Abstract
Background
There is growing interest in the collection and use of patient-reported outcome measures (PROMs) to support clinical decision making in patients with non-small cell lung cancer (NSCLC). However, an overview of research into the prognostic value of PROMs is currently lacking.
Aim
To explore to what extent, how, and how robustly the value of PROMs for prognostic prediction has been investigated in adults diagnosed with NSCLC.
Methods
We systematically searched Medline, Embase, CINAHL Plus and Scopus for English-language articles published from 2011 to 2021 that report prognostic factor study, prognostic model development or validation study. Example data charting forms from the Cochrane Prognosis Methods Group guided our data charting on study characteristics, PROMs as predictors, predicted outcomes, and statistical methods. Two reviewers independently charted the data and critically appraised studies using the QUality In Prognosis Studies (QUIPS) tool for prognostic factor studies, and the risk of bias assessment section of the Prediction model Risk Of Bias ASsessment Tool (PROBAST) for prognostic model studies.
Results
Our search yielded 2,769 unique titles of which we included 31 studies, reporting the results of 33 unique analyses and models. Out of the 17 PROMs used for prediction, the EORTC QLQ-C30 was most frequently used (16/33); 12/33 analyses used PROM subdomain scores instead of the overall scores. PROMs data was mostly collected at baseline (24/33) and predominantly used to predict survival (32/33) but seldom other clinical outcomes (1/33). Almost all prognostic factor studies (26/27) had moderate to high risk of bias and all four prognostic model development studies had high risk of bias.
Conclusion
There is an emerging body of research into the value of PROMs as a prognostic factor for survival in people with NSCLC but the methodological quality of this research is poor with significant bias. This warrants more robust studies into the prognostic value of PROMs, in particular for predicting outcomes other than survival. This will enable further development of PROM-based prediction models to support clinical decision making in NSCLC.
Similar content being viewed by others
Background
Lung cancer is the second most common cancer, with an estimated 2.2 million patients newly diagnosed worldwide, accounting for 11.4% of all new cancer cases [1]. Non-small cell lung cancer (NSCLC), accounts for approximately 85% of lung cancer cases [2]. NSCLC may cause a range of symptoms (e.g., chronic cough, pain, dyspnoea, fatigue), psychological issues and decreased physical function [3]. This can negatively affects health-related quality of life (HRQOL)[4]. Another reason for this decreased HRQOL is related to anticancer treatments and their associated side effects [4].
Patient-reported outcomes measures (PROMs) are tools to assess patients’ views on aspects of their health and condition, including HRQOL, symptom status, physical function and mental health [5]. In the field of lung cancer, they can be categorised into generic, cancer-specific and lung cancer-specific instruments [6]. They can be used as part of the clinical management of lung cancer, with the aim to improve patient-clinician communication, decision making and patient satisfaction [5]. The increasing use of PROMs as part of clinical care contributes to the paradigm shift from illness-focused to patient-centred care [7]. Randomised controlled trials comparing PROM-directed follow-up to usual care demonstrated that integrating PROMs in care pathways was associated with better symptom control, reduced emergency department attendance and hospitalisation, and improved survival [8, 9].
Decisions regarding the treatment of NSCLC often involve a trade-off between potential benefits (e.g., prolonged survival) and potential risks (e.g., treatment toxicity, decreased HRQOL) [10]. PROMs could inform discussions about this trade-off and support shared treatment decision-making by patients and healthcare professionals [6]. Integrated as predictors into prognostic models, PROMs could also provide insights into patient’s future course of disease based on a current assessment of their own health and condition [11, 12]. However, several steps are required before such prognostic models can be implemented in practice. These include identification of the association between candidate prognostic factors and outcomes, development and validation of the model, and evaluation of its clinical utility and impact [13, 14].
A 2021 systematic review, in line with previous studies [6, 15, 16], suggested PROMs provided prognostic information for overall survival in a range of cancer populations, including lung cancers [17]. However, it is unclear if high quality studies are available that investigated the value of PROMs for predicting all clinical outcomes. This review aimed to address this unmet need by examining to what extent, how and how robustly research has been conducted on the prognostic value of PROMs in patients with NSCLC. As this topic concerns how research has been conducted, one suitable approach, which we adopt, is a scoping review [18]. This review will contribute to understanding and unlocking the potential of PROMs to support and enhance clinical decision making and, ultimately, to improve the outcomes of patients with lung cancer.
