5 years after diagnosis and late recurrence. Breast Cancer. 2020;27(1):54–61.
Ku JY, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Prognostic value of neutrophil-to-lymphocyte ratio in older patients with head and neck cancer. J Geriatr Oncol. 2020;11(3):417–22.
Gao X, Pan Y, Han W, Hu C, Wang C, Chen L, Guo Y, Shi Y, Pan Y, Xie H, et al. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med. 2021;18(1):283–97.
Matsuda S, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T, Kitagawa Y. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol. 2015;22(1):302–10.
Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Zhang X, Wang D, Sun T, Li W, Dang C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer. 2022;22(1):684.
Wu H, Ding F, Lin M, Shi Z, Mei Z, Chen S, Jiang C, Qiu H, Zheng Z, Chen Y, et al. Use of the Advanced Lung Cancer Inflammation Index as a Prognostic Indicator for Patients With Cholangiocarcinoma. Front Surg. 2022;9:801767.
Pian G, Hong SY, Oh SY. Prognostic value of advanced lung cancer inflammation index in patients with colorectal cancer liver metastases undergoing surgery. Tumori. 2022;108(1):56–62.
Horino T, Tokunaga R, Miyamoto Y, Hiyoshi Y, Akiyama T, Daitoku N, Sakamoto Y, Yoshida N, Baba H. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann Gastroenterol Surg. 2022;6(1):83–91.
He K, Si L, Pan X, Sun L, Wang Y, Lu J, Wang X. Preoperative Systemic Immune-Inflammation Index (SII) as a Superior Predictor of Long-Term Survival Outcome in Patients With Stage I-II Gastric Cancer After Radical Surgery. Front Oncol. 2022;12:829689.
Deng Y, Sun Y, Lin Y, Huang Y, Chi P. Clinical implication of the advanced lung cancer inflammation index in patients with right-sided colon cancer after complete mesocolic excision: a propensity score-matched analysis. World J Surg Oncol. 2022;20(1):246.
Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, Imaoka Y, Yasuda H, Ohi M, Kusunoki M. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr. 2021;40(3):1130–6.
Tsai YT, Hsu CM, Chang GH, Tsai MS, Lee YC, Huang EI, Lai CH, Fang KH. Advanced Lung Cancer Inflammation Index Predicts Survival Outcomes of Patients With Oral Cavity Cancer Following Curative Surgery. Front Oncol. 2021;11:609314.
Tan X, Peng H, Gu P, Chen M, Wang Y. Prognostic Significance of the L3 Skeletal Muscle Index and Advanced Lung Cancer Inflammation Index in Elderly Patients with Esophageal Cancer. Cancer Manag Res. 2021;13:3133–43.
Ruan GT, Ge YZ, Xie HL, Hu CL, Zhang Q, Zhang X, Tang M, Song MM, Zhang XW, Liu T, et al. Association Between Systemic Inflammation and Malnutrition With Survival in Patients With Cancer Sarcopenia-A Prospective Multicenter Study. Front Nutr. 2021;8:811288.
Xie H, Huang S, Yuan G, Kuang J, Yan L, Wei L, Tang S, Gan J. The advanced lung cancer inflammation index predicts short and long-term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ. 2020;8:e10100.
Kusunoki K, Toiyama Y, Okugawa Y, Yamamoto A, Omura Y, Ohi M, Araki T, Kusunoki M. Advanced Lung Cancer Inflammation Index Predicts Outcomes of Patients With Colorectal Cancer After Surgical Resection. Dis Colon Rectum. 2020;63(9):1242–50.
Barth DA, Brenner C, Riedl JM, Prinz F, Klocker EV, Schlick K, Kornprat P, Lackner K, Stöger H, Stotz M, et al. External validation of the prognostic relevance of the advanced lung cancer inflammation index (ALI) in pancreatic cancer patients. Cancer Med. 2020;9(15):5473–9.
Topkan E, Mertsoylu H, Ozdemir Y, Sezer A, Kucuk A, Besen AA, Ozyilkan O, Selek U. Prognostic Usefulness Of Advanced Lung Cancer Inflammation Index In Locally-Advanced Pancreatic Carcinoma Patients Treated With Radical Chemoradiotherapy. Cancer Manag Res. 2019;11:8807–15.
Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kimura K, Amano R, Hirakawa K, Ohira M. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer. 2019;19(1):241.
Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1811–5.
Li Q, Ma F, Tsilimigras DI, Åberg F, Wang JF. The value of the Advanced Lung Cancer Inflammation Index (ALI) in assessing the prognosis of patients with hepatocellular carcinoma treated with camrelizumab: a retrospective cohort study. Ann Transl Med. 2022;10(22):1233.
Chen XY, Lin Y, Yin SY, Shen YT, Zhang XC, Chen KK, Zhou CJ, Zheng CG. The geriatric nutritional risk index is an effective tool to detect GLIM-defined malnutrition in rectal cancer patients. Front Nutr. 2022;9:1061944.
Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Kitamura A, Ichikawa T, Ide S, Kitajima T, Fujikawa H, Yasuda H, et al. Close Relationship Between Immunological/Inflammatory Markers and Myopenia and Myosteatosis in Patients With Colorectal Cancer: A Propensity Score Matching Analysis. JPEN J Parenter Enteral Nutr. 2019;43(4):508–15.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.
Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, Lin JR. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27(10):1347–57.
He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, Chen X, Rong R, Zhang B, Xia L. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30(1):439.
Wu Y, Li C, Zhao J, Yang L, Liu F, Zheng H, Wang Z, Xu Y. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol. 2016;14(1):289.
Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106(8):1439–45.
Li F, Du H, Li S, Liu J. The Association Between Metabolic Syndrome and Gastric Cancer in Chinese. Front Oncol. 2018;8:326.
Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, Bae JM, Kim S. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr. 2019;38(2):870–6.
Acknowledgements
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 82172855, 81870442), and Natural Science Foundation of Hubei Province, China (No. 2021CFB365).
Author information
Authors and Affiliations
Contributions
ZL, ZK, DW, and WW conceived and designed the study. ZL, ZK, CD, KT, WK, LR, and QZ were responsible for the collection and assembly of data, data analysis, and interpretation. ZL and ZK were involved in writing the manuscript. ZL, ZK, DW, and WW revised the manuscript. All the work was performed under DW and WW instructions. All authors read and approved the final manuscript. Lilong Zhang and Kailiang Zhao contributed equally to this work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Zhao, K., kuang, T. et al. The prognostic value of the advanced lung cancer inflammation index in patients with gastrointestinal malignancy. BMC Cancer 23, 101 (2023). https://doi.org/10.1186/s12885-023-10570-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10570-6