Abstract
Background
Trophoblast cell-surface antigen 2 (TROP2) is related to tumor proliferation enhancement and poor prognosis. An antibody targeting TROP2 was developed to treat metastatic triple-negative breast cancer (TNBC) which has a limited treatment modality. To characterize the TROP2 expressing tumors in TNBC, we analyzed TROP2 expression in three cohorts; (1) primary tumor without neoadjuvant chemotherapy, (2) primary tumor with neoadjuvant chemotherapy, and (3) metastatic tumor.
Methods
A total of 807 TNBC cases were evaluated for TROP2 immunohistochemical expression. We evaluated the TROP2 H-score distribution in the three cohorts. Tumors were divided into two groups based on TROP2 expression (high vs. low). We analyzed the relationship between clinicopathologic features and markers, including epidermal growth factor receptor, cytokeratin 5/6, p53, and Ki-67, and prognostic significance at high vs. low TROP2 expression.
Results
There was no difference in TROP2 H-score distribution between the three cohorts. Moderate-to-strong membranous expression of TROP2 in at least 10% of tumor cells was present in 662 cases (82.0%) in Cohort 1, 59 cases (89.4%) in Cohort 2, and 23 cases (88.5%) in Cohort 3. There was no significant difference in clinicopathologic features between high vs. low TROP2 in all cohorts. TROP2 H-score was an independent poor prognostic factor for overall survival in Cohort 3.
Conclusions
TNBC showed similar TROP2 expression regardless of neoadjuvant treatment or primary tumor/metastasis. Although the prognostic significance of TROP2 expression in metastatic TNBC has been revealed, further evaluation of the predictive value of TROP2 expression for targeted therapy is needed.
Similar content being viewed by others
Background
Breast cancer in women had the highest incidence rate (24.5%) and mortality rate (15.5%) of all cancers in 2020 [1]. Triple-negative cancer (TNBC) accounts for about 10–15% of all diagnosed breast cancer. This cancer lacks immunohistochemical expression of the estrogen receptor (ER) and progesterone receptor (PR) and does not overexpress human epidermal growth factor receptor 2 (HER2) immunohistochemically or in situ hybridization [2, 3]. TNBC tends to have larger tumors and more nodal metastasis than other subtypes. It has a bad prognosis with rapid distant recurrence [4]. The 5-year relative survival rate of TNBC is 77%, compared with 93% in other breast cancer subtypes [5]. TNBC is a heterogeneous disease that can be divided into four subtypes (luminal androgen receptor, basal-like immunosuppressed, basal-like immune-activated, and mesenchymal) by gene expression profile by Burstein et al. [6]. Because TNBC is negative for ER and HER2, it does not respond well to endocrine therapy and molecular targeted therapy, respectively. Chemotherapy is still the standard systemic treatment [7]. Especially in the case of metastatic TNBC, the median breast cancer-specific survival is only 12 months. New therapeutic agents are urgently needed [8].
Trophoblast cell-surface antigen 2 (TROP2) is one of the numerous targetable gene mutations or marker proteins studied in cancer therapy. TROP2 was discovered first in trophoblast cells as a surface marker. Gene TACSTD2 located on chromosome 1p32 encodes TROP2 [9, 10]. TROP2 consists of extracellular and transmembrane domains and a cytoplasmic tail [11]. Mitogen-activated protein kinase (MAPK) pathway is activated by TROP2 expression. Activated MAPK pathway leads to cancer cell proliferation, invasion, migration, and survival of cancer cells [12]. TROP2 is overexpressed in several carcinomas, such as colorectal, pancreatic, gastric, oral squamous cell carcinoma, ovarian, and breast cancers, compared with the corresponding normal tissue [13,14,15,16,17,18]. In these studies, carcinomas with high TROP2 expression showed poor prognosis. Sacituzumab govitecan which is an antibody–drug conjugate (ADC) consists of an anti-TROP2 antibody and a cytotoxic drug, SN-38. It eradicates TNBC in vitro and in vivo [19]. In a clinical trial of 108 metastatic TNBC patients who had received more than one treatment and Sacituzumab govitecan for metastatic TNBC, 33.3% of patients showed complete response or partial response [20]. Based on this result, sacituzumab govitecan-hziy (Trodelvy™) received standard approval in April 2021 for the treatment of metastatic TNBC in patients who have received more than one previous therapy. In previous research, 88% of the tumors showed moderate to strong TROP2 expression [21]. Although high TROP2 expression had association with improved progression-free survival, there was no control group and the sample size was small (n = 48). Confirmation of high TROP2 expression is not necessary for drug usage.
