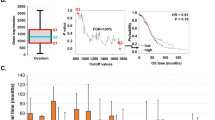
Abstract
Background
The BIRC5 gene encodes for the Survivin protein, which is a member of the inhibitor of apoptosis family. Survivin is found in humans during fetal development, but generally not in adult cells thereafter. Previous studies have shown that Survivin is abundant in most cancer cells, thereby making it a promising target for anti-cancer drugs and a potential prognostic tool.
Methods
To assess genetic alterations and mutations in the BIRC5 gene as well as BIRC5 co-expression with other genes, genomic and transcriptomic data were downloaded via cBioPortal for approximately 9000 samples from The Cancer Genome Atlas (TCGA) representing 33 different cancer types and 11 pan-cancer organ systems, and validated using the ICGC Data Portal and COSMIC. TCGA BIRC5 RNA sequencing data from 33 different cancer types and matching normal tissue samples for 16 cancer types were downloaded from Broad GDAC Firehose and validated using breast cancer microarray data from our previous work and data sets from the GENT2 web-based tool. Survival data were analyzed with multivariable Cox proportional hazards regression analysis and validated using KM plotter for breast-, ovarian-, lung- and gastric cancer.
Results
Although genetic alterations in BIRC5 were not common in cancer, BIRC5 expression was significantly higher in cancer tissue compared to normal tissue in the 16 different cancer types. For 14/33 cancer types, higher BIRC5 expression was linked to worse overall survival (OS, 4/14 after adjusting for both age and tumor grade and 10/14 after adjusting only for age). Interestingly, higher BIRC5 expression was associated with better OS in lung squamous cell carcinoma and ovarian serous cystadenocarcinoma. Higher BIRC5 expression was also linked to shorter progressive-free interval (PFI) for 14/33 cancer types (4/14 after adjusting for both age and tumor grade and 10/14 after adjusting only for age). External validation showed that high BIRC5 expression was significantly associated with worse OS for breast-, lung-, and gastric cancer.
Conclusions
Our findings suggest that BIRC5 overexpression is associated with the initiation and progression of several cancer types, and thereby a promising prognostic biomarker.
Similar content being viewed by others
Introduction
The BIRC5 (Baculoviral IAP Repeat Containing 5) gene located on chromosome 17 (17q25.3) encodes for the Survivin protein, which is a member of the IAPs (inhibitor of apoptosis family) that is normally expressed in humans during fetal development and in adult proliferating cells [1]. Survivin is a small protein with different isoforms, the majority of which are related to inhibition of apoptosis and promotion of cell proliferation [2]. Research during the past 20 years has shown that Survivin is highly expressed in most cancer cells [3, 4]. Although attempts have been made to develop small molecules targeting Survivin, there is no treatment currently in therapeutic use [5]. Recently, a study identified an association between BIRC5 expression and tumor-infiltrating lymphocytes (TILs) [6]. Moreover, we previously evaluated BIRC5 in breast cancer subtypes, thereby demonstrating that high BIRC5 expression is associated with worse prognosis in breast cancer patients [7].
Several studies have found that Survivin can be implicated in chemoresistance to platinum-based [8] or taxane-based [9] chemotherapy in ovarian cancer. In contrast, a previous study comparing Survivin expression in ovarian cancer patients (n = 435) treated with platinum/cyclophosphamide (PC) (n = 244) or taxane/platinum (TP) (n = 191) found that patients with high nuclear Survivin expression and an accumulation of TP53 in tumor cells that were treated with TP had a decreased risk of recurrence and death [10]. Furthermore, high nuclear Survivin expression and TP53 dysfunction had a higher likelihood of having high platinum sensitivity. A recent in vitro study on human cell lines of neuroendocrine tumors (NETs) showed increased BIRC5 expression in irradiated cells, additionally BIRC5 knockdown resulted in reduced cellular proliferation but not significantly increased radiosensitivity [11]. Kleinberg et al. [12] observed an association between high nuclear Survivin in tumor samples and improved progression-free survival (PFS) in chemotherapy-naïve patients. Expression analysis of the BIRC gene family in 30 patients with triple-negative breast cancer (TNBC) [13] identified higher gene expression of the BIRC gene family (including BIRC5) in patients (< 50 years old) with TNBC. In contrast, TNBCs with lymphovascular and fat tissue invasion had lower expression of BIRC genes. Although BIRC5 had the highest average expression of the tested genes, high BIRC5 expression had no significant association with tumor size. However, there was a significant difference in BIRC5 expression when comparing patients with no nodal metastasis (N0) with patients with micrometastases up to 1–3 axillary metastases and when comparing N0 with patients with 10 or more nodal metastases. There was also an association between histopathological grade in breast tumors and BIRC5 expression [13].
Copy number gains of three BIRC genes (BIRC2, BIRC3, and BIRC5) were identified in melanoma [14], while miR-195-5p/− 218-5p, and not genetic/epigenetic aberrations, was correlated in high BIRC5 levels in gastric cancer [15]. In the present study, we used publicly available -omics (genomics, transcriptomics) and survival data to examine BIRC5 genetic alterations and altered expression in 33 cancer types in relation to prognosis.
