Abstract
Background
The aim of this systematic review was to evaluate the evidence and clinical outcomes of screening interventions and implementation trials in sub-Saharan Africa (SSA) and also appraise some ethical issues related to screening in the region through quantitative and qualitative narrative synthesis of the literature.
Methods
We searched Pubmed, OvidMEDLINE, Embase, and Web of Science to identify studies published on breast cancer screening interventions and outcomes in SSA. Descriptive statistics were used to summarize the frequency and proportions of extracted variables, and narrative syntheses was used to evaluate the clinical outcomes of the different screening modalities. The mixed methods appraisal tool was used to assess the quality of studies included in the review.
Results
Fifteen studies were included, which consisted of 72,572 women in ten countries in SSA. 63% (8/15) of the included publications evaluated Clinical Breast Examination (CBE), 47% (7/15) evaluated mammography and 7% (1/15) evaluated ultrasound screening. The cancer detection rate was < 1/1000 to 3.3/1000 and 3.3/100 to 56/1000 for CBE and mammography screening respectively. There was a lot of heterogeneity in CBE methods, target age for screening and no clear documentation of screening interval. Cost-effective analyses showed that CBE screening linked to comprehensive cancer care is most cost effective. There was limited discussion of the ethics of screening, including the possible harms of screening in the absence of linkage to care. The gap between conducting good screening program and the appropriate follow-up with diagnosis and treatment remains one of the major challenges of screening in SSA.
Discussion
There is insufficient real-world data to support the systematic implementation of national breast cancer screening in SSA. Further research is needed to answer important questions about screening, and national and international partnerships are needed to ensure that appropriate diagnostic and treatment modalities are available to patients who screen positive.
Similar content being viewed by others
Background
Breast cancer is the most common cause of cancer death among women globally with a disproportionate burden of mortality in developing countries [1]. Whereas breast cancer survival rates are increasing in most developed countries, in part due to early detection and more effective treatment [2,3,4], Sub-Saharan Africa (SSA) has the worst mortality-to-incidence ratios globally [2]. This has been partly attributed to advanced stage at presentation with approximately 80% of patients presenting with locally advanced and metastatic disease at diagnosis [5].
Efforts to reduce the burden of disease in SSA has focused on improving survival by increasing the rates of early detection for breast cancer, combined with effective treatment for early-stage disease in order to improve the cure rates and survival for breast cancer. In developed countries mammography screening annually or biennially has significantly reduced breast cancer mortality rates by at least 20% [6], and is accepted as a gold standard for cost effective breast cancer screening. The World Health Organization (WHO) currently recommends systematic mammography screening for women between ages 40 to 75 years through population-based mammography screening program in well-resourced settings [7, 8]. However, in resource-limited settings mammography has been assessed as not cost-effective and it is recommended that early detection focus on downstaging through improved breast cancer awareness [9]. Current research does not show a survival benefit of Breast Self-Examination (BSE) [10, 11] and Clinical Breast Examination (CBE) [12], but may be promising in resource-limited settings through downstaging [13], and if adequate diagnostic and therapeutic facilities are in place [8].
Currently, we are not aware of countries in SSA with a systematic national breast cancer screening program. The debate on the most appropriate screening modality for breast cancer in SSA has centered around the need to pursue global justice, which proposes equalizing access and utilization of mammography screening in less developed countries such as SSA [14], versus pursuing screening modalities that is informed by the cost-effective intervention trials and research performed within the socioeconomic context and the resources available within the current healthcare infrastructure [15]. Furthermore, there are ethical concerns about implementing screening interventions in SSA where there is limited or lack of access to diagnostic services, treatment, follow-up and an adequate number of healthcare professionals to accommodate the increased patient workload anticipated from screening. Given these challenges in resource-restricted settings, experts suggest that a greater priority now, is to provide interventions that are plausible in achieving early detection and adequate treatment for the cancers currently diagnosed [16].
The aim of this systematic review was to evaluate the evidence and clinical outcomes of screening interventions and implementation trials in SSA and also appraise some ethical issues related to screening in the region through quantitative and qualitative narrative synthesis of the literature. Findings from this study will contribute to the knowledge of effective screening interventions in SSA, and guide health policy and funding decisions on screening modalities appropriate in SSA.
