Abstract
Background
Diminished immune defense plays an important role in cancer development. Cancer risk in immunocompromised patients may differ. Identifying individuals with elevated cancer risk can inform strategies for routine cancer screening. This study aimed to understand and compare cancer incidence and risk in three patient groups: recipients of solid organ transplant (SOT) or hematopoietic stem cell transplant (HSCT); diagnosis of primary or secondary immunodeficiency disorder (PID/SID); and recipients of tumor necrosis factor inhibitor (TNF-i) therapy.
Methods
This retrospective cohort study used the University of Utah Health System database and Huntsman Cancer Institute tumor registry. Patients aged ≥18 years with SOT/HSCT, PID/SID or ≥ 3 months of TNF-i therapy were included. The date of transplant, diagnosis of PID/SID, or 1st TNF-i medication order date was defined as the index date. We calculated cumulative cancer incidence by Kaplan-Meier method. A Cox-proportional hazard regression model with a stepwise variable selection process was used to identify independent risk factors associated with the time to onset of a new primary cancer.
Results
In total, 13,887 patients were included which comprised of 2982 (21%) SOT/HSCT, 7542 (54%) PID/SID and 3363 (24%) patients receiving TNF-i. The mean (SD) age ranged from 46.8 (15) years - 50.4 (18.2) years. The proportion of white patients ranged from 72.3–84.8%. The estimated cumulative cancer incidence was 11.5% in the SOT/HSCT cohort, 14.3% in the PID/SID cohort, and 8.8% in the TNF-i cohort. The multivariable model adjusted for age, benign in-situ disease, Charlson Comorbidity Index, hypertension/cardiovascular disease/end stage renal disease, gender, race/ethnicity, and renal cyst as significant risk factors. The adjusted hazard ratios for cancer development in SOT/HSCT and PID/SID cohorts compared to the TNF-i cohort over the full follow-up period were 1.57 (95% CI: 1.16–2.13) and 2.14 (95% CI: 1.65–2.77), respectively.
Conclusion
A significantly increased risk of cancer was observed in PID/SID patients and SOT/HSCT patients compared to TNF-i patients. Age ≥ 50 years, male gender, and clinical comorbidities were additional factors impacting cancer risk. PID/SID and SOT/HSCT patients may benefit from more intensive cancer screening.
Similar content being viewed by others
Background
Cancer is currently the second leading cause of death in the United States (US) [1]. Cancer remains the second most costly disease among Americans with a 27% rise in cost within the past 10 years [2,3,4]. The annual costs of cancer care are estimated to exceed $245 billion dollars by 2030 [5,6,7]. Delays in cancer detection can lead to decreased functional outcomes, productivity loss, treatment complications, reduced health-related quality of life, and increased healthcare costs [8]. However, cancer screening enables the earlier detection and treatment of cancer which may lead to survival benefits, reduce the complexity of care, and decrease long-term costs in cancer patients [9].
Prior epidemiologic studies suggest that patients with known and severe immune dysregulation following transplant have an increased susceptibility to cancer [10]. A large cohort study linking US transplant registries with 15 population based state and regional cancer registries has found a two-fold increased risk of cancer in transplant patients [11]. Transplant patients have been found to be susceptible to 32 different types of cancer [11], with higher incidence of oncogenic virus-related cancers (e.g., hepatocellular carcinoma, anogenital carcinoma, cervical cancer, GI cancer, head and neck cancer, and nasopharyngeal carcinoma) [12]. Although limited data is available, patients with primary or secondary immunodeficiency disorder (PID/SID) can likewise be at increased risk of cancer [10]. The estimated relative risk of cancer in adults with PID is 1.42 compared with the age-adjusted SEER population [13]. PID is primarily associated with an increased risk of lymphomas, which contributes up to 60% of PID cancer cases [13]. Acquired Immune Deficiency Syndrome (AIDS) is the most common SID, associated with significantly higher rates of cancer especially Kaposi’s sarcoma, non-Hodgkin’s lymphoma, lung, liver, melanoma, or stomach cancer [14, 15]. While immunomodulatory therapy such as tumor necrosis factor inhibitor (TNF-i) may increase the risk of cancer attributable to its mechanism of action, the impact on cancer risk in real-world TNF-i users is still debatable. Current screening recommendations currently do not address the increased cancer risk in immunocompromised patients or the incident cancer types.
