Abstract
Background
This French nationwide NETSARC exhaustive prospective cohort aims to explore the impact of systematic re-excision (RE) as adjuvant care on overall survival (OS), local recurrence free survival (LRFS), and local and distant control (RFS) in patients with soft tissue sarcoma (STS) with positive microscopic margins (R1) after initial resection performed outside of a reference center.
Methods
Eligible patients had experienced STS surgery outside a reference center from 2010 to 2017, and had R1 margins after initial surgery. Characteristics and treatment comparisons used chi-square for categorical variables and Kruskall-Wallis test for continuous data. Survival distributions were compared in patients reexcised (RE) or not (No-RE) using a log-rank test. A Cox proportional hazard model was used for subgroup analysis.
Results
A total of 1,284 patients had experienced initial STS surgery outside NETSARC with R1 margins, including 1,029 patients with second operation documented. Among the latter, 698 patients experienced re-excision, and 331 were not re-excised. Characteristics were significantly different regarding patient age, tumor site, tumor size, tumor depth, and histotype in the population of patients re-excised (RE) or not (No-RE). The study identified RE as an independent favorable factor for OS (HR 0.36, 95%CI 0.23–0.56, p<0.0001), for LRFS (HR 0.45, 95%CI 0.36–0.56, p<0.0001), and for RFS (HR 0.35, 95%CI 0.26–0.46, p<0.0001).
Conclusion
This large nationwide series shows that RE improved overall survival in patients with STS of extremities and trunk wall, with prior R1 resection performed outside of a reference center. RE as part of adjuvant care should be systematically considered.
Level of evidence II
Similar content being viewed by others
Introduction
Soft tissue sarcomas (STS) are a heterogenous group of malignant tumors gathering over 155 histotypes and molecular subtypes that constitute approximatively 1% of all malignancies [1]. Extremities and trunk-wall locations are the most frequent location of STS [2]. En-bloc surgical resection with clear margins (R0) after review by a multidisciplinary tumor board (MDTB) in an expert center is the mainstay of curative treatment [3]. Nevertheless even in expert centers, this objective is not always achievable for all patients: positive microscopic margins (R1) are reported in 16 to 34% of the cases in specialized centers [4,5,6] and in up to 70% in non-specialized centers [7].
In case of unplanned macroscopically complete resection outside of a specialized center, re-excision (RE) followed by radiotherapy is generally considered [3, 8]. Most studies reported that RE improves local tumor control, and better local and distant relapse free survival (LRFS) [5, 9,10,11,12,13]. Whether RE impact overall survival (OS) is conversely debated, raising the issue of surveillance measures or a more aggressive approach with systematic RE. Based on the indirect correlation between re-excision of residual tumor in tumor bed and progression and/or survival, several studies reported that patients benefit from RE [5, 9,10,11,12] after unplanned resection while others did not evidence that residual tumor in tumor bed re-excision was associated with improved disease specific survival,[13] distant metastasis risk, and overall survival [14] or reported similar overall survival (OS) in patients with unplanned initial resection re-operated or not [15].
While patients operated in high-volume multidisciplinary sarcoma centers have better outcome [16], many patients are initially operated out of a sarcoma center and the question of systematic re-operation is still debated, considering the inconsistent impact of RE on overall survival across series. We used the French nationwide prospective database NETSARC to assess overall survival (OS), local and distant relapse free survival (RFS), and local control (LRFS) in patients with R1 margins who had been operated for a trunk wall and limb soft tissue sarcoma outside of a reference center who experienced re-excision (RE) or not (no-RE).
Material and patients
Objectives
This study aims to assess the overall survival in patients with STS of the extremities or the trunk wall, who experienced initial surgery with R1-margins, performed outside of the French nationwide NETSARC reference centers, reexcised (RE) or not (No-RE). Secondary objectives included local and distant relapse free survival (RFS) and local recurrence free survival (LRFS). The data-collection and analysis received approval from the national Advisory Committee on Information Processing in Health Research (Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé, CCTIRS) n°10.403, September 16, 2010, and from the French data protection authority (Commission Nationale Informatique et Liberté, CNIL), n° 910390, July 15, 2013.