We tried to determine if primary tumors with or without neoadjuvant chemotherapy and metastatic tumors in TNBC have different TROP2 expression levels. We also examined the correlation between TROP2 expression and clinicopathologic features and conducted a survival analysis.
Materials and methods
Patients and clinical data
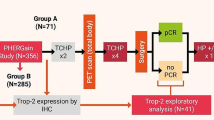
This study was done in three different TNBC cohorts. The first cohort (Cohort 1) comprised 715 patients who underwent surgical treatment for breast cancer and did not receive neoadjuvant chemotherapy between 2004 and 2011. The second cohort (Cohort 2) comprised 66 patients who had residual tumors after neoadjuvant chemotherapy and who underwent surgical treatment for residual breast cancer between 2011 and 2012. The third cohort (Cohort 3) comprised 26 patients who had surgery for metastatic breast cancer between 2001 and 2016 (Supplementary Table 1). All patients in Cohort 3 were diagnosed with metastasis after primary breast cancer, except for one patient who was diagnosed with metastasis of lung and primary breast cancer simultaneously. All patients were recruited from Asan Medical Center, and follow-up data was obtained. Paraffin blocks were obtained from the surgical specimens of all patients. Clinicopathologic data were obtained through review of medical records, including age at diagnosis, sex, tumor location, neoadjuvant chemotherapy, and death. Pathologic features (nuclear/histologic grade, TNM staging, lymphovascular invasion (LVI), tumor-infiltrating lymphocytes (TILs), epidermal growth factor receptor (EGFR), cytokeratin 5/6 (CK5/6), p53, and Ki-67 immunostains) were noted after pathologic review of the surgical specimens. TNBC diagnosis was based on immune-negative results in ER and PR, HER2 negative results in immunostains or silver in situ hybridization tests. The TIL level was calculated as the proportion of the area occupied by mononuclear inflammatory cells within the tumor stroma. For EGFR, membranous staining in more than 10% of tumor cells was considered positive. For CK5/6, any tumor cell being positive was interpreted as a positive. For p53, the percentage of positive tumor cells was classified into four hierarchical groups. For Ki-67, the percentage of positive tumor cells was classified into three hierarchical groups. Our study protocol was approved by the Institutional Review Board of Asan Medical Center (2013–0866).
Immunohistochemical staining and interpretation
Sections from tissue microarrays were immunostained using TROP2 rabbit monoclonal antibodies (EPR20043, Abcam, Cambridge, UK; dilution 1:2000). Immunostaining of the tumor specimens was performed using the autoimmunostainer Benchmark XT (Ventana Medical Systems, Tucson, AZ, USA) with Optiview Dab Detection Kit (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturers’ manuals and using the reagents provided in the kit.