Previous studies provide information on cancer risk in specific groups within immunocompromised patients (only solid organ (SOT) or hematopoietic stem cell transplant (HSCT), only PID or SID) [13, 14, 16,17,18]. However, limited data is available on cancer risk across overall patient groups (combined SOT and HSCT patients or combined PID and SID patients). In addition, little is known regarding the impact of patient characteristics / demographics and pre-existing clinical conditions on cancer risk in patients with dysregulated immune system. The aim of this study was to evaluate and compare cancer risk in three patient groups: recipients of SOT/HSCT, patients with PID/SID and patients treated with TNF-i therapy. As the impact of TNF-i on cancer risk has been found to be non-significant in multiple studies [19,20,21,22,23], we hypothesized that the risk of cancer in SOT/HSCT recipients and PID/SID patients will be higher compared to TNF-i recipients. Data from our study adds insights to the current evidence on the immunosuppression-cancer paradigm and informs strategy for routine cancer screening.
Methods
This was a retrospective cohort study using electronic health records from the University of Utah Enterprise Data Warehouse (EDW) and data from the Huntsman Cancer Institute Tumor Registry (HCI-TR). The EDW integrates historical and comprehensive health records of more than 1.5 million patients across the University of Utah Healthcare System (hospital and clinics), and the HCI. The HCI-TR registers all primary cancer cases diagnosed and/or treated by the HCI and works closely in cancer tracking activities and statistical reporting procedures with the Utah Cancer Registry, which is a Surveillance, Epidemiology and End Results (SEER) registry.
Overall study cohort included three groups, patients with SOT/HSCT, diagnosis of PID/SID and who received TNF-I therapy between July 1,2000 and February 20, 2018. SOT/HSCT or PID/SID were defined by the Current Procedural Terminology codes and the International Classification of Diseases Ninth and Tenth revision Clinical Modification (ICD-9-CM and ICD-10-CM) codes respectively. The TNF-i subgroup included patients who received at least 3 months of TNF-i therapy. The index date was defined as the date when an individual was first confirmed either as a transplant recipient, diagnosed with PID or SID or initiated TNF-i therapy. The pre-index period was defined from January 1, 2000 to the index date for all patients which allowed for the capture of baseline characteristics and potentially confounding variables [e.g., demographics, prior cancer, comorbidities, Charlson Comorbidity Index (CCI) score]. Patients with age ≥ 18 years at the index date, and with a minimum follow up of 90 days post-index date were included. The cohorts were kept mutually exclusive based on the risk hierarchy as indicated in prior studies [13, 16, 23]: SOT/HSCT patients that met the eligibility criteria for either the PID/SID or TNF-i cohorts were analyzed under SOT/HSCT cohort; PID/SID patients that also received TNF-i therapy, were included in the PID/SID cohort. The TNF-i cohort with presumably lower risk was used as a reference group for comparing risk in other two cohorts.
Following cohort identification, patients were screened through the HCI-TR to identify the first occurrence of a new primary cancer diagnosis (outcome) post-index date. For patients with prior cancer during the pre-index period, a new primary cancer diagnosis was required to meet the definition of the development of cancer. For each cohort, follow-up ended at cancer diagnosis, death, last date of follow-up or end of study period (February 2020) whichever occurred first.
Means with standard deviations (SD) and medians with interquartile ranges (IQR) were reported for continuous variables. Frequencies and percentages were reported for categorical variables. Statistical comparisons were performed using analysis of variance (ANOVA) tests for continuous variables and Chi-square test and Fisher’s Exact test for categorical variables. Differences in the baseline characteristics among three risk groups were considered statistically significant if P < 0.05. Number of new cancer cases over observation period were presented in incidence rate per 1000 person-years. To account for the follow-up period in calculating cumulative incidence, we generated Kaplan-Meier product-limit estimates where patients were censored at date of death or last follow-up without the onset of new cancer. We ran univariable Cox regression analysis to identify statistically significant confounders. The potential confounding effects were tested in multivariable Cox regression model. Age (18–49, 50–64, ≥65), BMI (< 18.5, 18.5–24.9, 25–29.9, ≥30) and CCI score (0, 1–2, 3–5, ≥6) were used as categorical variables in the model. Multivariable models were constructed using a stepwise forward selection approach, using p < 0.3 as the entry criterion and p < 0.2 as the staying criterion. Akaike Information Criteria (AIC) and Bayesian Information Criterion (BIC) were used to compare the prediction errors for nested and non-nested models. Lower values indicated that the model explains data with the consideration of the goodness-of-fit and degrees of freedom. All analyses were performed using the SAS statistical software version 9.4 (Cary, NC: SAS Institute) and STATA statistical software version 16 (College Station, TX: StataCorp LLC.).