Patients’ selection
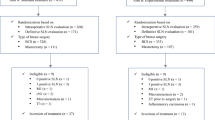
The study enrolled patients with localized STS of trunk and limbs prospectively registered in the NETSARC database between 07/2010 and 12/2017, with specified R1 margins after initial surgery performed outside a NETSARC reference center. Desmoid, well differentiated (atypical lipomatous tumors), dermato-fibrosarcoma protuberans were excluded because they are rarely life-threatening diseases. Patients with metastasis at diagnosis or unknown initial metastatic status were also excluded (Fig. 1).
The affiliation of the first surgeon was collected and categorized within or outside a NETSARC reference center; patients were considered as operated in a NETSARC reference center if the surgeon was registered in NETSARC network (https://NetSarc.sarcomabcb.org), and conversely, as operated in a non-expert center if the surgeon was not referenced in the NETSARC network.
NETSARC network and database
The French nationwide reference network for clinical and pathological sarcoma care NETSARC supported by the French Institute of Cancer (INCa) set up a nationwide database currently considered to be close to exhaustivity of all STS in France [2]. All sarcoma including suspicion for sarcoma are presented and reviewed by a multidisciplinary tumor board (MDTB) involving the 26 French cancer centers and registered at first presentation in a database by a dedicated team of clinical research assistants, at any time of the disease course (before diagnosis, before any treatment, after primary surgery, before adjuvant therapy, at the date of oncologic event or/and clinical trial screening).
In France, for each operated patient regardless of the institution, a centralized review with double-interpretation is deemed mandatory and pathological reports encourage the clinicians to present each case to MDTB. Thus, data from patients operated in or outside of NETSARC reference center network are collected in NETSARC database.
The database includes patient and tumor characteristics, surgery, relapse and survival. The wider tumor diameter defined tumor size. The National Federation of Cancer Centres (FNCLCC, Unicancer) specified 4 categories for histological grades: grade 1, 2, 3, and ungraded tumors. Sarcomas without grading resulted from histology grading failure or lack of critical elements to complete the diagnosis, as determined by experts.
The quality of surgical resection used the definition of the Union Internationale Contre le Cancer (UICC) [17], and margin status determination is based on pathology and surgery reports when available: R0 referred to clear margins – in the present study R0 margins qualified a monobloc resection and clear margins specified on pathological report; R1 margins referred to (possible) microscopic residual disease, with visible tumor cells on resection margins (positive microscopic margins) – in the present study R1 margins indicated margins not confirmed as R0 or R2. R2 resulted from fragmented resections, or operative/pathological reports suggesting or notifying macroscopic residual tumor and/or fragmented resection; cases with no margin characterization were excluded (missing data) (Fig. 1). Patients referred after first surgery, with any residual tumor, hematoma, and scar track are generally examined by magnetic resonance imaging and identification of all pathologic features (diagnosis and margins), primary surgical procedure, pre- (if available) and post-operative imaging, and patient general assessment are performed.
Statistical method
Qualitative variables were described with frequencies and percentages, and quantitative variables with average and range. Comparisons between groups used the chi-square test for qualitative variables and Kruskall-Wallis test for quantitative variables.