Methods
We conducted a scoping review [18] to identify and characterise published studies that evaluated the association between PROMs (as candidate prognostic factors) and outcomes, or that developed prognostic models including PROMs as predictors. We designed, conducted the review in accordance with the JBI’s guidance for conducting systematic scoping reviews and reported the review in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [19, 20].
Database search strategy
We systematically searched Medline and Embase via Ovid, CINAHL Plus and Scopus on Aug 9th, 2021. Based on terms used by other published reviews on related topics [21,22,23], our search syntax combined keywords and MeSH terms related to ‘NSCLC’, ‘PROMs’ and ‘prediction or prognosis’ (see full search syntax in Appendix 1). To complement our electronic search, we manually searched the reference lists of all included studies and relevant reviews.
Inclusion criteria for relevant studies
The inclusion criteria for assessing the relevance of studies were structured according to the Population, Concept and Context framework for scoping reviews [19]. We deemed articles relevant if (1) the proportion of adults ≥ 18 years old with NSCLC in the study population was 50% or higher, (2) any generic, cancer-specific or lung cancer-specific PROMs or their components were independent variables or predictors, (3) the study entailed prognostic prediction of any future outcome at the individual level, (4) it was a prognostic factor study or prediction model development or validation study, (5) it was a published original study in English with full-text available, including full-text conference papers and (6) published after 2011, as survival of patients with NSCLC improved since owing to recent advances in treatment [24,25,26]. Appendix 2 presents a detailed overview of inclusion and exclusion criteria.
Screening and study selection
After removing duplicates, all titles and abstracts were screened independently by two reviewers (KL, TW, JC), with KL screening all and TW and JC each screening half. For potentially relevant studies identified by screening titles and abstracts, two reviewers (KL and TW) assessed the full text independently and in duplicate. Discrepancies were resolved through consensus and discussed with a third reviewer (SNVDV), if needed. Reasons for exclusion were recorded for the full-text screening stage only. Both titles and abstracts and full text screening were conducted using Rayyan (https://www.rayyan.ai/).
Extracting and charting the results
For data charting, we were interested in what and how PROMs had been collected, what outcomes were evaluated in association with PROMs or predicted by PROMs, and what statistical methods had been used. For this, we used the items suggested by the Cochrane Prognosis Methods Group (https://methods.cochrane.org/prognosis/tools) and a similar prognostic scoping review in another clinical area [27]. We organised, categorised and charted data as follows: characteristics of the study, characteristics of PROMs as predictors, characteristics of outcomes, and statistical methods. Specific data items and their definition are listed in Appendix 3. Two reviewers (KL and SNVDV) tested the data charting form by independently charting the data from a randomly selected eligible study. Two reviewers (KL and TW) charted data independently and in duplicate. They resolved discrepancies in the charted data through discussion and discussed with a third reviewer (MS) if needed. All the data were charted in Excel spreadsheets.
Collating, summarising, and reporting the results
The extracted evidence was repeatedly reviewed in the process of collating and summarising the findings. Results were synthesised through a descriptive numerical summary analysis to present an overview of the current evidence on the value of PROMs used as prognostic factors.
Risk of bias assessment
To provide an overview of the quality of available research on the prognostic value of PROMs, we critically appraised included studies using one of two well-established risk of bias tools. For prognostic factor studies, we used the QUality In Prognosis Studies (QUIPS) tool recommended by the Cochrane Prognosis Methods Group [28] and rated the overall risk of bias in each study following the procedure suggested by Grooten and colleagues [29]. For prognostic model development studies, we applied the risk of bias assessment section of the Prediction model Risk Of Bias ASsessment Tool (PROBAST) [30]. KL appraised all studies, with TW appraising a randomly selected 25% in duplicate. Inter-rater agreement was calculated as the percentage of the agreed opinion on the domain level. The inter-rater agreement was 70% and achieved full inter-rater agreement after discussion of discrepancies with the third reviewer. We used the robvis web tool (https://mcguinlu.shinyapps.io/robvis/) [31] to plot risk of bias summary graphs.