Results
In total, 13,887 patients were included in the analysis: 2982 SOT/HSCT patients, 7542 PID/SID patients, and 3363 patients in the TNF-i cohort (Fig. 1). Out of 2982 transplant patients, 1891 (63.4%) were SOT and 1091 (36.6%) were HSCT patients. The most common SOT type was kidney transplant (60.4%), followed by liver (15.8%) and heart (12.4%). Among HSCT cohort, 710 (65.1%) had autologous and 381 (34.9%) had allogeneic stem cell transplant. The most common type of PID observed (29.4%) was ‘Other Combined Immunodeficiencies’ (ICD-9/10-CM: 277.6/D81.810), followed by ‘Other Specific Disease of Blood and Blood-forming Organs’ (ICD-9/10-CM: 289.89/D89.2) and ‘Unspecified Disorder of Immune Mechanism’ (ICD-9/10-CM: 279.9/D89.9). In the SID cohort, we included patients with HIV (45% of the PID/SID cohort) (ICD-9/10-CM: 042/B20). The median (IQR) duration of exposure to TNF-i was 1.6 years (1.0–3.0). The baseline characteristics of patients by risk cohort are described in Table S1 (Supplemental Material). The proportion of males was higher in the SOT/HSCT and PID/SID cohorts compared to the TNF-i cohort. The average age was higher in the SOT/HSCT (50.3) and PID/SID cohort (50.4) compared to the TNF-i cohort (46.8). Most of the patients in all 3 cohorts were white followed by Hispanic. More than a third of patients in the SOT/HSCT and TNF-i cohort had a BMI ≥30; while a normal BMI (18.5–24.9) was observed in 35.8% of patients in the PID/SID cohort. Prior history of cancer was observed in 38.0% of patients in the SOT/HSCT cohort, followed by 7.6% in the PID/SID cohort and 1.9% in the TNF-i cohort. Most patients (92.3%) in the HSCT cohort had prior history of cancer. The most common baseline cancer(s) in the SOT/HSCT and PID/SID cohort was cancer involving the hematopoietic system while those in the TNF-i cohort were skin, breast, head and neck cancer. Most patients in the SOT/HSCT, PID/SID and TNF-i cohorts had a CCI score of 3–5, 0 and 1–2, respectively. Hypertension (HTN)/ cardiovascular disease (CVD)/ end stage renal disease (ESRD), and diabetes mellitus (DM) were the most common comorbidities observed in all three cohorts. The median (IQR) follow up was 5.5 years (3.0–9.4), 6.2 years (3.7–10.0) and 4.4 years (3.2–6.9) in the SOT/HSCT, PID/SID and TNF-i cohorts, respectively. The baseline characteristics (gender, age, race/ethnicity, BMI), clinical characteristics (history of cancer, 4 categories (≥6, 3–5,1-2,0) of CCI), and most of the comorbidities were significantly different among three cohorts (p < 0.01).
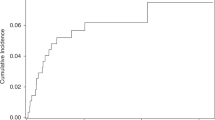
The estimated cumulative cancer incidence (Kaplan Meier estimates) was 11.5% in the SOT/HSCT cohort, 14.3% in the PID/SID cohort, and 8.8% in the TNF-i cohort (Fig. 2). The crude cancer incidence rate per 1000 person-years for the full follow-up period was 7.5 (95% CI: 6.4–8.7) in the SOT/HSCT, 8.0 (95% CI: 7.3–8.8) in the PID/SID, and 3.4 (95% CI: 2.7–4.3) in the TNF-i cohort. The unadjusted hazard ratios for cancer in the SOT/HSCT and PID/SID cohorts compared to the TNF-i cohort over the full follow-up period were 2.16 (95% CI: 1.63–2.87) and 2.32 (95% CI: 1.80–3.00), respectively.