Methods
Data collection
cBioPortal for Cancer Genomics repository
The cBioPortal for Cancer Genomics repository [16,17,18] was first used to analyze multi-omics data from The Cancer Genome Atlas (TCGA Pancancer) [19] for the BIRC5 gene. Genomic and transcriptomic data from approximately 9000 samples representing 33 different cancer types and 11 pan-organ systems were analyzed (Table 1). Esophageal squamous and adenocarcinoma were combined into esophageal carcinoma. Colon and rectal carcinoma were combined into colorectal carcinoma in available genomic data from cBioPortal, resulting in 32 different tumor groups. First, BIRC5 gene alteration frequency was determined on the DNA level for the different cancer studies. Genetic alterations were subsequently divided into mutation, fusion, amplification, deep deletion, multiple alterations for 8812 samples from 32 different cancer types. From the same platform, we downloaded DNA amplification data for BIRC5 in the different cancer types.
The cBioPortal repository was then used to identify genes that were co-expressed with BIRC5 for 32 tumor types corresponding to 9351 samples (Table 1). Only data for esophageal adenocarcinoma (and not esophageal squamous carcinoma) were available for this analysis, while cervical squamous cell carcinoma and endocervical adenocarcinoma were both included under cervical squamous cell carcinoma (CESC). Spearman’s correlation was used to identify genes with mRNA expression that were significantly correlated with BIRC5 mRNA expression. Pathway analysis was then performed using Reactome [20, 21] with BIRC5 and the top 100 co-expressed genes for every tumor type.
Broad GDAC Firehose and UCSC Xena Browser
RNA sequencing (RNA-seq) data (UNC RNASeqV2 level 3 expression (normalized RSEM) for BIRC5 expression were downloaded from Broad GDAC Firehose [22] for 8526 TCGA tumor samples corresponding to the 33 different cancer types, as well as matching normal tissue samples (n = 627) for 16 cancer types (n = 5507; Table 1). Liu et al. recently compiled genomic and clinical data for the TCGA dataset into a standardized version called the TCGA Pan-Cancer Clinical Data Resource (TCGA-CDR) [23]. Therefore, we downloaded survival and phenotype data for the TCGA dataset from UCSC Xena Browser [24, 25] and from National Cancer Institute – Genomic Data Commons [23, 26]. Although the survival data included four clinical end points, i.e. overall survival (OS), disease-specific survival (DSS), disease-free interval (DFI) and progression-free interval (PFI), OS (the time from diagnosis to death of any cause) and PFI (the time from diagnosis to new tumor event, e.g. progression of disease, local recurrence, distant metastasis, new primary tumors, or died with the cancer without a new tumor event) were used in the present study as these were deemed to be relatively accurate endpoints by Liu et al.
External validation
External validation of the results found using TCGA data was performed in four steps.
-
1.
Genomics datasets: Cancer genomics datasets from the International Cancer Genome Consortium (ICGC) and Catalogue of Somatic Mutations in Cancer (COSMIC) genome version GRCh38 were analyzed to validate potential somatic mutations of ‘high mutation impact’ or ‘pathogenic’ in the BIRC5 gene [27,28,29,30].
-
2.
Transcriptomics datasets: Two RNA microarray datasets (Affymetrix HG-U133A and Affymetrix HG-U133 Plus 2) for BIRC5 were retrieved from the GENT2 web-based tool for cancer and corresponding normal tissues [31, 32]. Cancer types with less than 10 normal and/or cancer samples were removed from the analysis (i.e. Affymetrix HG-U133A: adrenal gland, bladder, cartilage, larynx, muscle, pharynx, small intestine, soft tissue, tongue, urothelium; Affymetrix HG-U133 Plus 2: bone, eye, gall bladder, lymph node, muscle, pharynx, placenta, spleen, teeth, testis, vagina). After filtering, the Affymetrix HG-U133A dataset included 21 cancer types comprised of 16,539 cancer samples and 4283 normal samples, while the Affymetrix HG-133 Plus 2 dataset included 25 cancer types comprised of 35,523 cancer samples and 5063 normal samples.
-
3.
Genomics and transcriptomics breast cancer dataset: DNA microarray, SNP genotyping, and RNA-seq data for breast cancer [33] were reevaluated from our previous work to identify DNA copy number alterations, exonic variants, and gene fusions. Additionally, survival analysis was performed using BIRC5 expression and OS.
-
4.
Survival analysis: Survival analysis for BIRC5 gene expression and OS was performed using the KM plotter web-based tool [34] with RNA microarray data for breast- [35], ovarian- [36], lung- [37], and gastric cancer [38]. The following settings were selected in KM plotter: (1) BIRC5 (Affymetrix probe 202094_at), (2) ‘auto select best cutoff’ to stratify the patient cohort, (3) OS endpoint, and (4) only ‘Jetset’ best probe set [39]. No cutoffs were made with regards to tumor subtype or treatment.
Statistical analysis
Statistical analyses were performed using R/Bioconductor 3.12 (BiocManager 1.30.12) in RStudio (version 1.3.1073), where p-value < 0.05 was considered to be statistically significant. BIRC5 expression in cancer samples was compared to expression in corresponding normal tissue. Tumor samples with no corresponding normal samples were removed from the analysis (Table 1). Boxplots were then generated with R packages ggpubr version 0.4.0 [40] and rstatix version 0.6.0 [41] using Wilcoxon test adjusted with Benjamini-Hochberg correction. DNA amplification data for BIRC5 from TCGA Pancancer was matched with RNA sequencing data from Broad GDAC Firehose using Wilcoxon test to determine the effect of DNA amplification on BIRC5 gene expression.