Methods
Search strategy
The systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. An electronic search in Pubmed, OvidMEDLINE, Embase, and Web of Science was performed using Boolean search terms for Clinical Breast Examination or Self Breast Examination and Africa or mammogram* screen* and Africa or breast cancer screening and Africa or breast cancer screen* Africa. Studies were limited to research involving “Humans” and publications in English. There was no restriction on the year of publication and the search was carried out on articles up to April 24, 2019. Additional references were identified by reviewing the citation of key references. Publications that evaluated a screening intervention and clinical outcomes in asymptomatic women were included. Research conducted in countries outside of SSA were excluded, as well as publications that did not focus on breast cancer or included only symptomatic women. We also excluded publications that did not evaluate any of the following screening outcomes: survival, cancer cases diagnosed, stage shifting, positive predictive value and cost-effectiveness analysis (CEA). Reviews and conference proceedings were excluded. A two-step process was used to identify reviewed articles: Authors YMM and BD reviewed titles and abstracts that met inclusion criteria. Duplicates were removed and the full text articles were reviewed for data abstraction. All disagreements were resolved by further examination and discussions among the co-authors.
Outcomes variables
The following variables were extracted: 1. Context (author, year, country); 2. Screening protocol (study group, interval,, longest follow-up); 3. Participants (n for study group and control (if applicable), age at enrollment); 4. Screening outcomes assessed and results. Ethical issues were qualitatively appraised, which included data on access to appropriate diagnostic and treatment for those who screen positive and assessment of harm-to-benefit ratio.
Statistical analysis
Descriptive statistics were used to summarize the frequency and proportions of the extracted variables, and narrative synthesis was used to evaluate the clinical outcomes data of the included publications. The mixed methods appraisal tool (MMAT) [17] was used to assessed the quality of the studies included in the analysis. The assessment of quantitative non-randomized study was used to evaluate the quality of the included studies using five criteria.
Results
Study characteristics
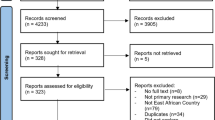
The flow chart for publications identified and reviewed for our analysis are presented in Fig. 1. 72,572 total participants (including 28,465 participants in the control group) were included from 15 articles, that evaluated screening intervention through research conducted in 10 countries and published from year 2008 to year 2018. This included three publications on CEA using mathematical modeling and one publication on cancer outcomes assessment using microsimulation. 63% (8/15) of the included publications evaluated CBE, 47% (7/15) evaluated mammography and 7% (1/15) evaluated ultrasound screening. The screening outcomes assessed included cancer detection rates, stage shifting, CEA and survival outcomes. Two intervention studies included had a control arm, and one study was a cross-sectional self-controlled design. Details on the study population, screening modality and outcomes are presented in Table 1.
Quality of the studies
The quality of the 11 quantitative studies included in our analysis was moderate as shown in Fig. 2. 88% of the publications used a sampling strategy relevant to the address the research question. Furthermore 67% of the articles used a sample representative of the target population. Three studies did not clearly define the age inclusion criteria for the participants enrolled in the study. Additionally, three studies evaluating breast cancer screening strategies included a lower limit of 15 years and 18 years for participants enrolled in the study. 33% of the publications had adequate documentation of risk of non-response, which was assessed as low. Finally, the majority of publications included in the review had adequate outcome data (> 80%) that were included in our analysis.
Narrative synthesis
Clinical breast examination
There are few studies evaluating real-world data on clinical outcomes of breast cancer screening interventions in SSA. Majority of the studies were pilot studies and did not evaluate a control group in the study design. Of the studies included majority evaluate CBE, however there was significant heterogeneity in the personnel conducting these examinations – which included training of sampled lay persons, community healthcare workers (CHW) within the community that participants were selected from, and training of healthcare professionals. The setting also differed in terms of whether these were conducted in the community vs. the hospital or clinic setting or “breast camps.” The screening uptake rate differed across studies, with high screening uptake rate reported in studies that included extensive education and engagement of community leaders, religious groups, village leaders and in studies where the communities chose the candidates to be trained as CHW performing these examinations [21, 25, 33]. Interestingly, in Sudan, villages screened by volunteers from other villages had low screening uptake rate [33]. There was also disparate data in studies that reported higher uptake when CHW were financially compensated vs. not. The CBE studies were mostly pilots and did not evaluate different screening intervals or have long follow-up. In Tanzania, CBE uptake rates increased with subsequent years of annual screening evaluated [34].