From the analysis of overall cohort, female patients (compared to male) [HR: 0.81 (95% CI: 0.70, 0.94)], patients of unknown/other race or ethnicity (compared to white) [HR:0.50 (95% CI: 0.32, 0.79)], and with CCI of 0 (compared to CCI 1–2) [HR: 0.74 (95% CI: 0.62, 0.90)] were associated with a lower risk of cancer onset. Patients with BMI 25–29 had 23% higher risk of cancer onset [HR: 1.23 (95% CI: 1.01, 1.48)] compared to the patients with BMI 18.5–24.9. Prior history of a cancer was significantly associated with cancer risk in the overall cohort [HR: 1.38 (95% CI: 1.11, 1.71)]. Age was the only covariate that was consistently associated with cancer risk across all 3 cohorts, with a significantly higher risk among patients ≥50 years old compared to those 18 to 49 years old [HR:3.04 (95% CI: 2.57, 3.59)]. In addition, patients who had benign in-situ disease [HR:2.28 (95% CI: 1.82, 2.85)], DM [HR:1.4 (95% CI: 1.13, 1.73)], HTN/CVD/ESRD [HR:1.6 (95% CI: 1.37, 1.88)], COPD/asthma/pneumonia/bronchitis [HR:1.55 (95% CI:1.21, 1.99)], use of diuretics [HR:2.67 (95% CI:1.27, 5.61)] and renal cyst [HR:2.54 (95% CI:1.51, 3.66)] had a higher risk of cancer compared to those who did not have those comorbidities (Table S2) (Supplemental Material).
Due to potential multicollinearity between comorbidities (CVD, ESRD, DM, COPD, pneumonia, bronchitis), baseline cancer and conditions used to calculate CCI score (cancer, metastatic cancer, myocardial infarction, congestive heart failure, peripheral vascular disease, renal disorder, DM, pulmonary disease), two multivariable models were developed. In one model, we adjusted for age, race/ethnicity, gender, BMI and CCI score. Controlling for other potential predictors, individuals with SOT/HSCT had 55% [HR:1.55 (95% CI: 1.15, 2.10)] and PID/SID had 121% [HR: 2.21 (95% CI: 1.70, 2.86)] higher risk of cancer compared to recipients of TNF-i therapy. Age was the only independent predictor selected by the model [HR:2.88 (95% CI: 2.41, 3.43)] (Fig. 3).
The second model adjusted for age, race/ethnicity, gender, baseline cancer, HTN/CVD/ESRD, DM, COPD/asthma/pneumonia/bronchitis, DM, renal cyst and benign in-situ disease. Controlling for other potential predictors, individuals with SOT/HSCT had 57% [HR:1.57 (95% CI: 1.16, 2.13)] and PID/SID had 114% [HR: 2.14 (95% CI: 1.65, 2.77)] higher risk of cancer compared to recipients of TNF-i therapy. In addition, age ≥ 50 years [HR: 2.89 (95% CI: 2.42, 3.44)], history of benign in-situ disease [HR: 1.67 (95% CI: 1.33, 2.11] and renal cyst [HR: 1.64 (95% CI: 1.04, 2.56] were significant predictors of cancer risk (Fig. 4).
A total of 701 patients developed new primary cancer; 161 in the SOT/HSCT cohort, 471 in the PID/SID cohort and 69 in the TNF-I cohort (Table S3). In the SOT/HSCT cohort, GI cancer (14.3%) was the most common newly diagnosed cancer followed by cancer of hematopoietic system and skin cancer (12.4% each). In the PID/SID cohort, cancer of hematopoietic system was predominant (22.3%) followed by GI (16.8%) and skin cancer (14.2%). In the TNF-i cohort, cancer of reproductive system (17.4%) was the most common followed by breast cancer (15.9%).