Multivariable Cox proportional hazards regression analysis was performed using R packages survival version 3.2–7 [42, 43], survminer version 0.4.9 [44], and Publish version 2020.12.23 [45]. Cox regression models were calculated using RNA sequencing data for BIRC5 expression with the OS and PFI endpoints, adjusting for age and/or tumor grade (if available). Only age was available for 21/33 cancer types (ACC, BRCA, COAD, DLBC, GBM, KICH, KIRP, LAML, LUAD, LUSC, MESO, PCPG, PRAD, READ, SARC, SKCM, TGCT, THCA, THYM, UCS, UVM), while both age and tumor grade were available for 12/33 cancer types (BLCA, CESC, CHOL, ESCA, HNSC, KIRC, LGG, LIHC, OV, PAAD, STAD, UCEC). Forest plots were generated using the R package forestplot version 1.10 [46]. Due to missing data, LAML was excluded in the PFI analysis. For the external breast cancer dataset [33], multivariable Cox regression models adjusted using age and tumor grade were calculated using BIRC5 expression and OS.
To identify clinicopathologic features that were associated with BIRC5 expression, BIRC5 expression was first categorized from RNA sequencing data as low BIRC5 (lower than median BIRC5 expression) and high BIRC5 (higher than median BIRC5 expression) by calculating the quantiles (0, 25, 50, 75, 100%) for BIRC5 expression; median BIRC5 expression (50%, quantile 2) was 0.4996274. Phenotype data were then retrieved for each cancer type from Xena Browser and matched with the RNA sequencing data in one file. Tableone script (version 0.13.0) in R was then used to identify clinicopathologic features associated with BIRC5 expression. However, 9/33 cancer types (COAD, DLBC, GBM, LAML, OV, READ, SKCM, TGCT, UCS) could not be analyzed in tableone due to that they only had samples with high BIRC5 expression.
Results
Genetic alterations in BIRC5 are relatively uncommon in cancer
To evaluate the prevalence of genetic alterations (i.e. inframe mutation, missense mutation, truncating mutation, fusion, amplification, and deep deletion) in the BIRC5 gene, genomic profiling data from cBioPortal were analyzed for 8812 cancer samples representing 32 different cancer types. BIRC5 was only found to be altered in 2% (n = 196) of cases, primarily DNA amplification (Fig. 1A). Uterine carcinosarcoma (UCS), invasive breast carcinoma (BRCA), liver hepatocellular carcinoma (LIHC), mesothelioma (MESO) and ovarian cancer (OV) had the highest alteration frequencies, i.e., 5.3, 4.2, 4.0, 3.5 and 3.1%, respectively. Deep deletions were more common in thymoma (THYM), but only 3/123 cases harbored genetic alterations in BIRC5 (2 cases with deep deletions and 1 case with DNA amplification). No genetic alterations were seen in adrenocortical carcinoma (ACC), cholangiocarcinoma (CHOL), diffuse large B-cell lymphoma (DLBC), kidney chromophobe (KICH), acute myeloid leukemia (LAML) or testicular germ cell tumors (TGCT). RNA expression levels were found to be higher for tumor samples with higher amplification of BIRC5 (P < 2.2e− 16; Fig. 1B).
Distribution of genetic alterations in the BIRC5 gene in 32 cancer types using the interactive web-based online tool cBioPortal (cbioportal.org). A Although only 196 of the 8812 cases (2%) had a gene alteration of any kind, DNA amplification was found to be most prevalent. Figure modified from cBioPortal [16, 17]. B Amplification of BIRC5 in relation to mRNA expression levels of BIRC5 (P < 2.2e-16)
To validate these findings, the ICGC Data Portal and COSMIC were used to identify somatic mutations of ‘high mutation impact’ or ‘pathogenic’ in the BIRC5 gene. ICGC data showed that eight patients affected by different cancer types harbored eight different BIRC5 mutations (Additional Table 1). Four of the eight cancer projects (BRCA, PRAD, SKCM, UCEC) were derived from TCGA data and the other four were from projects in China (colorectal cancer, COCA-CN; liver cancer, LICA-CN; nasopharyngeal cancer, NACA-CN) and Spain (chronic lymphocytic leukemia, CLLE-ES). For the TCGA data, high impact BIRC5 mutations were classified as missense for SKCM and UCEC and stop gain for BRCA and PRAD, which were in line with the findings in cBioPortal, BIRC5 mutations in the other datasets were classified as a frameshift mutation in COCA-CN, start loss mutation in LICA-CN, missense mutation in NACA-CN, and frameshift mutation in CLLE-ES. Furthermore, genome-wide screening data (array comparative genomic hybridization and SNP genotyping) were reevaluated from our previous work on breast cancer. DNA amplification in the BIRC5 gene was found in 15/229 (0.066%) breast cancer samples. None of the samples were shown to harbor deep deletions, mutations or fusions. COSMIC data revealed 210 unique cancer samples with somatic mutations in the BIRC5 gene, of which 33 were classified as pathogenic mutations (Additional Table 2). In total, 6 nonsense substitutions (breast, endometrium, hematopoietic and lymphoid, large intestine), 22 missense substitutions (breast, cervix, endometrium, esophagus, hematopoietic and lymphoid, kidney, large intestine, lung, prostate, skin, stomach, urinary tract), 3 synonymous substitutions (skin), and 1 unclassified mutation (head and neck) were identified. Eighteen of the 33 unique samples were derived from TCGA data.