The diagnosis date was the date of pathological diagnosis (biopsy or first surgery). Overall survival (OS) was defined as the time from the date of diagnosis to the date of the last follow-up or death due to any cause. Local and/or distant relapse free survival (RFS) was defined as the time from the date of diagnosis to the date of last follow-up or the date of first local progression, metastatic progression, or death, whichever occurred first. Local relapse free survival (LRFS) was computed from the diagnosis date to the date of last follow-up or the date of first local progression. OS and RFS were calculated using the Kaplan-Meier method. Duration of follow-up was estimated using the reverse Kaplan-Meier method and expressed with Q1-Q3 interval. Survival distributions were compared between groups using the Log–rank test and the multivariate analysis used the Cox proportional hazard model. Competing events to local recurrence are considered in a competing risk approach to estimate LRFS. The cumulative incidence function and non-parametric Gray’s test were used to estimate and to compare cumulative incidence function between the groups. Univariate and multivariate analysis explored whether first resection outside NETSARC impacted OS, RFS, and LRFS in R1 patients. Multivariate analysis used a Fine-Gray model [18], and included usual prognostic factors for sarcoma.
Sub-group analyses explored whether RE may benefit to specific subgroups of patients.
RE status was not available for 255 patients with R1 margins. Considering that all re-excision of R1 patients were carried out in NETSARC reference centers, missing data regarding RE status were considered as missing not at random. Characteristics of patients with no specified margin status are presented in Supplementary material S1 and a sensitivity analysis was performed considering these patients as not reoperated in Supplementary material S2.
A propensity score matching analysis was carried out and presented in supplementary material S3.
The cut-off date for data analysis was 2020, Novembre 9. Analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA) and significance for all statistical tests was evaluated using two-sided p values.
Results
Patients’ characteristics
Among the 1284 (68.3%) patients operated outside NETSARC centers with specified R1 margins at initial surgery, a total of 1029 had re-excision information available, 698 patients were re-excised (RE) and 331 patients had no re-excision (No-RE) (Fig. 1, Table 1)
Impact of RE on overall survival (OS) (Fig. 2A, Table 2)
The median follow-up between the two groups was similar (RE: 32.95, 9.1-59.5; No-RE: 30.58, 7.52-63.24 months). In univariate analysis, RE was associated with an improved OS (HR 0.33, 95%CI 0.22–0.49), p<0.0001). OS also significantly correlated with age at diagnosis (p<0.0001), tumor size (p <0.0001), tumor grade (p=0.00), histotype (p=0.03), and tumor location (p=0.02). The multivariate analysis identified RE as an independent favorable prognostic factor for OS (HR 0.36, 95%CI 0.23–0.56, p<0.0001), along with age at diagnosis, and tumor size, site, grade, and histotype (Table 2).
Impact of RE on local and/or distant Relapse Free Survival (RFS) (Fig. 2B)
RFS was significantly better in RE patients in univariate analysis (HR 0.43 95%CI 0.35–0.52, p<0.0001) and significantly associated with lower age at diagnosis, smaller size and depth of the tumor, lower grade, and histotype. The multivariate analysis showed that RE was independently associated with a better RFS (HR 0.45 95%CI 0.36–0.56, p<0.0001) along with age at diagnosis, tumor size, grade, and histotype (Table 2).
Impact of RE on Local Recurrence Free Survival (LRFS) (Fig. 2C)
The univariate analysis showed that patients with a first R1 resection outside NETSARC, and re-excision had a significantly better LRFS (HR 0.35, 95%CI 0.27–0.44, p<0.0001). Age at diagnosis, tumor size, grade and histotype associated with LRFS (Table 2). Multivariate analysis showed a significantly better LRFS for RE patients (HR 0.35, 95%CI 0.26–0.46, p<0.0001). Age at diagnosis, tumor size, and histotype significantly associated with LRFS (Table 2).
Sub-group analysis survival
A sub-group analysis explored the potential benefit of RE in specific patient subgroups. RE is associated with a significantly lower mortality risk regardless tumor location (depth, site), grade, and size (Fig. 3).
Sensitivity analysis
The characteristics of all the patients with R1 margins (N=1,284), including the 255 patients with no RE status available are presented in Supplementary material S1. Assuming the patients with RE status non available as not having been reoperated, the univariate analysis showed that OS was similar in the patients with RE status missing and No-RE patients (Supplementary material S2); in the multivariate analysis, RE remained a favorable prognostic factor for OS after adjustment on major prognosis factors (HR 0.36, 95% 0.23–0.56, p<0.0001 (Supplementary material S4).