Discussion
In our analysis of real-world data of 13,887 individuals, the risk of cancer was significantly higher in patients with PID/SID or SOT/HSCT relative to patients who received TNF-i therapy while controlling for other predictors of cancer risk. The crude incidence rate of cancer estimated in these two cohorts (PID/SID: 8.01 cases per 1000 person-years; SOT/HSCT: 7.48 per 1000 person-years) was almost twice as high as the age-adjusted cancer incidence in the region, (4.02 cases per 1000 person-years) [24]. The crude cancer incidence in the TNF-i cohort was 3.45 cases per 1000-person years which is close to the age-adjusted incidence in Utah. Similar trends in the escalation of cancer risk among transplant patients have been reported in the literature [11]; however, the increase in cancer risk among patients with PID/SID in our study appeared to be larger than findings from previous studies (RR:1.42 compared to SEER age-adjusted population) [13]. This higher estimate may be a reflection of overall risk from a combined PID/SID cohort compared to only PID patients in the prior study [13]. Another reason of this discrepancy may be heterogeneity in causes of immunodeficiency, which can influence cancer risk. In addition to the specific PID disorders that have shown to increase the risk of cancer (common variable immunodeficiency disorder, severe combined immunodeficiency disorder, Wiskott-Aldrich syndrome, hypogammaglobulinemia) [13, 25], the PID/SID cohort in this study also captured a variety of additional PID disorders (e.g., other common immunodeficiencies, other disorders of blood and blood forming organs, and unspecified disorder of immune mechanism etc).
Baseline demographics and clinical characteristics among three groups can influence cancer risk in three cohorts. To adjust for selection bias, we incorporated the clinically and statistically relevant variables e.g., age, gender, race/ethnicity, body mass index, prior history of cancer and certain comorbidities (e.g., cardiovascular disease, diabetes, benign in-situ- disease etc.) in our regression analysis. In the multivariate model while controlling for age, sex and other confounders, cancer risk in the SOT/HSCT and PID/SID cohort was significantly higher than the TNF-i cohort. Consistent with trends reported in the literature [26], age was found to be associated with cancer risk, with an effect size consistent across cohorts. Therefore, cohorts with a higher proportion of elderly patients (e.g., PID/SID and SOT/HSCT) possibly had a higher incidence of cancer. Similarly, cohorts with a higher proportion of males (SOT/HSCT) might have higher incidence of cancer as both the univariable and multivariable model found a lower risk of cancer in females. In addition, a history of benign or in-situ growth was associated with an increased cancer risk in the overall cohort. The progression of benign growths to malignant cancers is most studied among women with benign breast disease, who have been found to have more than twice the risk of developing subsequent breast cancer compared to their counterparts who do not have benign breast disease [27]. A similar association with higher cancer risk was also observed with a history of renal cysts, which have a risk of developing into renal cell carcinomas [28, 29].
One of the indications of organ transplantation (SOT and most cases of HSCT) is organ failure from cancer. HSCT is a recommended treatment for several types of hematologic cancers, e.g., acute leukemia, aggressive B cell lymphoma, multiple myeloma [30]. That’s why we included patients with prior history of cancer in our cohort to have a representation of real-world transplant patients. As patients with prior history of cancer may develop subsequent cancer of different types, prior history of cancer was included as one of the variables in our regression model. Although prior history of cancer was a statistically significant predictor of cancer in the univariate analysis, the effect was confounded by some other predictors e.g., age, benign disease in the multivariable model. In addition to prior history of cancer, other factors that may be associated with increased cancer risk in transplant especially HSCT patients include, age at HSCT, pre-HSCT exposure to chemotherapy and radiation, infection with oncogenic viruses (Epstein–Barr virus [EBV] and hepatitis B and C viruses), autologous versus allogeneic HSCT etc. [31].
There has been a lack of evidence to suggest an increased risk of cancer among patients who receive TNF-i therapy, both in our study cohort and in the literature [32]. Biologically, the role of TNF-alpha in cancer progression remains unclear and has been linked to both cancer-suppressing and -promoting pathways [33]. Therefore, insights from real-world evidence may shed light on the effect of long-term TNF-i therapy on cancer risk, guiding the clinical use of TNF-i agents especially among individuals who are already predisposed to higher risk of cancer.