The age of the target population at enrollment differed significantly for studies that reported this in their inclusion criteria. Of note studies have shown that more than half of patients diagnosed in SSA are ≤ 50 years [35]. Appropriately studies extended their inclusion criteria of women enrolled in asymptomatic screening. The lower limit at inclusion ranged from 15–30 years old.
There was also heterogeneity in the screening outcomes that were assessed and reported. The cancer diagnoses rate varied from < 1/1000 examinations to 8.7/1000 screened for CBE. This was unadjusted for the age groups of the women that were evaluated in each study. One showed a significantly higher proportion of early-stage disease (ie. stage I & II) in the study group compared to the control group [34]. One microsimulation study evaluated survival and absolute risk reduction in Eastern SSA. One study included total cost of the screening intervention only, which was assessed as $45,000 / year [34].
Mammography screening and ultrasound
Five studies included an assessment of mammography or ultrasound screening. These studies all evaluated opportunistic screening analyzed prospectively or retrospectively and one cross-sectional study. The studies varied in the age distribution of the population screened and the reported cancer detection rate that ranged from 3.3/1000 to 56/1000 examinations. None of these studies reported screening intervals assessed.
Cost effectiveness analysis
Three studies were modeling studies that used imputed data to analyze cost effectiveness of screening and treatment modalities for breast cancer in Ghana, Uganda (E. Africa region) and SSA. Although the methodology differed, the summarized data showed that the most cost-effective screening modality in SSA is biennial CBE and treatment of all stages for women aged 40–69, which corresponds to the highest reduction in disability-adjusted life years (DALY) and mortality. Mammography may be cost effective in parts of SSA that are assessed as middle-income countries and cost between $Int2248 and $4596 per DALY averted [29].
Ethical appraisal
There were several ethical issues appraised from the studies included in our analysis. The data presented on breast screening in SSA remains insufficient to recommend systematic national screening programs in SSA. Further studies, specifically real-world data are needed to assess the clinical benefit of screening in this population. The benefit of screening can be realized in countries where there are integrated treatment programs established for breast cancer cases diagnosed early through screening [19]. Furthermore the benefit of downstaging and early detection can be fully achieved in programs where diagnostic and health system delays are minimal. In this regard, a model of breast cancer camps that included multidisciplinary teams that served as a one-stop shop for diagnosis may be most effective in SSA [26], given that several studies have shown prolonged systems delays for cancer patients [36,37,38]. Finally, there is no established age criteria for when CBE or mammography should be initiated in SSA, given that most patients present before age 50 [35]. This is important to assess including the psychological and associated costs of screening a lower limit of younger women whose risk of breast cancer does not make this a cost-effective intervention in that population. In studies that reported positive predictive value of the different modalities, a PPV for CBE by a trained laywoman compared to a physician had a 48% PPV, which raises the additional issue of the high rates false positive examinations and associated psychological implications. One study evaluated ultrasound screening, which is not an evidence-based screening modality and also reported PPV of 33%. All the studies evaluated expounded on the potential benefits of screening, but included limited discussion of potential harms.
Discussion
This review showed that there are few articles that have examined breast cancer screening outcomes in SSA and the current data remains insufficient to recommend systematic national screening programs in any country in SSA. There is also a deficit of data analyzing the ethical issues of the effective rollout of national screening programs in SSA. Given the current debate surrounding breast cancer screening in developed countries, it is critical that future studies and recommendations that propose implementation of any breast cancer screening modality in SSA carefully evaluate the existing data for different screening strategies to ensure that the benefit to harm ratio is acceptable and cost-effective for the target populations being screened in order to realize significant survival benefits.
The following issues remain to be addressed by current screening interventions:
-
1.
What is the best screening modality? The current data support that based on financial, human personnel and health infrastructure resources, CBE in the immediate future may be the most appropriate screening intervention in SSA. WHO does not recommend population-based mammography screening in limited-resource settings but offers consideration for CBE for women ages 50–69 in this setting [8]. An analysis of national cancer control plans globally, showed that only 5% and 18% of low-income countries and lower-middle-income plans respectively, included any population-based breast cancer screening [39]. Furthermore, few low- and middle-income counties have specific guidelines for CBE screening. For instance, Malaysia recommends CBE for women ≥ 35 years old and risk-stratified mammography screening programs starting at 40 years [40].
-
2.