In brief, sections of 4 μm were mounted on silanized slides and dried for 10 min at RT, followed by 20 min in an incubator at 65℃. Sections were conducted by HIER (CC1) for 32 min and incubated for 16 min with anti-TROP2 in the autoimmunostainer. Normal skin tissue was used for positive control and normal cerebral cortex was used for negative control of TROP2 immunostain (Supplementary Figure 1) [22, 23]. Immunostained slides were scanned on a PANNORAMIC 250 Flash III (3DHISTECH, Budapest, Hungary) with PANNORAMIC Scanner Software (3DHISTECH, Budapest, Hungary). TROP2 immunoexpression was evaluated in cells showing membranous expression. The score was obtained by using the semi-quantitative H-score method [24]. The staining intensity was as followed (0, no; 1 + , weak; 2 + , moderate; and 3 + , strong). The percentage of tumor cells showing expression was multiplied by each intensity group. The final scores were calculated by summing the values of each group. The mean TROP2 expression in the entire cohort (167) was used to discriminate between the TROP2 high group and low group.
Statistical analysis
All statistical analysis was conducted using SPSS 20.0 statistical software (SPSS Inc, Chicago, IL, USA). The Mann–Whitney U test was performed to compare the difference in TROP2 expression between two independent cohorts. The correlations between TROP2 expression and clinicopathologic features were analyzed using the χ2 test. Log-rank tests and Kaplan–Meier curves were used for comparing survival differences between the TROP2 high and low groups. Overall time was calculated from the date of primary tumor surgery to the date of death in Cohort 1 and Cohort 2. In Cohort 3, overall time was defined as duration calculated from the date of the metastatic lesion surgery to the date of death. Univariate regression analysis using the Cox proportional hazards model was applied for estimating the hazard ratios (HRs) of the clinicopathologic features and TROP2 expression. P < 0.05 was considered statistically significant.
Results
Clinicopathologic features and TROP2 immunoexpression in three TNBC cohorts
In Cohort 1, 714 women and one man ranged in age from 23–76 (median 47) years. In Cohort 2, 66 women ranged in age from 23–70 (median 40) years. In Cohort 3, 26 women ranged in age from 25–70 (median 48) years. In Cohort 1 and Cohort 2, all patients received a breast-conserving operation or mastectomy. In Cohort 3, all patients had surgical treatment of the metastatic site. In Cohort 1, TNBC histologic type was mostly invasive breast carcinoma of no special type (IBC-NST) (83.4%), followed by metaplastic carcinoma, and carcinoma with apocrine differentiation (9.2%, and 3.6%, respectively) based on the WHO classification of breast tumors, 5th edition. In Cohort 2, IBC-NST was 92.4% of TNBC, while the rest was metaplastic carcinoma (7.6%). In Cohort 3, all the TNBC was IBC-NST. The follow-up period ranged from 9–187 (median 128) months in Cohort 1, 12–102 (median 84) months in Cohort 2, and 5–113 (median 26) months in Cohort 3.
TROP2 expression was identified in the cellular membrane and cytoplasm of tumor cells. In previous studies, membranous TROP2 expression was related to an unfavorable prognosis in breast cancer. Membranous expression is relevant for the use of the ADC [15, 25]. Therefore, TROP2 expression was evaluated for membranous expression only, not for cytoplasmic expression (Fig. 1). The median [1st quartile, 3rd quartile] H-score was 180 [110, 220], 200 [150, 226], and 182 [130, 205] in Cohort 1, Cohort 2, and Cohort 3, respectively. A Mann–Whitney U test showed that H-score was not significantly different in the three cohorts (Fig. 2). Considering TROP2 expression in more than 10% of tumor cells is positive, moderate-to-strong intensity of TROP2 expression occurred in 662 cases (82.0%) in Cohort 1, 59 cases (89.4%) in Cohort 2, and 23 cases (88.5%) in Cohort 3 (Table 1a). When the expression was divided into positive or negative by 10% of any TROP2 expression intensity, positive staining was evident in 694 cases (97.1%) in Cohort 1, 65 cases (98.5%) in Cohort 2, and 24 cases (92.3%) in Cohort 3 (Table 1b).
The clinicopathologic features for the three cohorts are summarized in Table 2. TROP2 expression was not significantly associated with age, histologic subtype, nuclear and histologic grades, stage, LVI, or TIL levels.