Discussion
Our results issued from the French nationwide prospective database NETSARC registering all sarcoma and connective tissue tumors since 2010 show a significantly improved OS, RFS, and LRFS with a median follow-up of 31 months in patients with an initial R1 resection conducted outside of a reference center for a limb or trunk wall soft tissue sarcoma, and RE as part of adjuvant treatment. The benefit of RE on OS is observed in almost all subgroup of patients i.e. all other thing being equal, meaning regardless of age, tumor size (deep/superficial seated), location (lower/upper or trunk), grade (1/2 or 3), and histology. Our series is the first and largest so far to our knowledge, using direct comparison of prospectively registered patients with and without RE after a first surgery outside of a reference center, and prompt us to systematically consider RE in patients with potential microscopic margins (R1) initially operated outside a reference center.
Once unplanned resection has been carried out, there is a general consensus in the literature for the need of further resection to remove the potential residual tumor and achieve resection with appropriate margins in order to improve oncologic outcome, i.e. local control and disease specific survival. Most of the authors recommend RE based on the high incidence (31 to 72%) of residual tumor in the re-excision specimen [9, 10, 13, 19,20,21,22,23]. Residual tumor is considered as an unfavorable prognosis factor, with impact on local recurrence [19], but also recurrence free survival, metastasis free survival, and overall survival [5, 9,10,11, 24], as recently reported in the systematic review of Sacchetti and colleagues [25].
In addition, RE has also been recommended based on equivalent or even better oncologic outcome in patients reexcised after unplanned resection compared with patients with only primary resection. Several subsequent studies showed similar or even better control of local recurrence, metastasis free survival, and survival [9, 13, 26] with re-excision of unplanned first surgery compared with one stage surgery [27,28,29].
Overall, most studies indirectly support the idea that RE after unplanned resection improves not only local tumor control but also disease-free specific survival whereas some studies questioned the association between RE and OS or distant metastasis, and would consider the option of postponing RE. Meanwhile, Lewis et al. reported no correlation between residual tumor on re-excision specimen and disease specific or recurrence free survival in a series of 407 re-excised sarcomas [13]. Recently, Danieli and colleagues also showed that a residual disease in the RE tumor-bed was not associated with higher risk of distant metastasis and lower OS in a large cohort of patients surgically treated from 1997 to 2017 [14], and authors proposed to consider postponing reexcision after macroscopic complete unplanned excision until local recurrence occurs, on a case-by-case basis.
Decanter et al. recently investigated systematic RE after unplanned resection of extremities and superficial trunk STS in patients first operated out of reference centers [15], and reported that systematic RE in sarcoma specialized centers offered better local control but did not impact OS. However, results were issued from a different study population including 395 (70%) R0 patients and 168 (29%) R1 patients after first surgery. Indeed, the present study included only confirmed sarcomas with R1 margins after first resection; R2 and unknown margins resections, as well as tumors of intermediate malignancy, atypical lipomatous tumors, dermato-fibrosarcoma protuberans tumors, desmoid tumors and patients with metastasis at diagnosis were excluded. This highly selected population of R1-patients and not all unplanned surgical procedures carried out outside reference centers as usually reported in the whole literature, is particularly appropriate to report RE benefit in patients at higher risk.
The present study does not allow to conclude that all patients with R1 resection initially operated outside reference centers might be re-operated, and better identification of subgroups of patients for whom RE should be recommended, or conversely discouraged, is required. The subgroup analysis conducted in RE patients to address this issue showed similar HR for all subgroups considering tumor depth, location, grade, and size. Nevertheless, exploration in patients with good prognosis (i.e. small and superficial and low-grade tumors) was limited by the too reduced sample size of patients and events (disease-related death), and further studies need to focus on this specific topic. So far, RE has to be discussed for all patients after unplanned R1 resection outside of NETSARC center.