What target populations should be considered for screening? Majority of guidelines for mammography screening in developed countries recommend screening asymptomatic women aged ≥ 50 years. Screening recommendations from few low- and middle- income countries indicate that a lower age at screening initiation may be appropriate and generalizable to the SSA region where typically ≥ 50% of women present at age ≤ 50 years [35]. Screening interventions will therefore have to incorporate the at-risk population younger than 50, but assess a lower limit that does not include young women in whom the risk of breast cancer is low. In balancing this risk it is important to consider the prevention paradox, by assessing the specific risk distribution based on a country’s population distribution and breast cancer incidence and mortality pattern. Although there are no national population-based screening programs in SSA, the Cancer Association of South Africa recommends annual mammography screening starting at age 40 and biennial in women ≥ 55 years old [41]. There also some consideration for initiation of screening at 35 years such as current CBE guidelines in Malaysia [40], and based on recent data from a cluster randomized trial of CBE every two years in Indian women aged 35–64 years who were followed up for 20 years [13]. In a sub-analysis, women < 50 years who attended all prescribed rounds of screening had a breast cancer specific mortality benefit compared to no benefit in women who did not attend all rounds of screening [42].
-
3.
What strategies are effective in increasing screening uptake? The data presented here shows that screening conducted by lay healthcare workers selected from the screening villages result in high screening uptake. Furthermore, CBE coupled with extensive mass campaigns and engagement of community/village leaders are also very effective. This is in line with the ethical recommendation of community engagement in resource poor settings. Community engagement serves as way of empowering the community to be active participants of intervention programs and ensuring that interaction with community is a continuous process even after the screening intervention is over [43]. Similarly, modelling breast cancer camps where multidisciplinary teams served as a one-stop shop for diagnosis underscores capacity building which is a vital requirement emphasized in resource poor settings. Collective approach to diagnosis and treatment encourages communication and exchange of ideas among team members which fosters knowledge, skills and expertise.
Subsequent studies need to provide clear data on training protocols for examiners, compensation, examiner-to-participant ratio, factors related to effective CBE uptake. Follow-up should be reported on linkage to diagnostic services. Finally, larger studies are needed to report significant differences in early-stage vs late-stage cancer diagnosis rate in the screened vs unscreened population, as well as survival and absolute risk reduction through screening. CEA should also incorporate more real-world data for the specific countries using actual healthcare costs within the public systems where available.
The main ethical challenge in screening interventions in SSA is the gap between conducting good screening program and the appropriate follow up with diagnosis and treatment. This challenge is similar to the one encountered in clinical trials in developing countries where availability of proven interventions is often not feasible or occur on a fragmented scale [44]. It is important therefore to develop research and advocate for policies that would incorporate screening and appropriate diagnosis and treatment. Achieving this will rely on fidelity to frameworks for ethical implementation of screening such as the Australia population-based screening framework [45], that are aligned with the WHO Screening Programmes guide, which advocates for a detailed contextual assessment of ethical considerations, benefits and potential harm prior to implementation of national systematic screening programs [46]. Most screening recommendations in SSA follow guidelines from North America and Europe, there is a critical need for a contextual adaption, which will ensure the incorporation of context specific values and true benefits of screening in improving the health outcomes of women. All studies should also address the potential harms of screening before recommending national screening programs. In our review, only two studies reported a positive predictive value of CBE. However, a complete assessment of harm should be incorporated into the initial study design and should include sensitivity and specificity evaluations. In SSA, elevated false positive rates may result in psychological distress and healthcare system strain due to multiple diagnostic follow-ups. Conversely high false negative rates from a suboptimally implemented or ineffective screening program may result in more prolonged diagnostic delays in a region where this is a significant problem [47]. In addition, diagnosing women at early stages can result in potential harm if there are delayed referrals and lack of access to adequate treatment which can be attributed to weak healthcare infrastructure in LMICs. Whereas the argument for overdiagnosis has remained central in the mammography screening debate [48], CBE is unlikely to increase diagnosis of in-situ only disease and therefore may be a lower consideration of the harm assessment in SSA. The variability of harm in different contexts further supports the need for contextual assessment in formulating screening policies in SSA. The question is less of whether there is a potential benefit and more of whether prior studies have addressed ethical considerations for implementing CBE screening in SSA. In fact, CEA analysis suggests that treatment alone of all stages in SSA might not be as cost effective, and that screening coupled with treatment of all stages constitute the most effective strategies. Further research and funding is needed to answer some of these important questions related to screening, and national government and international partnerships are needed to ensure that appropriate diagnostic and treatment modalities are available for breast cancer patients that are being diagnosed in their countries.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Change history
22 March 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12885-022-09412-8
Abbreviations
- ARR:
-
Absolute Risk Reduction
- BHW:
-
Breast Health Worker
- CBE:
-
Clinical Breast Examination
- CEA:
-
Cost Effectiveness Analysis
- CHW:
-
Community Health Worker
- DALY:
-
Disability-Adjusted Life Years
- DCIS:
-
Ductal Carcinoma in situ
- HLY:
-
Healthy Life Years
- IDC:
-
Invasive Ductal Carcinoma
- NR:
-
Not Reported
- PPV:
-
Positive Predictive Value
- SSA:
-
Sub Saharan Africa
- WHO:
-
World Health Organization
- YLS:
-
Years Life Saved
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomark Prev. 2015;24(10):1495–506. https://doi.org/10.1158/1055-9965.EPI-15-0535.