BIRC5 levels are elevated in cancer compared to normal samples
To determine whether BIRC5 expression patterns differ in cancer and normal tissues, RNA-seq data was used for 16/33 TCGA cancer types (n = 5507) containing gene expression data for corresponding normal tissue (n = 627; Table 1). For all analyzed cancer types, BIRC5 levels were shown to be significantly higher in cancer tissue than corresponding normal tissues (Fig. 2A). These findings were confirmed using our previously published RNA microarray data for breast cancer, an Affymetrix HG-U133A RNA microarray dataset with 21 cancer types, and an Affymetrix HG-U133 Plus 2 RNA microarray dataset comprised of 25 cancer types (Fig. 2B-D). However, no statistically significant difference in expression patterns was found for heart and pancreas tissues in the Affymetrix HG-U133A dataset, as well as for adipose, endometrium, oral, and small intestinal tissues in the Affymetrix HG-U133 Plus 2 dataset. Moreover, normal samples derived from blood, prostate, testis, thyroid, and uterus also displayed significantly higher BIRC5 expression patterns than their tumor counterparts.
Box plots showing BIRC5 expression in (A) 16 different TCGA cancer types, (B) breast cancer compared to corresponding normal tissue. BIRC5 expression is significantly higher in all of the analyzed cancer types than normal tissue. C Affymetrix HG-U133A RNA microarray dataset with 21 cancer types, and (D) Affymetrix HG-U133 Plus 2 RNA microarray dataset comprised of 25 cancer types. BIRC5 expression is significantly higher in the majority of the analyzed cancer types than normal tissue. The Wilcoxon test was used to calculate statistically significant (Benjamini-Hochberg adjusted p-values) differences in BIRC5 expression between cancer and normal tissue. ns = not significant (P > 0.05); *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001
BIRC5 is frequently co-expressed with genes involved in cell cycle and DNA replication
To identify genes recurrently co-expressed with BIRC5 in cancer, the top 100 co-expressed genes in the 32 cancer types were extracted from the Spearman correlation analysis (Q < 0.05) in cBioPortal. When combining the top 100 co-expressed genes for each cancer type, some genes occurred more than once, e.g. AURKB (encoding for Aurora kinase B [47]) and CDC20 (encoding for Cell division cycle 20 [48]) were the most frequent among the combined list of 3200 genes. In total, 117/3200 genes were negatively correlated with BIRC5 and the remaining genes were positively correlated (Additional Table 3). When duplicates were removed and BIRC5 was included in the list, 629 genes remained. The Reactome Pathway Database was then used to identify signaling pathways associated with BIRC5 and the co-expressed genes. In total, 436/629 genes involving 1039 pathways were identified in Reactome, including pathways playing a pivotal role in cell cycle and DNA replication were found to be overrepresented (Fig. 3, Additional Table 3, Additional file 1).
Pathway analysis for genes co-expressed with BIRC5 using Reactome. Yellow depicts pathways containing genes co-expressed with BIRC5. Figure downloaded from www.reactome.org [20]
Survival analysis demonstrates the prognostic relevance of BIRC5 expression in cancer
To assess the prognostic significance of BIRC5 expression in the 33 cancer types, multivariable Cox regression analysis was performed for OS or PFI after adjusting for age and/or tumor grade. In total, 14/33 cancer types were found to be associated with more unfavorable OS in patients with tumor samples expressing BIRC5, whereas BIRC5 expression was linked to a protective effect in 2/33 cancer types (LUSC and OV; Fig. 4). After adjusting for both age and tumor grade, BIRC5 expression was found to have an adverse effect on OS in KIRC, LGG, LIHC, and PAAD (hazard ratio (HR) > 1, P < 0.05), whereas BIRC5 expression was associated with a protective effect in OV (HR = 0.86; 95% confidence interval (95% CI): 0.75–1.00; P = 0.047). After adjusting for age alone, HR > 1 (P < 0.05) were found for ACC, KICH, KIRP, LUAD, MESO, PCPG, PRAD, SARC, SKCM and UVM, with the highest HR values found for PCPG (HR = 4.46; 95% CI: 2.24–13.32) and ACC (HR = 2.91; 95% CI: 2.02–4.18). Moreover, adjusting for age showed that BIRC5 expression was associated with a protective effect in LUSC (HR = 0.90; 95% CI: 0.83–0.99). These data were validated using multivariable Cox regression analysis (adjusted for age and tumor grade) with RNA expression data from our previous work on breast cancer, as well as the web-based KM plotter tool for four different tumor types (breast-, ovarian-, lung-, and gastric cancer). The breast cancer dataset demonstrated that BIRC5 expression was significantly associated with adverse OS rates (BIRC5 probe ILMN_1803124: HR = 5.49, 95% CI: 2.32–12.97, P < 0.001; BIRC5 probe ILMN_2349459: HR = 1.85, 95% CI: 1.27–2.6, P = 0.0014), while the Kaplan-Meier analysis of the four cancer types revealed that low BIRC5 expression was significantly associated with better overall survival for breast-, lung and gastric cancer (Fig. 5).
Kaplan-Meier curves from the KM plotter web-based tool showing the association between BIRC5 expression and overall survival (OS) for (A) breast cancer, (B) ovarian cancer, (C) lung cancer, and (D) gastric cancer. BIRC5 expression was associated with significantly more unfavorable OS for breast cancer, lung cancer, and gastric cancer
Intriguingly, BIRC5 expression was only associated with significantly more unfavorable PFI in 14/33 cancer types, with 4/14 cancer types (KIRC, LGG, LIHC, and PAAD) after adjusting for both age and tumor grade and 10/14 cancer types (ACC, KICH, KIRP, LUAD, MESO, PCPG, PRAD, SARC, THCA and UVM) after adjusting for age (Fig. 6). The highest HR was seen for UVM (HR = 4.69; 95% CI: 2.24–9.82) and PCPG (HR = 4.34; 95% CI: 2.34–8.04).