Correlation between TROP2 expression and other markers (CK5/6, EGFR, p53, and Ki-67) in Cohort 1 and Cohort 2
CK5/6 and EGFR immunostains are commonly used as basal markers in TNBC. If EGFR or CK5/6 was positive, then it was considered a basal-like tumor [26]. In Cohort 1, the proportion of basal-like tumors was higher in the TROP2 low group (55.4%) than the high group (44.5%) (p = 0.004). In Cohort 2, the proportion of basal-like tumors was higher in the TROP2 low group (30.0%) than in the high group (25.0%), but this was not statistically significant (p = 0.675). When the tumor suppressor gene TP53 is mutated, mutant p53 protein can increase cell proliferation and survival, which contributes to tumor aggressiveness and metastatic potential [27]. Missense mutations can result in high p53 protein expression. This protein is not expressed in deletion mutations, however [28]. Ki-67 immunostain is frequently used as a cellular proliferation cancer marker. A study examining these markers and TROP2 in Cohort 1 and Cohort 2 (Table 3) demonstrated no correlation between them.
TROP2 as a prognostic factor of metastatic TNBC
To identify the prognostic value of TROP2 expression, we used log-rank test and the Kaplan–Meier method to analyze overall survival. The TROP2 high group showed poor OS in Cohort 3, but this was not statistically significant (p = 0.059) (Fig. 3).
Survival rate of each TNBC cohort. High expression of TROP2 was related to poor prognosis. In the three cohorts, there were no significant differences between TROP2 expression and survival rate. a Survival rate of Cohort 1 (p = .676, log-rank) b Survival rate of Cohort 2 (p = .627, log-rank) c Survival rate of Cohort 3 (p = .059, log-rank)
In the univariate Cox hazards model, TROP2 expression was not a statistically significant prognostic parameter for OS in either the H-score and high vs. low groups in Cohorts 1 and 2. In Cohort 3, TROP2 expression showed statistically significant unfavorable prognosis in H-score (HR = 1.010, 95% confidence interval (CI) = 1.001–1.020, p = 0.037) and trended towards an unfavorable prognosis in the high vs. low groups (HR = 2.593, 95% CI = 0.963–7.227, p = 0.069) (Table 4). Multivariate analysis including age and TILs demonstrated that TROP2 expression in the H-score group (HR = 1.009, 95% CI = 1.000–1.018, p = 0.047) was associated with poor overall survival in Cohort 3.
Discussion
In this study, we investigated TROP2 expression in three different TNBC cohorts. TROP2 expression acquired by H-score showed no difference in the distribution between the three TNBC cohorts. When TROP2 expression in at least 10% of tumor cells is considered as positive, percentage of dominant intensity as moderate to strong expression in the three cohorts was from 80.4% to 89.4%. These results were similar to another study of a metastatic TNBC cohort [21]. In that study, 88% had moderate to strong TROP2 expression. When the expression was divided into positive or negative according to the criteria of 10% of any staining intensity, the positivity was from 92.3% to 98.5%.
There was no difference in clinicopathologic features between the TROP2 high and low groups in primary TNBC tumors (Cohorts 1 and 2). However, in one study of TROP2 membranous expression in invasive ductal breast cancer, the high/low expression of TROP2 was related to histological grade, lymph node metastasis, distant metastasis, TNM staging, cyclin D1, and p53 status [15]. In another study, TROP2 expression was associated with TNBC in breast cancer [24].