Results
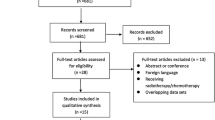
Figure 1 shows that our search yielded 2,769 unique titles, of which 97 articles were eligible for full-text screening. The most common reasons for excluded full-text articles were the wrong publication type (n = 27) and the lack of PROMs as predictors (n = 21). Finally, we included 33 articles, reporting the results of 31 unique studies and 33 unique prognostic factor analyses and prediction models (referred to in the remainder of the manuscript as ‘analyses and models’).
Characteristics of included studies
Table 1 shows the characteristics of the 31 included studies [15, 16, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The majority of studies were prognostic factor studies (n = 27, 88%), had a study population consisting of NSCLC patients only (n = 23, 74%), and used data from an observational study (n = 18, 58%). The median sample size was 237.5 (range, 35 to 6290). There were no studies externally validating a prediction model. Study-level information on characteristics is available in Appendix 4.
Characteristics of PROMs as predictors in analyses and models
Figure 2 shows the 33 analyses and models (from 31 studies) used a total of 17 different PROMs [15, 16, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)– Core 30 (EORTC QLQ-C30) was most commonly used (n = 16, 48%), followed by the EORTC QLQ-Lung cancer module (EORTC QLQ-LC13) (n = 9, 27%) and the 36-item Short Form Health Survey SF-36 (n = 7, 21%).
PROMs used as predictors in prognostic factor analyses and prognostic models (n = 33). More than one PROMs could be used in an analysis or model. EORTC QLQ-C30, The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30; EORTC QLQ-LC13, EORTC Quality of Life Questionnaire–Lung Cancer Module; SF-36, 36-item Short Form Health Survey; FACT-L, Functional Assessment of Cancer Therapy–Lung; HADS, Hospital Anxiety and Depression Scale; LCSS, Lung Cancer Symptom ScaleEQ-5D, EuroQol-5D; EQ-VAS, EuroQol-Visual Analogue Scale; FACT-G, Functional Assessment of Cancer Therapy–General; PTGI, Posttraumatic Growth Inventory; ADL, Katz’s Activities of Daily Living, DT, Distress Thermometer; FACT-Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity Questionnaire; MDASI-LC, M. D. Anderson Symptom Inventory - Lung Cancer; PHQ-9, Patient Health Questionnaire-9; SF-12, 12-item Short Form Health Survey; SOBQ, The University of California San Diego Shortness of Breath Questionnaire.
Table 2 summarises the characteristics of PROMs that were used. Most analyses and models included generic PROMs as predictors (n = 18, 55%), followed by the lung-cancer specific PROMs (n = 16, 48%). HRQOL was the most investigated aspect, measured in nearly all included analyses and models (n = 31, 94%). The majority of the analyses and models applied the subdomain scores (n = 18, 54%) of PROMs and collected PROMs only once at baseline (i.e. preoperative, pretreatment, or at enrolment; n = 24, 73%). Information at the analysis/model level is available in Appendix 5.
Characteristics of predicted outcomes
Almost all analyses and models (n = 32, 97%) aimed to predict overall survival. Only one study focused on predicting self-rated health status (as measured by the EuroQol-Visual Analogue Scale and the EORTC global health status), which were measured 1-year postoperatively [47]. The median follow-up time for these studies ranged from 180.3 days to 4.4 years, and the maximum length of observation ranged from 381 days to 12 years. One model had progression-free survival and treatment-related adverse events as secondary outcomes [44]. Appendix 6 contains analysis/model-level information on the characteristics of predicted outcomes.
Statistical methods using for prognostic modelling using PROMs
Table 3 summarises the characteristics of statistical methods used for analyses and models. Almost all analyses and models (n = 31, 93.9%) fitted a multivariable model. The Cox proportional hazards regression was the most used predictive modelling technique (n = 31, 93.9%). Over half of the analyses and models did not report the selection procedure of predictors in multivariable models (n = 17, 52%). Information on the analysis/model-level is presented in Appendix 7. Figure 3 shows the types of covariates that were included in analyses and models in addition to PROMs, with demographics (n = 30, 91%) and tumour characteristics (n = 23, 70%) being most common. Other types of covariates that were adjusted for in at least in two analyses/models were comorbidities, smoking status, physiological measurements, genetic biomarkers, and complications. Detailed information of covariates on the level of analysis and models can be found in Appendix 7.