Clinicopathological features and BIRC5 expression
We then determined a relationship between clinicopathological features and BIRC5 expression stratified as high BIRC5 (higher than median BIRC5 expression) and low BIRC5 (lower than median BIRC5 expression) expression. Intriguingly, it was apparent that most cancer samples were classified as BIRC5 high (Additional Table 4), with 9/33 tumor types (COAD, DLBC, GBM, LAML, OV, READ, SKCM, TGCT, UCS) only containing samples in the high BIRC5 group. In most, but not all, tumor types, we observed a trend that patients in the high BIRC5 group were generally younger at initial pathological diagnosis, e.g. ESCA (mean age 64.21 in high BIRC5 group vs 78.50 in low BIRC5 group; P = 0.001), KIRP (61.21 vs 64.48 years; P = 0.01), LGG (42.31 vs 47.83 years; P = 0.01), PCPG (44.15 vs 54.05 years; P < 0.001). Moreover, multiple cancer types were also found to be associated with BIRC5 expression levels and T stage (BLCA, BRCA, KIRC, LUAD, PAAD, PRAD, STAD), N stage (BRCA, ESCA, PRAD, THCA), M stage (ESCA, KICH, KIRP, LUAD, LUSC), tumor stage (ESCA, KIRC, KIRP, LIHC, PAAD, UCEC), tumor grade (ESCA, KIRC, KIRP, LIHC, PAAD, UCEC), therapy success (BLCA, KIRP, LUAD, LUSC, PAAD, PRAD, STAD), and race/ethnicity (BRCA, KIRP, LGG, LIHC, PRAD, STAD).
Discussion
Here, we applied a pan-cancer multiomics approach in 33 different cancer types to examine molecular mechanisms that can ultimately lead to the high BIRC5 gene expression patterns observed in cancer. We show that, although genetic alterations are uncommon in the BIRC5 gene, DNA amplification is associated with higher RNA levels of BIRC5. However, the clinical impact of genetic alterations such as DNA amplification in the BIRC5 gene is still unclear. In agreement with previous studies [3], our results also show that BIRC5 expression levels are higher in cancer tissue than normal tissue. In several different cancer types, we observe an association between higher BIRC5 expression and unfavorable OS. Taken together, our findings demonstrate the prognostic relevance of BIRC5 expression in a variety of cancer types from different organ systems.
The highest HR values for OS and BIRC5 expression were found for adrenocortical carcinoma (ACC) and pheochromocytoma and paraganglioma (PCPG), both of which are hormone-producing tumors [49]. A previous study using immunohistochemistry to evaluate Survivin levels in ACC samples revealed overexpression of Survivin in carcinomas compared to adenomas or normal glands, with worse prognosis for patients with tumors expressing higher Survivin levels (not statistically significant). Knockdown of Survivin in an ACC cell line resulted in higher apoptotic rates [50]. Another study comparing Survivin expression in healthy adrenal medulla and pheochromocytoma/paraganglioma (malignant and benign) showed no significant difference between malignant or benign tumors. However, a more recent study showed an association between increased Survivin expression and worse prognosis in pheochromocytoma [51]. For uveal melanoma where we show an association between BIRC5 expression and worse PFI, two previous studies showed conflicting results, one did not find any difference in immunohistochemical expression and tumor activity [52] and the other indicated the possible involvement of Survivin in Cisplatin-resistance using human uveal melanoma cell lines [53]. Our results show that high BIRC5 expression is associated with worse prognosis in all three analyzed types of kidney cancer. This is in line with previous results from a meta-analysis on 10 studies containing 1063 renal cancer cases, which demonstrated that high Survivin expression is associated with TNM stage and Fuhrman grade [54]. Other studies have also found a connection with more aggressive renal tumors and high Survivin expression [55,56,57]. Our study proposes Survivin/BIRC5 as a promising biomarker using RNA sequencing data. Survivin/BIRC5 could be an addition to other biological detection indicators. A study investigating cervical cancer cell lines found that Survivin showed more intense fluorescence in cancer cells than in normal cervical cells. Although the authors found Survivin to have a clinical sensitivity of 72.5% and a specificity of 77%, the sensitivity increased to 98% when combining Survivin with HPV16E6 and 96.1% when only using HPV16E6 [58].
Unlike the tumor types discussed above, higher BIRC5 expression seems to be beneficial for OS for patients with ovarian cancer. A previous study found an association between high Survivin and response to taxane-platinum treatment [10]. However, recently, two meta-analyses found that high Survivin expression in ovarian cancer is associated with poor prognosis and worse tumor stage [59, 60]. Further studies on BIRC5 expression/Survivin protein levels are needed in order to determine its prognostic significance for ovarian cancer or its possible connection to chemotherapy response. Interestingly, it has been shown that wild type of the tumor suppressor gene p53 could subdue Survivin expression [61], suggesting that non-functional p53 in cancer could result in higher Survivin expression. Similar conclusions were also determined in a recent study showing elevated Survivin expression in mice with p53-mutated esophageal squamous cell carcinoma, which could play a role in aiding lung metastasis [62]. A clinical study examined the genome and transcriptome of 198 lung squamous cell carcinomas and found that BIRC5 amplification was prevalent in tumors with p53 mutations [63].