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA: A Cancer Journal for Clinicians. 2017;67(6):439–48. https://doi.org/10.3322/caac.21412.
Guo F, Kuo YF, Chen Y, Shih T, Giordano SH, Berenson AB. Trends in Breast Cancer Mortality by Stage at Diagnosis Among Young Women in the United States. Cancer Published online. 2018. https://doi.org/10.1002/cncr.31638.
Jedy-Agba E, McCormack V, Adebamowo C, dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. The Lancet Global Health. 2016;4(12):e923–35. https://doi.org/10.1016/S2214-109X(16)30259-5.
Humphrey L, Helfand M, … BCA of internal, 2002 undefined. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Am Coll Physicians. https://pubmed.ncbi.nlm.nih.gov/12204020/.
Recommendations by age group and resource setting - WHO Position Paper on Mammography Screening - NCBI Bookshelf. Accessed December 19, 2021. https://www.ncbi.nlm.nih.gov/books/NBK269538/#recommendations.s5
World Health Organization. WHO | Breast cancer: Early Diagnosis and Screening. WHO. Published online 2018. Accessed May 18, 2019. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
Corbex M, Burton R, Sancho-Garnier H. Breast cancer early detection methods for low and middle income countries, a review of the evidence. The Breast. 2012;21(4):428–34. https://doi.org/10.1016/j.breast.2012.01.002.
Larkin M. Breast self examination does more harm than good, says task force. Lancet (London, England). 2001;357(9274):2109. https://doi.org/10.1016/S0140-6736(00)05238-7.
Thomas DB, Gao DL, Ray RM, et al. Randomized Trial of Breast Self-Examination in Shanghai: Final Results. JNCI Journal of the National Cancer Institute. 2002;94(19):1445–57. https://doi.org/10.1093/jnci/94.19.1445.
Barton MB, Harris R, Fletcher SW. The rational clinical examination Does this patient have breast cancer? The screening clinical breast examination: should it be done? How? JAMA. 1999;282(13):1270–80.
Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical Breast Examination: Preliminary Results from a Cluster Randomized Controlled Trial in India. JNCI Journal of the National Cancer Institute. 2011;103(19):1476–80. https://doi.org/10.1093/jnci/djr304.
Sangiovanni A. Global Justice, Reciprocity, and the State. 2007.
Wendler D, Emanuel EJ, Lie RK. The standard of care debate: can research in developing countries be both ethical and responsive to those countries’ health needs? Am J Public Health. 2004;94(6):923–8. https://doi.org/10.2105/ajph.94.6.923.
Gelband H, Sankaranarayanan R, Gauvreau CL, et al. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: key messages from Disease Control Priorities, 3rd edition. Lancet (London, England). 2016;387(10033):2133–44. https://doi.org/10.1016/S0140-6736(15)00755-2.
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon MP, Griffiths F, Nicolau B, O'Cathain A, Rousseau MC, Vedel I. Improving the content validity of the mixed methods appraisal tool: a modified e-Delphi study. J Clin Epidemiol. 2019;111:49–59.e1. https://doi.org/10.1016/j.jclinepi.2019.03.008. Epub 2019 Mar 22.
Apffelstaedt JP, Steenkamp V, Baatjes K. Performance data of screening mammography at a dedicated breast health centre. South African medical journal. 2008;98(12):950–3.
Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. Lancet Oncol. 2013;14(4):363–70. https://doi.org/10.1016/S1470-2045(12)70583-1.