The types of incident cancer observed in the SOT/HSCT and PID/SID cohorts (e.g., GI, cancer of hematopoietic system and skin cancer) support the possible association of immunodeficiency and subsequent oncogenic virus-or bacterial infection related cancer. GI cancer can be associated with Epstein Barr virus (EBV) or Helicobacter pylori infection [13, 16]. Increased risk of lymphoma and leukemia can be related to EBV driven lymphoproliferation [13]. Three pathogenic human viruses have been linked with skin neoplasms; human papilloma virus, Kaposi’s sarcoma associated herpes virus and human T cell leukemia virus type 1. Viruses can alter keratinocytes by activation of cancer promoting genes and accelerated viral carcinogenesis is mostly observed in immune deficient hosts [34]. Cancer of reproductive system and breast cancer was identified most frequently in the TNF-i cohort along with skin cancer. The most common cancers of the reproductive system such as, cervical and anal cancer may be associated with human papilloma virus [35]. Also, the TNF-i cohort comprised more than 60% of females and females are more susceptible to breast cancer and cancer of the reproductive system. Based on the incident cancer types, a broader cancer screening approach may be warranted in this patient population as over 50% of the cancers in the SOT/HSCT and PID/SID population would not be detected through current cancer screening recommendations (i.e., breast, lung, colorectal, cervical, prostate).
Findings of our research should be considered in the context of the retrospective observational research design, which is subject to misclassification due to the coding, incomplete records, and unobserved confounders. Patient characteristics differed significantly between the cohorts, potentially complicating comparisons between the groups of patients. To address this limitation, covariates that potentially influence the onset of cancer were controlled for in our analysis. The differences in the size of the cohorts may affect the statistical significance of the associations between the covariates, exposure, and outcomes. In addition, we could not adjust for some of the highly reported risk factors, (e.g, smoking, alcohol history, family history) as the database did not provide enough information to capture these variables appropriately. Using data specific to Utah population may limit the generalizability of our result.
Conclusion
Based on our results, patients with PID/SID or SOT/HSCT are susceptible to cancers. The types of incident cancer identified in our and prior studies suggest a limitation of routine single cancer screening tests that target only certain patient groups and address only five cancer types. A higher incidence of cancer has led to recommendations for more intensive cancer screening among SOT/HSCT patients in a few clinical guidelines [36]. Furthermore, earlier detection of cancer may be particularly valuable among patients with PID/SID and SOT/HSCT as cancer can be more challenging to treat among these patients due to the variety of cancer types that may develop and a higher risk of severe infections secondary to the chemotherapy regimens. Future studies in the form of disease and economic modeling may help delineate the clinical and economic value of implementing a broader cancer screening approach in elevated risk patient populations such as SOT/HSCT and PID/SID patients.
Availability of data and materials
Raw data are not publicly available due to restriction related to patient privacy or consent. Derived de-identified data can be available upon reasonable request from the corresponding author.
Abbreviations
- US:
-
United States
- USPTF:
-
United States Preventive Service Task Force
- MCED:
-
Multi Cancer Early Detection
- PID/SID:
-
Primary Immunodeficiency Disorder/ Secondary Immunodeficiency Disorder
- TNF-i:
-
Tumor Necrosis Factor- inhibitor
- AIDS:
-
Acquired Immune Deficiency Syndrome
- SOT/HSCT:
-
Solid Organ Transplant/ Hematopoietic Stem Cell Transplant
- EDW:
-
Electronic Data Warehouse
- HCI-TR:
-
Huntsman Cancer Institute-Tumor Registry
- SEER:
-
Surveillance, Epidemiology and End Results
- ICD-CM:
-
International Classification of Disease-Clinical Modification
- CCI:
-
Charlson Comorbidity Index
- BMI:
-
Body Mass Index
- AIC :
-
Akaike Information Criteria
- BIC:
-
Bayesian Information Criteria
- HTN:
-
Hypertension
- CVD:
-
Cardiovascular Disease
- ESRD:
-
End Stage Renal Disease
- DM:
-
Diabetes Mellitus
- COPD:
-
Chronic Obstructive Pulmonary Disease
- GI:
-
Gastrointestinal
- EBV:
-
Epstein Barr Virus
- HPV:
-
Human Papilloma Virus
References
Beer TM. Novel blood-based early cancer detection: diagnostics in development. Am J Manag Care. 2020;26(14 Suppl):S292–s9.