In a reference center, R1-margins are mostly anticipated by a pre-treatment decided by MDTB; unexpected R1-margins rarely occur [30]; such cases likely translate tumoral biomarker of aggressiveness [31]. The signification of R1 surgery carried out of a reference center deems different: based on the improvement of LRFS, as well as RFS and OS after RE, R1 status would more likely be considered as a marker of inadequate surgery rather than a marker of aggressiveness or what we retrospectively considered as R1 margins includes in fact some R0 margins’ resections.
There are several limitations in the current study. Firstly, despite prospective data collection, this multicenter retrospective design leads to some selection biases that may affect results: RE decision and to what extent bed tumor should be re-excised is a critical process which is complex to track retrospectively; RE decision does not rely on the same arguments for all patients and all surgeons. The large sample size, the guidelines shared between centers may reduce, but not completely erase this bias. Secondly, we can not exclude that some patients failed to be referred to reference centers by clinicians, or to be registered by pathologist and ultimately missed. Nevertheless, the nationwide incidence of STS suggests that NETSARC network established a closest to exhaustive national collection from 2013 [2]. Thirdly, assessment of R1 margins relying on data from first pathology, surgical, macro- and microscopic analysis and discussion between surgeons and pathologist is a critical issue and increased accuracy would be expected [32]; Notably, uncertainty remains between R1 and R0 in case of thin margins [33], and margin classification outside of reference centers may be questionable. R2 margins-resections are easy to identify and rule out. In case of any doubt between R0 and R1, resection was considered R1. Finally, RE impact on OS, on local and distant recurrence, actually implies to consider the complete adjuvant treatment strategy and surveillance modalities associated with RE process, which were not captured in the present work. Nevertheless little and controversial impact of chemotherapy on oncologic outcome is reported in the literature, and radiotherapy is considered not to impact OS, the primary objective of this study. RE results must be assessed in the light of these consensus statements on adjuvant therapy [3, 8]. Finally, we relied on multivariate analysis to adjust for observable selection bias. A propensity score method confirming significant impact of RE on OS, RFS and LRFS has also been used to control the selection bias despite the literature reviews have reported equivalent results to traditional regression for eliminating the bias on observed variables (supplementary material S3). However, none of these methods consider the bias due to unobserved variables, i.e. not collected in the study [34].
To address the issue of RE after surgery out of a reference center, other nationwide studies from other countries are necessary. In parallel, commitment to continuous quality improvement for extensive data collection must be applied, namely access to reliable data with accurate margin status qualification from any operative and pathology reports will contribute to minimize missing data for patients treated outside of a reference center. Finally, earlier referral of patients prior to any surgery would ensure appropriate quality of information mandatory for more relevant in-depth studies.
In conclusion, the present study highlights the importance of re-excision as part of an “adjuvant” multidisciplinary treatment after R1 margins surgical treatment of a sarcoma of extremities and trunk wall outside of a sarcoma reference center to improve survival and reduce relapse. All subgroups of patients are eligible to discuss RE.
Availability of data and materials
The nationwide database NETSARC (netsarc.org) that support the findings of this study contains information that could compromise privacy of the research participants. The anonymised data sets are available upon reasonable request from the data protection officer of the Léon Bérard cancer center at DPD@lyon.unicancer.fr.
References
Fletcher CDM, Baldini EH, Blay JY, Gronchi A, Lazar AJ, Messiou C, Pollock RE, Singer SE. WHO classification of soft tissue tumours. Introduction. In: Soft Tissue and Bone Tumours. Who Classification of Tumours. 5h Edition. WHO Classification of Tumours Editorial Boards; 2020.
de Pinieux G, Karanian M, Le Loarer F, et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PloS One. 2021;16(2):e0246958. https://doi.org/10.1371/journal.pone.0246958.
Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2018, 29;(Suppl 4):iv268–9. https://doi.org/10.1093/annonc/mdy321.