There are several in vitro studies that show how BIRC5 overexpression or silencing could affect cancer cell lines. For instance, TP53 has been linked to BIRC5 in both glioblastoma multiforme (GBM) cells and 5-fluorouracil resistant cholangiocarcinoma (CHOL) cell lines [64, 65]. In vitro studies have suggested that BIRC5/Survivin could be implicated in chemotherapy resistance of Irinotecan in colon adenocarcinoma (COAD), Oxaliplatin in esophageal squamous and esophageal adenocarcinoma (ESCA) and Cisplatin in hepatocellular carcinoma (LIHC) [66,67,68]. In breast cancer cell lines, Survivin, as well as FOXM1 and XIAP have been shown to contribute to drug-resistance [69]. Silencing of Survivin in HeLa cells (cervical carcinoma cells) was shown to result in an increased sensitivity to radiation therapy [70]. Several studies on thyroid carcinoma (THYR) cell lines demonstrate the involvement of Survivin in inhibiting cell proliferation [71,72,73] and an in vivo study using human gastric adenocarcinoma cell lines in mice xenografts showed that inhibition of Survivin expression could promote cell death [74].
In conclusion, BIRC5 is indeed overexpressed in most cancer types, which frequently correlates with patient clinical outcome. Although publicly available TCGA data are useful for explorative pan-cancer studies, these findings need to be examined further in specific tumor types at the protein level to assess the clinical utility of BIRC5/Survivin. A limitation of the current study was the lack of large datasets similar to the TCGA dataset that contained both gene expression and clinical data to validate the prognostic relevance of BIRC5 expression in cancer. In future studies, it would also be interesting to evaluate the impact of BIRC5 expression levels on chemotherapy efficacy. In oncology, there is a constant need for better predictive markers in order to choose the right course of treatment [75]. Some treatment regimens are not only associated with acute toxicity, but also long-lasting chronic complications [76, 77]. Our study suggests BIRC5 as a promising prognostic biomarker for several cancer types, but these findings need to be investigated further.
Availability of data and materials
The data used in this study have already been deposited in Gene Expression Omnibus (accession GSE97293), as stated in our previous publication [33]. The data bases referenced in the methods section of this article are all open access.
References
BIRC5 - The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000089685-BIRC5. Accessed 21 Sep 2021.
Khan S, Ferguson Bennit H, Asuncion Valenzuela MM, Turay D, Diaz Osterman CJ, Moyron RB, et al. Localization and upregulation of survivin in cancer health disparities: a clinical perspective. Biologics. 2015;9:57–67.
Wheatley SP, Altieri DC. Survivin at a glance. J Cell Sci. 2019;132(7):jcs223826. https://doi.org/10.1242/jcs.223826.
Mittal RD, Jaiswal P, Goel A. Survivin: A molecular biomarker in cancer. Indian J Med Res. 2015;141(4):389.
Li F, Aljahdali I, Ling X. Cancer therapeutics using survivin BIRC5 as a target: what can we do after over two decades of study? J Exp Clin Cancer Res. 2019;38(1):368.
Xu L, Yu W, Xiao H, Lin K. BIRC5 is a prognostic biomarker associated with tumor immune cell infiltration. Sci Rep. 2021;11(1):390.
Oparina N, Erlandsson MC, Fäldt Beding A, Parris T, Helou K, Karlsson P, et al. Prognostic Significance of BIRC5/Survivin in Breast Cancer: Results from Three Independent Cohorts. Cancers. 2021;13(9):2209.
Chen L, Liang L, Yan X, Liu N, Gong L, Pan S, et al. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23(2):256–63.
Du J, Li B, Fang Y, Liu Y, Wang Y, Li J, et al. Overexpression of Class III β-tubulin, Sox2, and nuclear Survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC Cancer. 2015;15:536.
Felisiak-Golabek A, Rembiszewska A, Rzepecka IK, Szafron L, Madry R, Murawska M, et al. Nuclear survivin expression is a positive prognostic factor in taxane-platinum-treated ovarian cancer patients. J Ovarian Res. 2011;4(1):20.
Hanif A, Lee S, Gupta M, Chander A, Kannisto ED, Punnanitinont A, et al. Exploring the role of survivin in neuroendocrine neoplasms. Oncotarget. 2020;11(23):2246–58.
Kleinberg L, Flørenes VA, Silins I, Haug K, Trope CG, Nesland JM, et al. Nuclear expression of survivin is associated with improved survival in metastatic ovarian carcinoma. Cancer. 2007;109(2):228–38.
Makuch-Kocka A, Kocki J, Brzozowska A, Bogucki J, Kołodziej P, Płachno BJ, Bogucka-Kocka A. The BIRC Family Genes Expression in Patients with Triple Negative Breast Cancer. Int J Mol Sci. 2021;22(4):1820.
Sheen YS, Tan KT, Tse KP, Liao YH, Lin MH, Chen JS, et al. Genetic alterations in primary melanoma in Taiwan. Br J Dermatol. 2020;182(5):1205–13.