TNBC can be divided into basal type and non-basal type subgroups. EGFR and CK5/6 were commonly used as basal markers, but there are no standardized immunohistochemical measurements of EGFR and CK5/6. As a result, EGFR and CK5/6 expression in TNBC ranged from 24–72% and 13–78%, respectively [29]. Although the prevalence of basal markers in TNBC is variable, EGFR had a significant independent prognostic value for disease-free survival in TNBC [30]. CK5/6 is related to a poor prognosis in TNBC [31]. We evaluated the relationship between basal type and TROP2 high and low groups. For tumors positive for EGFR and/or CK5/6, the TROP2 low group had higher ratio of basal type than the TROP2 high group (55.4% in the TROP2 low group and 44.5% in the TROP2 high group in Cohort 1; 30.0% in the TROP2 low group and 25.0% in the TROP2 high group in Cohort 2). While there were no reports on the association of TROP2 and basal markers in breast cancer, one study of lung adenocarcinoma found that the EGFR mutant was in 54% of the TROP2 low group and 46% of the TROP2 high group, but this was not statistically significant (p = 0.26) [32].
The TP53 mutation occurs in approximately 30% of breast cancer cases and 75%-80% of TNBC cases [33]. One study reported that TNBC was more prevalent in cases of high Ki-67 expression (82.5%) than non-TNBC (42.5%) (high expression; ≥ 20%) [34]. We evaluated the p53 and Ki-67 expression in the TROP2 high and low groups. There were no significant differences noted in Cohort 1 and Cohort 2.
TROP2 H-score was a statistically significant unfavorable prognostic marker in the metastatic TNBC cohort (Cohort 3), which had the smallest number of cases. In some studies, the high TROP2 expression group had a shorter survival time than the low group in breast cancer [24, 25]. TROP2 immunohistochemical overexpression has poor overall survival in many solid tumors, like the stomach, nasopharynx, gallbladder, and cervix [35].
Our study results may differ from other studies for several reasons. First, we analyzed only TNBC, but other studies analyzed all breast cancer subtypes. In one study comparing membranous and cytoplasmic TROP2 expression in TNBC and non-TNBC, TNBC had higher TROP2 expression than non-TNBC [24]. More research is needed to confirm the difference between TNBC and non-TNBC. Second, different antibodies have been used in various researches [15, 24, 25]. If a common antibody had been used instead, the results might be more comparable. Finally, we only used excisional specimens to construct the tissue microarray, which might have resulted in a bias.
Although there were some limitations in our study, this is the most comprehensive examination of TROP2 expression in TNBC. We assessed clinicopathologic features and prognosis but also common markers, such as EGFR, CK5/6, p53, and Ki-67. To the best of our knowledge, we compare the TROP2 expression in primary and metastatic TNBCs for the first time. Furthermore, this is the first assessment of TROP2 expression in patients with primary TNBC who received neoadjuvant chemotherapy. However, the results would be better if we analyzed primary and matched metastatic tumors and pre- and post-neoadjuvant chemotherapy tumors together.
A background study on TROP2 expression in a metastatic TNBC cohort receiving the TROP2 ADC reported that most tumors (88%) were moderately or strongly positive for TROP2 expression. This is a major reason that TROP2 expression is not a condition for using the drug [21]. In our study, 84.6% was a moderate-to-strong positive for TROP2 in the metastatic cohort. We conducted the study on only metastatic specimens in Cohort 3 and found that high TROP2 expression had a poor prognosis. In a previous study, a longer progression-free survival was observed in the moderate to strong TROP2 group, so the patients with high TROP2 expression can benefit from the drug. To accurately establish drug indications and usage guidelines, further research should clarify if patients with low TROP2 expression can benefit from the drug.
Conclusions
We showed that TROP2 expression of TNBC is similar regardless of neoadjuvant treatment or primary tumor/metastasis. TROP2 expression was revealed as a poor prognostic factor in metastatic TNBC, therefore some patients with high TROP2 expression may benefit from ADC for TROP2. Further evaluation of the predictive value of TROP2 expression and establishment of indication for targeted therapy should be performed.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TROP2:
-
Trophoblast cell-surface antigen 2
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- ADC:
-
Antibody–drug conjugate
- LVI:
-
Lymphovascular invasion
- TILs:
-
Tumor-infiltrating lymphocytes
- EGFR:
-
Epidermal growth factor receptor
- CK5/6:
-
Cytokeratin 5/6
- IBC-NST:
-
Invasive breast carcinoma of no special type
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–56.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34.