Early detection facts and figures. Available from: https://www.canaryfoundation.org/wp-content/uploads/EarlyDetectionFactSheet.pdf. Accessed 15 Dec 2021.
Lentz R, Benson B, S K. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120(1):85–92.
Brill JV. Screening for cancer: the economic, medical, and psychosocial issues. Am J Manag Care. 2020;26(14 Suppl):S300–s6.
Parikh DA, Ragavan M, Dutta R, Edwards JG, Dickerson J, Maitra D, et al. Financial toxicity of Cancer care: an analysis of financial burden in three distinct health care systems. JCO Oncol Pract. 2021;17(10):e1450–e9.
Ezell S. Seizing the transformative opportunity of multi-cancer early detection. Washington DC: Information Technology & Innovation Foundation; 2019. Available from: https://itif.org/publications/2021/04/19/seizing-transformative-opportunity-multi-cancer-early-detection.
Angela M, Yabroff R. Cancer Care Costs in the United States Are Projected to Exceed $245 Billion by 20302020. Available from: https://www.aacr.org/about-the-aacr/newsroom/news-releases/cancer-care-costs-in-the-united-states-are-projected-to-exceed-245-billion-by-2030/. Accessed 15 Dec 2021.
Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087.
Hudis CA. World Cancer day 2019: emphasis on early detection. Huntington: The ASCO Post; 2019. Available from: https://ascopost.com/News/59711. [cited 2021]
Prakash O, Gill J, Farr G. Immune disorders and susceptibility to neoplasms. Ochsner J. 2002;4(2):107–11.
Voss AM. Cancer risk among immunosuppressed populations. 2012;2021(10-29-2021) Available from: https://dceg.cancer.gov/news-events/news/2012/immunosuppressed-populations. Accessed 15 Dec 2021.
Engels EA. Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am J Transplant. 2019;19(12):3223–32.
Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States immune deficiency network registry. J Allergy Clin Immunol. 2018;141(3):1028–35.
Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–504.
Wang CC, Silverberg MJ, Abrams DI. Non-AIDS-defining malignancies in the HIV-infected population. Curr Infect Dis Rep. 2014;16(6):406.
Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901.
Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and risk of skin Cancer in organ transplant recipients: systematic review and Meta-analysis. Am J Transplant. 2016;16(12):3490–503.
Aguiar B, Santos Amorim T, Romãozinho C, Santos L, Macário F, Alves R, et al. Malignancy in kidney transplantation: A 25-year single-center experience in Portugal. Transplant Proc. 2015;47(4):976–80.
Dreyer L, Mellemkjær L, Andersen AR, Bennett P, Poulsen UE, Juulsgaard Ellingsen T, et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides - a follow-up study from the DANBIO registry. Ann Rheum Dis. 2013;72(1):79–82.
Kim SC, Pawar A, Desai RJ, Solomon DH, Gale S, Bao M, et al. Risk of malignancy associated with use of tocilizumab versus other biologics in patients with rheumatoid arthritis: A multi-database cohort study. Semin Arthritis Rheum. 2019;49(2):222–8.
Muller M, D'Amico F, Bonovas S, Danese S, Peyrin-Biroulet L. TNF inhibitors and risk of malignancy in patients with inflammatory bowel diseases: A systematic review. J Crohns Colitis. 2021;15(5):840–59.
Peleva E, Exton LS, Kelley K, Kleyn CE, Mason KJ, Smith CH. Risk of cancer in patients with psoriasis on biological therapies: a systematic review. Br J Dermatol. 2018;178(1):103–13.
Singh N, Li CI. Impact of rheumatoid arthritis and biologic and targeted synthetic disease modifying antirheumatic agents on cancer risk and recurrence. Curr Opin Rheumatol. 2021;33(3):292–9.
Utah At A Glance: American Cancer Society: Cancer Statistics Center. Available from: https://cancerstatisticscenter.cancer.org/#!/state/Utah. Accessed 15 Dec 2021.
Salavoura K, Kolialexi A, Tsangaris G, A M. Development of cancer in patients with primary immunodeficiencies. Anticancer Res. 2008;28(2b):1263–9.