Brakohiapa EK, Armah GE, Clegg-Lamptey JNA, Brakohiapa WO. Pattern of breast diseases in Accra: review of mammography reports. Ghana Med J. 2013;47(3):101–6.
Luyeye Mvila G, Postema S, Marchal G, et al. From the set-up of a screening program of breast cancer patients to the identification of the first BRCA mutation in the DR Congo. BMC Public Health. 2014;14(1):759. https://doi.org/10.1186/1471-2458-14-759.
Apffelstaedt JP, Hattingh R, Baatjes K, Wessels N. Results of a pilot programme of mammographic breast cancer screening in the Western Cape. South African medical journal. 2014;104(4):297–8.
Apffelstaedt SP, Dalmayer L, Baatjes K. Mammographic screening for breast cancer in a resource-restricted environment. South African medical journal. 2014;104(4):294–6.
Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer. 2015;136(12):2875–9. https://doi.org/10.1002/ijc.29348.
Gutnik L, Lee C, Msosa V, et al. Clinical breast examination screening by trained laywomen in Malawi integrated with other health services. J Surg Res. 2016;204(1):61–7. https://doi.org/10.1016/j.jss.2016.04.017.
Sayed S, Moloo Z, Ngugi A, et al. Breast Camps for Awareness and Early Diagnosis of Breast Cancer in Countries With Limited Resources: A Multidisciplinary Model From Kenya. Oncologist. 2016;21(9):1138–48. https://doi.org/10.1634/theoncologist.2016-0004.
Omidiji OA, Campbell PC, Irurhe NK, Atalabi OM, Toyobo OO. Breast cancer screening in a resource poor country: Ultrasound versus mammography. Ghana Med J. 2017;51(1):6–12.
Pinder LF, Nzayisenga JB, Shibemba A, et al. Demonstration of an algorithm to overcome health system-related barriers to timely diagnosis of breast diseases in rural Zambia. Angeletti PC, ed. PLOS ONE. 2018;13(5):e0196985. https://doi.org/10.1371/journal.pone.0196985.
Ginsberg GM, Lauer JA, Zelle S, Baeten S, Baltussen R. Cost effectiveness of strategies to combat breast, cervical, and colorectal cancer in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344(mar02 2):e614–e614. https://doi.org/10.1136/bmj.e614.
Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Tropical Med Int Health. 2012;17(8):1031–43. https://doi.org/10.1111/j.1365-3156.2012.03021.x.
Ralaidovy AH, Gopalappa C, Ilbawi A, Pretorius C, Lauer JA. Cost-effective interventions for breast cancer, cervical cancer, and colorectal cancer: new results from WHO-CHOICE. Cost Effectiveness and Resource Allocation. 2018;16(1):38. https://doi.org/10.1186/s12962-018-0157-0.
Birnbaum JK, Duggan C, Anderson BO, Etzioni R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Glob Health. 2018;6(8):e885–93. https://doi.org/10.1016/S2214-109X(18)30257-2.
Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. Lancet Oncol. 2013;14(4):363–70. https://doi.org/10.1016/S1470-2045(12)70583-1.
Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer. 2015;136(12):2875–9. https://doi.org/10.1002/ijc.29348.
Vanderpuye V, Grover S, Hammad N, et al. An update on the management of breast cancer in Africa. Infectious Agents and Cancer. 2017;12(1):13. https://doi.org/10.1186/s13027-017-0124-y.
Tapela NM, Peluso MJ, Kohler RE, et al. A Step Toward Timely Referral and Early Diagnosis of Cancer: Implementation and Impact on Knowledge of a Primary Care-Based Training Program in Botswana. Front Oncol. 2018;8:187. https://doi.org/10.3389/fonc.2018.00187.
Gatera M, Bhatt S, Ngabo F, et al. Successive introduction of four new vaccines in Rwanda: High coverage and rapid scale up of Rwanda’s expanded immunization program from 2009 to 2013. Vaccine. 2016;34(29):3420–6. https://doi.org/10.1016/j.vaccine.2015.11.076.
Bhatia RK, Rayne S, Rate W, et al. Patient Factors Associated With Delays in Obtaining Cancer Care in Botswana. Journal of Global Oncology. 2018;4:1–13. https://doi.org/10.1200/JGO.18.00088 JGO.18.00088.