Stoeckle E, Gardet H, Coindre J-M, et al. Prospective evaluation of quality of surgery in soft tissue sarcoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2006;32(10):1242–8. https://doi.org/10.1016/j.ejso.2006.05.005.
Stojadinovic A, Leung DHY, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235(3):424–34. https://doi.org/10.1097/00000658-200203000-00015.
Traub F, Griffin AM, Wunder JS, Ferguson PC. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer. 2018;124(19):3868–75. https://doi.org/10.1002/cncr.31648.
Blay J-Y, Soibinet P, Penel N, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(11):2852–9. https://doi.org/10.1093/annonc/mdx484.
von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2018;16(5):536–63. https://doi.org/10.6004/jnccn.2018.0025.
Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13(1):110–7. https://doi.org/10.1245/ASO.2006.03.030.
Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90(2):203–8. https://doi.org/10.1302/0301-620X.90B2.19760.
Rehders A, Stoecklein NH, Poremba C, Alexander A, Knoefel WT, Peiper M. Reexcision of soft tissue sarcoma: sufficient local control but increased rate of metastasis. World J Surg. 2009;33(12):2599–605. https://doi.org/10.1007/s00268-009-0262-5.
Scoccianti G, Innocenti M, Frenos F, et al. Re-excision after unplanned excision of soft tissue sarcomas: Long-term results. Surg Oncol. 2020;34:212–7. https://doi.org/10.1016/j.suronc.2020.04.026.
Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231(5):655–63. https://doi.org/10.1097/00000658-200005000-00005.
Danieli M, Barretta F, Fiore M, et al. Unplanned Excision of Extremity and Trunk Wall Soft Tissue Sarcoma: To Re-resect or Not to Re-resect? Ann Surg Oncol. 2021;28(8):4706–17. https://doi.org/10.1245/s10434-020-09564-6.
Decanter G, Stoeckle E, Honore C, et al. Watch and Wait Approach for Re-excision After Unplanned Yet Macroscopically Complete Excision of Extremity and Superficial Truncal Soft Tissue Sarcoma is Safe and Does Not Affect Metastatic Risk or Amputation Rate. Ann Surg Oncol. 2019;26(11):3526–34. https://doi.org/10.1245/s10434-019-07494-6.
Blay J-Y, Honoré C, Stoeckle E, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(7):1143–53. https://doi.org/10.1093/annonc/mdz124.
Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94(9):2511–6. https://doi.org/10.1002/cncr.10492.
Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5(3):47. https://doi.org/10.21037/atm.2016.08.62.
Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66(2):81–7 10.1002/(sici)1096-9098(199710)66:2<81::aid-jso2>3.0.co;2-h.
Grimer R, Parry M, James S. Inadvertent excision of malignant soft tissue tumours. EFORT Open Rev. 2019;4(6):321–9. https://doi.org/10.1302/2058-5241.4.180060.
Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol Off J Am Soc Clin Oncol. 1985;3(10):1344–8. https://doi.org/10.1200/JCO.1985.3.10.1344.
Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78(5):650–5. https://doi.org/10.2106/00004623-199605000-00003.
Venkatesan M, Richards CJ, McCulloch TA, et al. Inadvertent surgical resection of soft tissue sarcomas. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2012;38(4):346–51. https://doi.org/10.1016/j.ejso.2011.12.011.
Potter BK, Hwang PF, Forsberg JA, et al. Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. J Bone Joint Surg Am. 2013;95(20):e151. https://doi.org/10.2106/JBJS.L.01149.
Sacchetti F, Alsina AC, Morganti R, et al. Re-excision after unplanned excision of soft tissue sarcoma: A systematic review and metanalysis. The rationale of systematic re-excision. J Orthop. 2021;25:244–51. https://doi.org/10.1016/j.jor.2021.05.022.
Funovics PT, Vaselic S, Panotopoulos J, Kotz RI, Dominkus M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: A single institution experience with 682 patients. J Surg Oncol. 2010;102(6):626–33. https://doi.org/10.1002/jso.21639.