Zou J, Liao X, Zhang J, Wang L. Dysregulation of miR-195-5p/−218-5p/BIRC5 axis predicts a poor prognosis in patients with gastric cancer. J Biol Regul Homeost Agents. 2019;33(5):1377–85.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discovery. 2012;2(5):401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. https://doi.org/10.1126/scisignal.2004088.
cBioPortal. https://www.cbioportal.org. Accessed 13 May 2021 and 21 Feb 2022.
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45(10):1113–20.
Fabregat A, Sidiropoulos K, Viteri G, Forner O, Marin-Garcia P, Arnau V, et al. Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinformatics. 2017;18(1):142.
Reactome. https://reactome.org. Accessed 8 Oct 2021.
Broad GDAC Firehose. https://gdac.broadinstitute.org/. Accessed 11 May 2021.
Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell. 2018;173(2):400–416.e411.
UCSC Xena browser. https://xenabrowser.net/datapages/. Accessed 9 May 2021 and 20 Dec 2021.
Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8.
National Cancer Institute - Genomic Data Commons. https://gdc.cancer.gov/about-data/publications/PanCan-Clinical-2018. Accessed 9 May 2021.
ICGC Data Portal. https://dcc.icgc.org. Accessed 20 Feb 2022.
Zhang J, Bajari R, Andric D, Gerthoffert F, Lepsa A, Nahal-Bose H, et al. The International Cancer Genome Consortium Data Portal. Nat Biotechnol. 2019;37(4):367–9.
COSMIC - Catalogue Of Somatic Mutations In Cancer. https://cancer.sanger.ac.uk/cosmic. Accessed 23 and 24 Feb 2022.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2018;47(D1):D941–7.
GENT2 - Gene Expression database of Normal and Tumor tissues. http://gent2.appex.kr/gent2/. Accessed 23 Feb 2022.
Park SJ, Yoon BH, Kim SK, Kim SY. GENT2: an updated gene expression database for normal and tumor tissues. BMC Med Genet. 2019;12(Suppl 5):101.
Parris TZ, Rönnerman EW, Engqvist H, Biermann J, Truvé K, Nemes S, et al. Genome-wide multi-omics profiling of the 8p11-p12 amplicon in breast carcinoma. Oncotarget. 2018;9(35):24140–54.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. Kaplan Meier-plotter [Breast Cancer]. 2010.
Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. In., vol. 123: Springer; 2010. p. 725–31.
Gyorffy B, Lánczky A, Szállási Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19(2):197–208.
Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8(12):e82241.
Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7(31):49322–33.
Li Q, Birkbak NJ, Gyorffy B, Szallasi Z, Eklund AC. Jetset: Selecting the optimal microarray probe set to represent a gene. BMC Bioinformatics. 2011;12(1):474.
Kassambara A. ggpubr: 'ggplot2' Based Publication Ready Plots. R package version 0.4.0.; 2020.
Kassambara A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.6.0; 2020.
T T: A Package for Survival Analysis in R. R package version 3.2–7. 2020.
Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000.
Kassambara A, Kosinski M, Biecek P. survminer: Drawing Survival Curves using 'ggplot2'; 2021.
Gerds TA, Ozenne B. Publish: Format Output of Various Routines in a Suitable Way for Reports and Publication; 2021.
Gordon M, Lumley T. forestplot: Advanced Forest Plot Using 'grid' GraphicsR package version 1.10 edn; 2020.
AURKB - The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000178999-AURKB. Accessed 23 Sep 2021.
CDC20 - The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000117399-CDC20. Accessed 23 Sep 2021.
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–g34.
Sbiera S, Kroiss M, Thamm T, Beyer M, Majidi F, Kuehner D, et al. Survivin in adrenocortical tumors - pathophysiological implications and therapeutic potential. Horm Metab Res. 2013;45(2):137–46.
Qin ZK, Zhou FJ, Dai YP, Chen W, Hou JH, Han H, et al. Expression and clinical significance of survivin and PTEN in adrenal tumors. Ai Zheng. 2007;26(10):1143–7.
Ben Simon GJ, Abulafia A, Pe'er J. Apoptosis inhibitor, survivin, in posterior uveal melanoma: comparison among primary tumors, tumors resistant to brachytherapy, tumors with liver metastases, and liver metastases. Curr Eye Res. 2006;31(3):251–7.
Li H, Niederkorn JY, Neelam S, Alizadeh H. Downregulation of survivin expression enhances sensitivity of cultured uveal melanoma cells to cisplatin treatment. Exp Eye Res. 2006;83(1):176–82.
Ma C, Lu B, Sun E. Clinicopathological and prognostic significance of survivin expression in renal cancer patients: a meta-analysis. Postgrad Med J. 2017;93(1098):186–92.
Wang GC, Hsieh PS, Hsu HH, Sun GH, Nieh S, Yu CP, et al. Expression of cortactin and survivin in renal cell carcinoma associated with tumor aggressiveness. World J Urol. 2009;27(4):557–63.
Parker AS, Kosari F, Lohse CM, Houston Thompson R, Kwon ED, Murphy L, et al. High expression levels of survivin protein independently predict a poor outcome for patients who undergo surgery for clear cell renal cell carcinoma. Cancer. 2006;107(1):37–45.
Byun SS, Yeo WG, Lee SE, Lee E. Expression of survivin in renal cell carcinomas: association with pathologic features and clinical outcome. Urology. 2007;69(1):34–7.
Han S, Li L, Jia X, Ou W, Ma J, Wang H, et al. A molecular beacon-based method for screening cervical cancer. J Nanosci Nanotechnol. 2012;12(11):8282–6.