Romero Y, Trapani D, Johnson S, et al. National cancer control plans: a global analysis. Lancet Oncol. 2018;19(10):e546–55. https://doi.org/10.1016/S1470-2045(18)30681-8.
Htay MNN, Donnelly M, Schliemann D, et al. Breast Cancer Screening in Malaysia: A Policy Review. Asian Pacific Journal of Cancer Prevention : APJCP. 2021;22(6):1685. https://doi.org/10.31557/APJCP.2021.22.6.1685.
Lipschitz S. Screening mammography with special reference to guidelines in South Africa. SA Journal of Radiology. 2018;22(2):1370. https://doi.org/10.4102/SAJR.V22I2.1370.
Mittra I, Mishra GA, DIkshit RP, et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. The BMJ. 2020;368. doi:https://doi.org/10.1136/BMJ.N256
Tindana PO, Singh JA, Tracy CS, et al. Grand Challenges in Global Health: Community Engagement in Research in Developing Countries. PLoS Med. 2007;4(9): e273. https://doi.org/10.1371/journal.pmed.0040273.
Kahn K, Ryan G, Beckett M, et al. Bridging the gap between basic science and clinical practice: a role for community clinicians. Implementation science : IS. 2011;6:34. https://doi.org/10.1186/1748-5908-6-34.
Population-based screening framework | Australian Government Department of Health. Accessed December 19, 2021. https://www.health.gov.au/resources/publications/population-based-screening-framework
world health organization screening framework - Google Search. Accessed December 19, 2021. https://www.google.com/search?q=world+health+organization+screening+framework&oq=world+health+organization+screening+framework&aqs=chrome..69i57j33i22i29i30l2.7680j0j7&sourceid=chrome&ie=UTF-8
Pace LE, Mpunga T, Hategekimana V, et al. Delays in Breast Cancer Presentation and Diagnosis at Two Rural Cancer Referral Centers in Rwanda. Oncologist. 2015;20(7):780–8. https://doi.org/10.1634/THEONCOLOGIST.2014-0493.
Morris E, Feig SA, Drexler M, Lehman C. Implications of Overdiagnosis: Impact on Screening Mammography Practices. Population Health Management. 2015;18(Suppl 1):S 3-11. https://doi.org/10.1089/POP.2015.29023.MOR.
Acknowledgements
Not applicable
Funding
Dr. Yehoda Martei’s research work is supported by an NIH K01 Award—K01TW011481. Bege Dauda’s research is supported by the Penn Center for Global Genomics and Health Equity (PennGGHE) with funding from Genentech, Inc. and the Penn Medicine Office of Inclusion, Diversity, and Equity.
Author information
Authors and Affiliations
Contributions
YMM and BD wrote the first draft of the manuscript and performed data analysis. All authors performed interpretation of the data, revising drafts of manuscript and approval of final version.
Authors’ information
Yehoda M. Martei, MD MSCE, is a Ghanaian medical oncologist at the University of Pennsylvania in Philadelphia. Her academic research focus is on implementation strategies for optimizing therapy delivery and breast cancer treatment outcomes in sub-Saharan Africa.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no financial and non-financial competing interests.
Bege Dauda, PhD, is a post-doctoral fellow in the Medical Ethics Division at the University of Pennsylvania in Philadelphia. His academic focus is on ethico-legal challenges and opportunities of benefit sharing in genomic research in Africa, focusing on the H3 Africa initiative. He also has an interest in the ethics of sample sharing and transfer as well as capacity building in research in Low- and Middle-Income Countries.
Verna Vanderpuye, MD, is a radiation and medical oncology physician with over 20 years of experience in treating cancer patients, training young physicians, nurses and other allied health workers and research into cancer in Africa. She has served on committees which influence care and research including the World Health Organization, American Society of Clinical Oncology, European Society of Clinical Oncology and the African Organization for Research and Treatment in Cancer. Dr. Vanderpuye works to improve delivery of academic content to African practitioners and researchers on the continent and is helping to move forward the agenda of implementation research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The funding section of this article has been updated.
Supplementary Information
Additional file 1: Supplementary Table 1.
Reasons for exclusion of titles and abstracts from initial screen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Martei, Y.M., Dauda, B. & Vanderpuye, V. Breast cancer screening in sub-Saharan Africa: a systematic review and ethical appraisal. BMC Cancer 22, 203 (2022). https://doi.org/10.1186/s12885-022-09299-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09299-5