Manoso MW, Frassica DA, Deune EG, Frassica FJ. Outcomes of re-excision after unplanned excisions of soft-tissue sarcomas. J Surg Oncol. 2005;91(3):153–8. https://doi.org/10.1002/jso.20323.
Arai E, Nishida Y, Tsukushi S, Wasa J, Ishiguro N. Clinical and Treatment Outcomes of Planned and Unplanned Excisions of Soft Tissue Sarcomas. Clin Orthop. 2010;468(11):3028–34. https://doi.org/10.1007/s11999-010-1392-7.
Nakamura T, Kawai A, Hagi T, Asanuma K, Sudo A. A comparison of clinical outcomes between additional excision after unplanned and planned excisions in patients with soft-tissue sarcoma of the limb: a propensity matching cohort study. Bone Joint J. 2021;103-B(12):1809–14. https://doi.org/10.1302/0301-620X.103B12.BJJ-2021-0037.R1 PMID: 34847719.
Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001;83(8):1149–55. https://doi.org/10.1302/0301-620x.83b8.12028.
O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866–75. https://doi.org/10.1002/cncr.28793.
Stoeckle E, Coindre J-M, Kind M, Kantor G, Bui BN. Evaluating surgery quality in soft tissue sarcoma. Recent Results Cancer Res Fortschritte Krebsforsch Progres Dans Rech Sur Cancer. 2009;179:229–42. https://doi.org/10.1007/978-3-540-77960-5_14.
Lintz F, Moreau A, Odri G-A, Waast D, Maillard O, Gouin F. Critical study of resection margins in adult soft-tissue sarcoma surgery. Orthop Traumatol Surg Res OTSR. 2012;98(4 Suppl):S9–18. https://doi.org/10.1016/j.otsr.2012.04.006.
Shah B, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58(6):550–9.
Acknowledgement
The authors thank Sophie DARNIS PhD for helpful comments and valuable help for medical editorial assistance.
Funding
NETSARC (INCA & DGOS) and RREPS (INCA & DGOS), RESOS (INCA & DGOS) and LYRICAN (INCA-DGOS-INSERM 12563), Association DAM’s, Ensemble contre Le GIST, Eurosarc (FP7-278742), la Fondation ARC, Infosarcome, InterSARC (INCA), LabEx DEVweCAN (ANR-10-LABX-0061), Ligue de l’Ain contre le Cancer, La Ligue contre le Cancer, EURACAN (EC 739521) funded the study. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
All authors were involved in writing and reviewing the report and in making the decision to submit for publication. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NETSARC (netsarc.org) is a network of 26 reference sarcoma centers with specialized multidisciplinary tumor boards (MDTB) funded by the French NCI (INCa). Since 2010, presentation to MDTB and second pathological review are mandatory for sarcoma patients. This research was conducted in accordance with relevant guidelines and local regulation. Namely, the study received approval from the national Advisory Committee on Information Processing in Health Research (Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé (CCTIRS) n°10.403, on September 16, 2010, in accordance with the law n°94-548 (1st July 1994) and from the French data protection authority (Commission Nationale Informatique et Liberté, CNIL), n° 910390, on July 15, 2013, in accordance with the provisions of French Law of 6 January 1978 "Loi Informatique et Libertés", as amended. In accordance with the local regulation, the dataset of the study was pseudonymized. Anonymised data sets are available upon reasonable request from the data protection officer of the Léon Bérard cancer center at DPD@lyon.unicancer.fr.
Consent for publication
Not applicable.
Competing interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary material S1, S2, S3 and S4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gouin, F., Stoeckle, E., Honoré, C. et al. Overall survival in patients with re-excision of positive microscopic margins of limb and trunk wall soft tissue sarcoma operated outside of a reference center: a nationwide cohort analysis. BMC Cancer 22, 1034 (2022). https://doi.org/10.1186/s12885-022-10121-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10121-5