Wareham NE, Li Q, Sengeløv H, Da Cunha-Bang C, Gustafsson F, Heilmann C, et al. Risk of de novo or secondary cancer after solid organ or allogeneic haematopoietic stem cell transplantation. J Cancer Res Clin Oncol. 2019;145(12):3125–35.
Louro J, Román M, Posso M, Comerma L, Vidal C, Saladié F, et al. Differences in breast cancer risk after benign breast disease by type of screening diagnosis. Breast. 2020;54:343–8.
Hurst FP, Jindal RM, Fletcher JJ, Dharnidharka V, Gorman G, Lechner B, et al. Incidence, predictors and associated outcomes of renal cell carcinoma in long-term Dialysis patients. Urology. 2011;77(6):1271–6.
Goh A, Vathsala A. Native renal cysts and dialysis duration are risk factors for renal cell carcinoma in renal transplant recipients. Am J Transplant. 2011;11(1):86–92.
Bair SM, Brandstadter JD, Ayers EC, Stadtmauer EA. Hematopoietic stem cell transplantation for blood cancers in the era of precision medicine and immunotherapy. Cancer. 2020;126(9):1837–55.
Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633–9.
Waljee AK, Higgins PDR, Jensen CB, Villumsen M, Cohen-Mekelburg SA, Wallace BI, et al. Anti-tumour necrosis factor-α therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: a nationwide, population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5(3):276–84.
Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, Ségui B. The TNF paradox in Cancer progression and immunotherapy. Front Immunol. 2019;10:1818.
Molho-Pessach V, Lotem M. Viral carcinogenesis in skin cancer. Curr Probl Dermatol. 2007;35:39–51.
Infection associated cancer ESMO 2016. Available from: https://www.esmo.org/oncology-news/archive/infection-associated-cancers. Accessed 15 Dec 2021.
Acuna SA, Huang JW, Scott AL, Micic S, Daly C, Brezden-Masley C, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. 2017;17(1):103–14.
Acknowledgements
Not Applicable.
Funding
This study was funded by GRAIL, LLC., a subsidiary of Illumina Inc., currently held separate from Illumina Inc. under the terms of the Interim Measures Order of the European Commission dated 29 October 2021. The sponsor contributed to study conception and design, protocol review, data interpretation and review of final study report, abstract, poster and manuscript.
Author information
Authors and Affiliations
Contributions
SI: Developed concept, proposal, protocol, analysed and interpreted data, wrote study report, abstract, poster, manuscript. CW: Reviewed concept, proposal, protocol, extracted and interpreted data, reviewed relevant study documents and manuscript. KK: Wrote statistical analysis plan, analysed and interpreted data, reviewed relevant study documents and manuscript. KCC: Developed and reviewed concept, proposal, protocol, interpreted data and reviewed relevant study documents and manuscript. BMW: Reviewed relevant study documents and manuscript. MST: Analysed and interpreted data and reviewed manuscript. CJT: Analysed and interpreted data, wrote study report and reviewed manuscript. DTN: Performed literature search, reviewed manuscript. DIB: Developed and reviewed concept, proposal, protocol, interpreted data and reviewed relevant study documents and manuscript. DDS: Developed and reviewed concept, proposal, protocol, interpreted data and reviewed relevant study documents and manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Given the retrospective nature of the study, the requirement for obtaining informed consent was waived by the University of Utah Institutional Review Board. The study was approved by the Institutional Review Board of the University of Utah (IRB_00142563). Additionally, we confirm that all methods were performed in accordance with relevant guidelines and regulations declared in BMC Cancer.
Consent for publication
Not Applicable.
Competing interests
Sabrina Ilham, Connor Willis, Kibum Kim, Malinda S. Tan, Chia Jie Tan, Diana I. Brixner, David D. Stenehjem received research funding from GRAIL, LLC. to conduct this study. Karen C. Chung and Brenda M. Wood are employees of GRAIL, LLC.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline characteristics of patients stratified by risk cohort. Table S2. Independent predictors of cancer in the overall cohort by Cox Regression Analysis. Table S3. Type of new primary cancer diagnosed in each cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ilham, S., Willis, C., Kim, K. et al. Cancer incidence in immunocompromised patients: a single-center cohort study. BMC Cancer 23, 33 (2023). https://doi.org/10.1186/s12885-022-10497-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10497-4