Risk of bias assessment
Figure 4 summarises the risk of bias in the included prognostic factor studies (n = 27). Overall, only 1 (4%) had a low risk of bias, whereas 23 (85%) had a high risk of bias, mainly due to bias of handling the existing prognostic factors, of which almost all the studies (n = 26, 96%) had moderate to high bias, except for the study by Greer and colleagues [42]. The vast majority had moderate to high risk of biases due to prognostic factor measurement (n = 23, 85%) and attrition (n = 18, 67%). The results of the risk of bias appraisal at the prognostic factor study level are presented in Appendix 8.
Summary of risk of bias in prognostic factor studies (n = 27). Plotted by the robvis web tool (https://mcguinlu.shinyapps.io/robvis/)
The risk of bias in the prognostic model development studies (n = 4) is presented in Fig. 5. The overall risk of bias in all four was high due to the high risk of bias in the statistical analysis, which was usually a result of not reporting the overfitting and optimism in model performance.
Summary of risk of bias in prediction model development studies (n = 4). Plotted by the robvis web tool (https://mcguinlu.shinyapps.io/robvis/)
Discussion
This scoping review found that PROMs for prognostic prediction in adults with NSCLC were investigated in a substantial number of studies. The majority were prognostic factor studies, while a few developed prognostic models; no external validation study was identified. Cancer-specific PROMs, such as the EORTC QLQ-C30, and those measured at baseline and summarised as a subdomain score were most frequently used as predictors. Almost all studies used multivariable Cox proportional hazards regression to predict overall survival only, whereas prediction of other clinical outcomes appeared underinvestigated. The risk of bias in included studies was mostly moderate to high.
We found that the EORTC QLQ-C30 was the most frequently used PROMs for prognostic predicting in patients with NSCLC. This finding was consistent with previous reviews [17, 61,62,63]. This may be explained by the fact that the EORTC QLQ-C30 is also the most commonly used PROM as part of the clinical management of lung cancer [6], meaning that it is a well-established PROM for which routine collection is more likely to be available.
With only one high-quality prognostic factor study identified in this review [36], more high-quality research is warranted to examine the prognostic value of PROMs. Future prognostic studies need to report how the prognostic factors are selected. In the event that factors are selected automatically (data-driven), the method for factor selection should be reported, ideally avoiding univariable selection methods. [64, 65]. Additionally, further studies need to take into account the risks associated with the categorising or dichotomising continuous predictors, which leads to loss of information and increases the risk of false positives [66].
The four prognostic models using PROMs as predictors in our review were all only internally validated [32, 37, 56, 57]. This finding is consistent with previous reviews suggesting that the vast majority of the prognostic models using PROMs as predictors in oncology lack external validation [17, 63, 67]. External validation is imperative because good performance during development is not guaranteed when transporting a model to other settings [68]. This lack of external validation limits the clinical implementation and application of PROM-based prognostic models, leaving their potential value for enhancing clinical decision-making untapped.
Previous reviews highlighted the significance of PROMs in the prediction of the overall survival in patients with cancer [17, 63]. Although we did not limit the present review to survival as the predicted outcome, we only identified one study that predicted self-reported health status one year after surgery in patients with NSCLC [47]. Survival is not the only outcome considered relevant when making treatment decisions in patients with NSCLC [69]. This warrants an increased effort to predict a wider range of outcomes in addition to survival, such as treatment response [70], treatment toxicity [71], and early treatment discontinuation [72], which in turn would better support patients and doctors in making complex treatment decisions.