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–98.
Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25(Suppl 1):S142–50.
Van Mechelen M, Van Herck A, Punie K, Nevelsteen I, Smeets A, Neven P, et al. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res Treat. 2020;181(1):115–25.
Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981;78(8):5147–50.
Calabrese G, Crescenzi C, Morizio E, Palka G, Guerra E, Alberti S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet Cell Genet. 2001;92(1–2):164–5.
Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796(2):309–14.
Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253.
Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12(10):3057–63.
Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99(8):1290–5.
Lin H, Huang JF, Qiu JR, Zhang HL, Tang XJ, Li H, et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol. 2013;94(1):73–8.
Bignotti E, Todeschini P, Calza S, Falchetti M, Ravanini M, Tassi RA, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46(5):944–53.
Mühlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62(2):152–8.
Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21(2):186–91.
Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–512.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380(8):741–51.
Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2017;35(19):2141–8.
Ito T, Tanegashima K, Tanaka Y, Hashimoto H, Murata M, Oda Y, et al. Trop2 Expression in Extramammary Paget’s Disease and Normal Skin. Int J Mol Sci. 2021;22(14):7706.
Stepan LP, Trueblood ES, Hale K, Babcook J, Borges L, Sutherland CL. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59(7):701–10.
Zhao W, Kuai X, Zhou X, Jia L, Wang J, Yang X, et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep. 2018;40(2):759–66.
Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE. 2014;9(5):e96993.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74.
Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D’Incalci M, et al. Targeting triple negative breast cancer: is p53 the answer? Cancer Treat Rev. 2013;39(5):541–50.
Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24(9):1248–53.
da Silva JL, Cardoso Nunes NC, Izetti P, de Mesquita GG, de Melo AC. Triple negative breast cancer: A thorough review of biomarkers. Crit Rev Oncol Hematol. 2020;145:102855.
Liu D, He J, Yuan Z, Wang S, Peng R, Shi Y, et al. EGFR expression correlates with decreased disease-free survival in triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2012;29(2):401–5.
Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203(2):661–71.
Inamura K, Yokouchi Y, Kobayashi M, Ninomiya H, Sakakibara R, Subat S, et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget. 2017;8(17):28725–35.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Ilie SM, Bacinschi XE, Botnariuc I, Anghel RM. Potential clinically useful prognostic biomarkers in triple-negative breast cancer: preliminary results of a retrospective analysis. Breast Cancer (Dove Med Press). 2018;10:177–94.
Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, Lu PH. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci Rep. 2016;6:33658.
Acknowledgements
Not applicable.
Funding
No additional funding sources were used during this study.
Author information
Authors and Affiliations
Contributions
YJ, GG, and HJL conceived and designed the study. YJ, JH, GG, and HJL contributed to sample preparation and data check. YJ and HJL carried out the study and interpreted the data. YJ, UJ, and HJL drafted the manuscript. The authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures conducted in this study using human data were in accordance with the Declaration of Helsinki. Because this retrospective study utilized all patients’ information after thorough de-identification and had no harm to the involved patients, Institutional Review Board of Asan Medical Center (2013–0866) approved exemption from informed consent.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Clinicopathologic features in three different cohorts. Supplementary Figure 1. Representative TROP2 expression in normal skin (positive control) and normal cerebral cortex (negative control). Original magnification x400. a skin (TROP2 antibody), b cerebral cortex (TROP2 antibody), c skin (rabbit IgG), d cerebral cortex (rabbit IgG).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeon, Y., Jo, U., Hong, J. et al. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 22, 1014 (2022). https://doi.org/10.1186/s12885-022-10076-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10076-7