He X, Yang K, Wang H, Chen X, Wu H, Yao L, Ma S. Expression and clinical significance of survivin in ovarian cancer: A meta-analysis. PLoS One. 2018;13(5):e0194463. https://doi.org/10.1371/journal.pone.0194463.
Li XJ, Pang JS, Li YM, Ahmed FA, He RQ, Ma J, et al. Clinical value of survivin and its underlying mechanism in ovarian cancer: A bioinformatics study based on GEO and TCGA data mining. Pathol Res Pract. 2018;214(3):385–401.
Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21(17):2613–22.
Tang Q, Efe G, Chiarella AM, Leung J, Chen M, Yamazoe T, et al. Mutant p53 regulates Survivin to foster lung metastasis. Genes Dev. 2021;35(7–8):528–41.
Haakensen VA-O, Khadse A, Sandhu V, Halvorsen AA-O, Solberg SK, Jørgensen LH, Brustugun OT, Kure EH, Helland Å. Molecular characterisation of TP53 mutated squamous cell carcinomas of the lung to identify putative targets for therapy. Int J Cancer. 2020;147(10):2957–66.
Guvenc H, Pavlyukov MS, Joshi K, Kurt H, Banasavadi-Siddegowda YK, Mao P, et al. Impairment of glioma stem cell survival and growth by a novel inhibitor for Survivin-Ran protein complex. Clin Cancer Res. 2013;19(3):631–42.
Namwat N, Amimanan P, Loilome W, Jearanaikoon P, Sripa B, Bhudhisawasdi V, et al. Characterization of 5-fluorouracil-resistant cholangiocarcinoma cell lines. Chemotherapy. 2008;54(5):343–51.
Wallin A, Svanvik J, Holmlund B, Ferreud L, Sun XF. Anticancer effect of SN-38 on colon cancer cell lines with different metastatic potential. Oncol Rep. 2008;19(6):1493–8.
Ngan CY, Yamamoto H, Takagi A, Fujie Y, Takemasa I, Ikeda M, et al. Oxaliplatin induces mitotic catastrophe and apoptosis in esophageal cancer cells. Cancer Sci. 2008;99(1):129–39.
Or YY, Chow AK, Ng L, Fan ST, Yau TC, Poon RT, et al. Survivin depletion inhibits tumor growth and enhances chemosensitivity in hepatocellular carcinoma. Mol Med Rep. 2014;10(4):2025–30.
Nestal de Moraes G, Delbue D, Silva KL, Robaina MC, Khongkow P, Gomes AR, et al. FOXM1 targets XIAP and Survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015;27(12):2496–505.
Song H, Xin XY, Xiao F, Wang DT, Yue QH, Han X. Survivin gene RNA interference inhibits proliferation, induces apoptosis, and enhances radiosensitivity in HeLa cells. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):83–9.
Kim YJ, Hwang HJ, Kang JG, Kim CS, Ihm SH, Choi MG, et al. Enigma Plays Roles in Survival of Thyroid Carcinoma Cells through PI3K/AKT Signaling and Survivin. Anticancer Res. 2018;38(6):3515–25.
Li JY, Shi J, Sang JF, Yao YZ, Wang XC, Su L. Role of survivin in the pathogenesis of papillary thyroid carcinoma. Genet Mol Res. 2015;14(4):15102–11.
Le F, Luo P, Ouyang Q, Zhong X. LncRNA WT1-AS Downregulates Survivin by Upregulating miR-203 in Papillary Thyroid Carcinoma. Cancer Manag Res. 2020;12:443–9.
Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, et al. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63(22):7724–32.
Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44(7):946–53.
Beyer AM, Bonini MG, Moslehi J. Cancer therapy-induced cardiovascular toxicity: old/new problems and old drugs. Am J Physiol Heart Circ Physiol. 2019;317(1):H164–h167.
Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017;81(6):772–81.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of Gothenburg. This study was supported by grants from Walter Andersons fund for scientific research (W2019–03), Lions Cancerfond Väst (LCV 2021:18), Sparbanken Sjuhärads foundation for research (21–02), Assar Gabrielsson Research Foundation for Clinical Cancer Research (FB21–88).
Author information
Authors and Affiliations
Contributions
A.F.B., T.Z.P, K.H., Z.E.: Study concept and experimental design; A.F.B., T.Z.P., P.L.: Analysis and interpretation of data; A.F.B., T.Z.P, K.H., Z.E., P.L.: Writing of the manuscript, preparation of figures and statistical analysis; A.F.B.: Acquisition of funding. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures for the breast cancer validation cohort were done in agreement with the Declaration of Helsinki and approved by the Medical Faculty Research Ethics Committee (Gothenburg, Sweden, application number S164–02). Due to the retrospective study design, the ethical review board approved a waiver of written consent to use the breast tumor specimens.
Consent for publication
Not applicable.
Competing interests
No competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Report Reactome 8th of October 2021.
Additional file 2: Additional Table 1
BIRC5 high impact mutations according to ICGC Data Portal. Additional Table 2 BIRC5 pathogenic mutations according to COSMIC. Additional Table 3 Top 100 genes co-expressed with BIRC5 for each cancer type. Additional Table 4 BIRC5 expression and clinicopathological features.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fäldt Beding, A., Larsson, P., Helou, K. et al. Pan-cancer analysis identifies BIRC5 as a prognostic biomarker. BMC Cancer 22, 322 (2022). https://doi.org/10.1186/s12885-022-09371-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09371-0