This scoping review has several limitations. Firstly, our search strategy needed to balance sensitivity (or comprehensiveness) against specificity (or precision). So, although we had a comprehensive search strategy based on previously published reviews, some relevant studies may have been missed. further studies of interest may have been missed because we excluded those reported as conference abstracts, in non-peer reviewed journals or in a non-English language. Secondly, this review did not distinguish between cancer stages, despite prognosis of patients with early/locally advanced stages NSCLC being different to that of patients with advanced stages [73, 74]. Therefore, we cannot draw conclusions on if and how the prognostic value of PROMs has been investigated differs between stages of NSCLC.
Conclusion
This scoping review identified an emerging body of research how PROMs have been used as a prognostic factor for predicting a range of clinical outcomes in patients with NSCLC but the methodological quality of this research is poor. This warrants more robust studies investigating the prognostic value of PROMs, in particular for predicting outcomes other than survival. This will enable further development of PROM-based prediction models to support clinical decision making in NSCLC.
Data availability
The data supporting the conclusions of this study are included within this article and its supplementary information files.
Abbreviations
- EORTC QLQ-C30 :
-
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30.
- EORTC QLQ-LC13 :
-
EORTC QLQ-Lung cancer 13.
- HRQOL :
-
Health-related quality of life.
- NSCLC :
-
Non-small cell lung cancer.
- PROBAST :
-
Prediction model risk of bias assessment tool.
- PROMs :
-
Patient-reported outcome measures.
- QUIPS :
-
Quality in prognosis studies.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer. J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85. https://doi.org/10.5334/aogh.2419.
Cooley ME. Symptoms in Adults with Lung Cancer: A Systematic Research Review. J Pain Symptom Manag. 2000;19:137–53. https://doi.org/10.1016/S0885-3924(99)00150-5.
Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–8. https://doi.org/10.2147/OTT.S100685.
Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015;350:g7818. https://doi.org/10.1136/bmj.g7818.
Bouazza YB, Chiairi I, El Kharbouchi O, De Backer L, Vanhoutte G, Janssens A, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer. 2017;113:140–51. https://doi.org/10.1016/j.lungcan.2017.09.011.
Øvretveit J, Zubkoff L, Nelson EC, Frampton S, Knudsen JL, Zimlichman E. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care. 2017;29:874–9. https://doi.org/10.1093/intqhc/mzx108.
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. JCO. 2016;34:557–65. https://doi.org/10.1200/JCO.2015.63.0830.
Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, et al. Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. JNCI: J Natl Cancer Inst. 2017;109:djx029. https://doi.org/10.1093/jnci/djx029.
Schmidt K, Damm K, Prenzler A, Golpon H, Welte T. Preferences of lung cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care (Engl). 2016;25:580–91. https://doi.org/10.1111/ecc.12425.
Moons KGM, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. https://doi.org/10.1136/bmj.b375.
Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595. https://doi.org/10.1136/bmj.e5595.
Kent P, Cancelliere C, Boyle E, Cassidy JD, Kongsted A. A conceptual framework for prognostic research. BMC Med Res Methodol. 2020;20:172. https://doi.org/10.1186/s12874-020-01050-7.
Craddock M, Crockett C, McWilliam A, Price G, Sperrin M, van der Veer SN, et al. Evaluation of Prognostic and Predictive Models in the Oncology Clinic. Clin Oncol (R Coll Radiol). 2022;34:102–13. https://doi.org/10.1016/j.clon.2021.11.022.
Sloan JA, Zhao X, Novotny PJ, Wampfler J, Garces Y, Clark MM, et al. Relationship Between Deficits in Overall Quality of Life and Non–Small-Cell Lung Cancer Survival. J Clin Oncol. 2012;30:1498–504. https://doi.org/10.1200/JCO.2010.33.4631.
Ediebah DE, Coens C, Zikos E, Quinten C, Ringash J, King MT, et al. Does change in health-related quality of life score predict survival? Analysis of EORTC 08975 lung cancer trial. Br J Cancer. 2014;110:2427–33. https://doi.org/10.1038/bjc.2014.208.
Efficace F, Collins GS, Cottone F, Giesinger JM, Sommer K, Anota A, et al. Patient-Reported Outcomes as Independent Prognostic Factors for Survival in Oncology: Systematic Review and Meta-Analysis. Value in Health. 2021;24:250–67. https://doi.org/10.1016/j.jval.2020.10.017.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. https://doi.org/10.1186/s12874-018-0611-x.
Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evid Implement. 2015;13:141–6. https://doi.org/10.1097/XEB.0000000000000050.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–73. https://doi.org/10.7326/M18-0850.
Zhou L, Wang X-L, Deng Q-L, Du Y-Q, Zhao N-Q. The efficacy and safety of immunotherapy in patients with advanced NSCLC: a systematic review and meta-analysis. Sci Rep. 2016;6:32020. https://doi.org/10.1038/srep32020.
Nama V, Nordin A, Bryant A. Patient-reported outcome measures for follow‐up after gynaecological cancer treatment. Cochrane Database of Systematic Reviews. 2013. https://doi.org/10.1002/14651858.CD010299.pub2.
Ingui BJ, Rogers MAM. Searching for Clinical Prediction Rules in Medline. J Am Med Inform Assoc. 2001;8:391–7. https://doi.org/10.1136/jamia.2001.0080391.
Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond). 2018;18:s41–6. https://doi.org/10.7861/clinmedicine.18-2s-s41.
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. https://doi.org/10.21037/tlcr.2016.06.07.
Qin H, Wang F, Liu H, Zeng Z, Wang S, Pan X, et al. New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res. 2018;10:2234–45.
Jenkins DA, Mohamed S, Taylor JK, Peek N, Veer SN van der. Potential prognostic factors for delayed healing of common, non-traumatic skin ulcers: A scoping review. Int Wound J. 2019;16:800–12. https://doi.org/10.1111/iwj.13100.
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing Bias in Studies of Prognostic Factors. Ann Intern Med. 2013;158:280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
Grooten WJA, Tseli E, Äng BO, Boersma K, Stålnacke B-M, Gerdle B, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—aspects of interrater agreement. Diagn Prognostic Res. 2019;3:5. https://doi.org/10.1186/s41512-019-0050-0.
Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann Intern Med. 2019;170:51–8. https://doi.org/10.7326/M18-1376.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods n.d.;n/a. https://doi.org/10.1002/jrsm.1411.
Agarwal JP, Chakraborty S, Laskar SG, Mummudi N, Arora J, Badhe R, et al. Prognostic value of a patient-reported functional score versus physicianreported Karnofsky Performance Status Score in brain metastases. Ecancermedicalscience. 2017;11:779.
Arraras JI, Hernandez B, Martinez M, Cambra K, Rico M, Illarramendi JJ, et al. Quality of Life in Spanish advanced non-small-cell lung cancer patients: determinants of global QL and survival analyses. SpringerPlus 2016;5.
Arrieta O, Angulo LP, Núñez-Valencia C, Dorantes-Gallareta Y, Macedo EO, Martínez-López D, et al. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann Surg Oncol. 2013;20:1941–8. https://doi.org/10.1245/s10434-012-2793-5.
Barney BJ, Wang XS, Lu C, Liao Z, Johnson VE, Cleeland CS, et al. Prognostic value of patient-reported symptom interference in patients with late-stage lung cancer. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 2013;22:2143–50.
Braun DP, Gupta D, Staren ED. Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer. 2011;11:353. https://doi.org/10.1186/1471-2407-11-353.
Brunelli A, Salati M, Refai M, Xiumé F, Berardi R, Mazzanti P, et al. Development of a patient-centered aggregate score to predict survival after lung resection for non–small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146:385–90.e2. https://doi.org/10.1016/j.jtcvs.2013.04.007.
Eser S, Göksel T, Erbaycu AE, Baydur H, Başarık B, Yanık A, et al. Comparison of generic and lung cancer-specific quality of life instruments for predictive ability of survival in patients with advanced lung cancer. SpringerPlus. 2016;5:1833. https://doi.org/10.1186/s40064-016-3492-7.
Fernando HC, Landreneau RJ, Heron DE, Daly BDT, Mandrekar SJ, Hillman SL, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: Results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg. 2015;149:718–26.
Fiteni F, Vernerey D, Bonnetain F, Vaylet F, Sennélart H, Trédaniel J, et al. Prognostic value of health-related quality of life for overall survival in elderly non-small-cell lung cancer patients. Eur J Cancer. 2016;52:120–8. https://doi.org/10.1016/j.ejca.2015.10.004.
Friis RB, Hjøllund NH, Pappot H, Taarnhøj GA, Vestergaard JM, Skuladottir H. Patient-Reported Outcome Measures Used in Routine Care Predict for Survival at Disease Progression in Patients With Advanced Lung Cancer. Clin Lung Cancer. 2021;22:e169–79. https://doi.org/10.1016/j.cllc.2020.09.014.
Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Gallagher ER, et al. Perceptions of health status and survival in patients with metastatic lung cancer. J Pain Symptom Manag. 2014;48:548–57.
Gupta D, Braun DP, Staren ED. Association between changes in quality of life scores and survival in non-small cell lung cancer patients. Eur J Cancer Care. 2012;21:614–22.
Hopkins AM, Wagner J, Modi N, Rowland A, Sorich MJ, Kichenadasse MJ. O http://orcid.org/0000-0003-1999-866X Ganessan AO-Hopkins, Ashley M; ORCID: http://orcidorg/0000-0001-7652-4378 AO-Sorich. Patient-reported outcomes as a prognostic marker of survival in patients with advanced nonsmall cell lung cancer treated with immunotherapy. Int J Cancer. 2020;147:3085–9.
Jeon H, Eo W, Shim B, Kim S, Lee S. Prognostic Value of Functional Assessment of Cancer Therapy-General (FACT-G) in Advanced Non-Small-Cell Lung Cancer Treated with Korean Medicine. Evidence-Based Complementary & Alternative Medicine (ECAM) 2020:1–9.
Kerstjens HAM, Hiltermann TJN, Geerse OP, Brandenbarg D, Berendsen AJ, Duijts SFA, et al. The distress thermometer as a prognostic tool for one-year survival among patients with lung cancer. Lung Cancer. 2019;130:101–7.
Kobayashi K, Nakaoka K, Yanagihara T, Ueda S, Saeki Y, Maki N, et al. Preoperative predictors of restoration in quality of life after surgery for lung cancer. Thorac Cancer. 2021;12:835–44.
Lemonnier I, Guillemin F, Arveux P, Clement-Duchene C, Velten M, Woronoff-Lemsi MC, et al. Quality of life after the initial treatments of non-small cell lung cancer: a persistent predictor for patients’ survival. Health Qual Life Outcomes. 2014;12:73. https://doi.org/10.1186/1477-7525-12-73.
Li T-C, Li C-I, Tseng C-H, Lin K-S, Yang S-Y, Chen C-Y, et al. Quality of life predicts survival in patients with non-small cell lung cancer. BMC Public Health. 2012;12:790.
Moller A, Sartipy U. Quality of life six months after lung cancer surgery is associated with long-term survival. Acta Oncol. 2012;51:1029–35.
Movsas B, Hu C, Sloan J, Bradley J, Komaki R, Masters G, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016;2:359–67. https://doi.org/10.1001/jamaoncol.2015.3969.
O’Mahony S, Nathan S, Bonomi P, Batus M, Fidler MJ, Wells K, et al. Survival Prediction in Ambulatory Patients With Stage III/IV Non-Small Cell Lung Cancer Using the Palliative Performance Scale, ECOG, and Lung Cancer Symptom Scale. Am J Hosp palliat Care. 2016;33:374–80.
Pinheiro LC, Reeve BB. Investigating the prognostic ability of health-related quality of life on survival: a prospective cohort study of adults with lung cancer. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2018;26:3925–32. https://doi.org/10.1007/s00520-018-4265-3.
Pinheiro LC, Zagar TM, Reeve BB. The prognostic value of pre-diagnosis health-related quality of life on survival: a prospective cohort study of older Americans with lung cancer. Qual Life Research: Int J Qual Life Aspects Treat Care Rehabilitation. 2017;26:1703–12. https://doi.org/10.1007/s11136-017-1515-7.
Pompili C, Salati M, Refai M, Berardi R, Onofri A, Mazzanti P, et al. Preoperative quality of life predicts survival following pulmonary resection in stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;43:905–10.
Schild SE, Tan AD, Wampfler JA, Ross HJ, Yang P, Sloan JA. A new scoring system for predicting survival in patients with non-small cell lung cancer. Cancer Med. 2015;4:1334–43.
Sim J, Kim YA, Kim JH, Lee JM, Kim MS, Shim YM, et al. The major effects of health-related quality of life on 5-year survival prediction among lung cancer survivors: applications of machine learning. Sci Rep. 2020;10:10693. https://doi.org/10.1038/s41598-020-67604-3.
Spigel DR, Patel JD, Reynolds CH, Garon EB, Hermann RC, Govindan R, et al. Quality of life analyses from the randomized, open-label, phase III PointBreak study of pemetrexed-carboplatin-bevacizumab followed by maintenance pemetrexed-bevacizumab versus paclitaxel-carboplatin-bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10:353–9. https://doi.org/10.1097/JTO.0000000000000277.
Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015;54:340–8. https://doi.org/10.3109/0284186X.2014.953259.
Yun YH, Kim YA, Sim JA, Shin AS, Chang YJ, Lee J, et al. Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer. 2016;16:505. https://doi.org/10.1186/s12885-016-2504-x.
Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–63.
Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:1–21.
Mierzynska J, Piccinin C, Pe M, Martinelli F, Gotay C, Coens C, et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol. 2019;20:e685–98. https://doi.org/10.1016/S1470-2045(19)30656-4.
Heinze G, Dunkler D. Five myths about variable selection. Transpl Int. 2017;30:6–10. https://doi.org/10.1111/tri.12895.
Heinze G, Wallisch C, Dunkler D. Variable selection - A review and recommendations for the practicing statistician. Biom J. 2018;60:431–49. https://doi.org/10.1002/bimj.201700067.
Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080.
Mallett S, Royston P, Waters R, Dutton S, Altman DG. Reporting performance of prognostic models in cancer: a review. BMC Med. 2010;8:21. https://doi.org/10.1186/1741-7015-8-21.
Ramspek CL, Jager KJ, Dekker FW, Zoccali C, van Diepen M. External validation of prognostic models: what, why, how, when and where? Clin Kidney J. 2020;14:49–58. https://doi.org/10.1093/ckj/sfaa188.
Cramer-van der Welle CM, van Loenhout L, van den Borne BE, Schramel FM, Dijksman LM. “Care for Outcomes”: systematic development of a set of outcome indicators to improve patient-relevant outcomes for patients with lung cancer. BMJ Open. 2021;11:e043229. https://doi.org/10.1136/bmjopen-2020-043229.
Li H, Galperin-Aizenberg M, Pryma D, Simone CB, Fan Y. Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiother Oncol. 2018;129:218–26. https://doi.org/10.1016/j.radonc.2018.06.025.
Yin J-Y, Li X, Li X-P, Xiao L, Zheng W, Chen J, et al. Prediction models for platinum-based chemotherapy response and toxicity in advanced NSCLC patients. Cancer Lett. 2016;377:65–73. https://doi.org/10.1016/j.canlet.2016.04.029.
Pennock MM, Halmos B, Iii WRB, Cheng H, Gucalp R, Ohri N. Predictors of Early Durvalumab Discontinuation After Chemoradiotherapy for Non-Small Cell Lung Cancer. Int J Radiation Oncology*Biology*Physics. 2021;111:e448–9. https://doi.org/10.1016/j.ijrobp.2021.07.1265.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–21.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237. https://doi.org/10.1093/annonc/mdy275.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study concept and design: KL, DCW, FG, CFF, MS, JY and SNVDV; searching databases: KL and SNVDV, screening and study selection: KL, TW, JCM and SNVDV, data charting: KL, TW and SNVDV, critical appraisal: KL, TW and MS, writing—original draft: KL, writing—review and editing: KL, DCW, FG, CFF, MS, JY and SNVDV.
The corresponding author confirms that all listed authors meet the authorship criteria, and that no other eligible authors have been omitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, K., Wang, T., Coomber-Moore, J. et al. Prognostic value of patient-reported outcome measures (PROMs) in adults with non-small cell Lung Cancer: a scoping review. BMC Cancer 22, 1076 (2022). https://doi.org/10.1186/s12885-022-10151-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10151-z