Abstract
Background
Axillary vein/subclavian vein (AxV/SCV) and Internal jugular vein (IJV) are commonly used for implantable venous access port (IVAP) implantation in breast cancer patients for chemotherapy. Previous research focused on comparison of complications while patient comfort was ignored. This study aims to compare patient comfort, surgery duration and complications of IVAP implantation between IJV and AxV/SCV approaches.
Methods
Two hundred forty-eight breast cancer patients were enrolled in this randomized controlled study from August 2020 to June 2021. Patients scheduled to undergo IVAP implantation were randomly and equally assigned to receive central venous catheters with either AxV /SCV or IJV approaches. All patients received comfort assessment using a comfort scale table at day 1, day 2 and day 7 after implantation. Patient comfort, procedure time of operation as well as early complications were compared.
Results
Patient comfort was significantly better in the AxV/SCV group than that of IJV group in day 1 (P < 0.001), day 2 (P < 0.001) and day 7(P = 0.023). Procedure duration in AxV/SCV group was slightly but significantly shorter than IJV group (27.14 ± 3.29 mins vs 28.92 ± 2.54 mins, P < 0.001). More early complications occurred in AxV/SCV group than IJV group (11/124 vs 2/124, P = 0.019). No difference of complications of artery puncture, pneumothorax or subcutaneous hematoma between these two groups but significantly more catheter misplacement in AxV/SCV group than IJV group (6/124 vs 0/124, P = 0.029). Absolutely total risk of complications was rather low in both groups (8.87% in AxV/SCV group and 1.61% in IJV group).
Conclusions
Our study indicates that patients with AxV/SCV puncture have higher comfort levels than IJV puncture. AxV/SCV puncture has shorter procedure duration but higher risk of early complications, especially catheter misplacement. Both these two approaches have rather low risk of complications. Consequently, our study provides an alternative choice for breast cancer patients to reach better comfort.
Similar content being viewed by others
Background
Breast cancer is the most common malignant tumor and leads to the most cancer deaths in females [1, 2]. Systemic intravenous chemotherapy has been recommended to many of invasive breast cancer patients to reduce the recurrence risk and improve patient prognosis, with the exception of those patients who are at low recurrence risk [3, 4]. As we know, it is dangerous to administer chemotherapy through peripheral veins because there are severe side effects such as chemotherapy drugs extravasation, unacceptable pain and psychological trauma [5, 6]. Hence, long-term CVC infusion ports are usually recommended for breast cancer patients for their convenience and safety during chemotherapy [7].
The most commonly used puncture sites of central venous catheters are subclavian veins (SCVs) and internal jugular veins (IJVs) [8]. In fact, axillary vein (AxV), the direct continuation of the SCV, has also been considered as an important alternative option of CVC puncture [9,10,11]. Ultrasound guidance is the standard technique during CVC insertion via both IJV and SCV [12, 13]. Compared with anatomical landmark guidance, procedure guided by ultrasound has been proved to improve patient safety and comfort [14].
Previous studies usually focused on the comparison of complications and safety between puncture via SCV or AxV and IJV [11, 15, 16]. However, to our knowledge, there was no study focusing on the comparison of patient comfort. Consequently, this work aims to compare patient comfort after IJV or AxV /SCV puncture with the help of ultrasound guidance. At the same time, we also compare procedure duration and complications between these two groups. The protocol of this study has been peer reviewed and published [17].
Methods
Patient selection
Inclusion criteria in this study were: (I) age 18–70 years old; (II) histologically confirmed diagnosis of invasive breast cancer; (III) with indication of chemotherapy according to the latest NCCN guideline; (IV) with the ability to understand our comfort scale questionnaire and comply with the study protocol.
Cases with one of these following situations or more were excluded: (I) confirmed bilateral breast cancer; (II) confirmed distant metastasis; (III) have coagulation diseases history such as deep venous thrombosis (DVT).
Study design
This was a prospective single center randomized controlled study. Breast cancer patients received port implantation from August 2020 to June 2021 were randomly assigned to AxV /SCV group or IJV group by simple randomization in a 1:1 ratio with computerized system for random numbers generation. Two designated doctors (Dr. Bao and Dr. Deng) with expertise in IVAP implantation performed the procedures for all patients. Patients enrolled completed all preoperative examinations before port implantation, including coagulation screening, blood routine examination, electrocardiograph, and puncture vascular ultrasound. After implantation surgery, all patients received comfort assessment at day 1, day 2 and day 7 with a comfort scale table. The study flowchart is shown in Fig. 1.
Implantation procedure
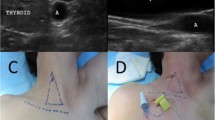
All the procedures were performed strictly following the aseptic principles in the out-patient operation room. Vital signs were monitored using electrocardiogram monitor, noninvasive blood pressure cuff and pulse oximetry continuously. For IJV group, patients were positioned with the head turning to the left in a neutral supine position while for AxV/SCV group, patients were positioned in a neutral supine position. Lidocaine (2%) was used for local anesthesia and no sedatives were used during procedure. One of the three designated brands of infusion ports, BARD (Bard Limited, Salt Lake City, USA), B Braun (B.Braun Medical, Chasseneuil-du-Poitou, France) and Medcomp (Medical Components, Inc., Harleysville, USA) was used for patients according to the surgeon’s preference. Guide wire and catheter insertion were performed with the ultrasound guidance (MYLAB 30CV, Esaote Medical Technology Co. LTD, China) (Fig. 2).
Center venous catheter puncture with ultrasound guidance. A-C Center venous catheter puncture through IJV with ultrasound guidance. D-F Center venous catheter puncture through AxV/SCV with ultrasound guidance. ICV: Internal carotid artery. IJV: Internal jugular vein. AxA: Axillary artery. AxV: Axillary vein. SCV: Subclavian vein
For patients in IJV group, we inserted the guide wire and then catheter was replaced through right IJV. For AxV/SCV group patients, we inserted the guide wire and then replaced with catheter through AxV or right SCV. All patients took a chest X-ray right after the procedure (Fig. 3) and then we checked the early complications.
Outcome measures
The primary outcome measure of this study is patient comfort at Day 7, 1 week after CVC puncture. Secondary outcome measures are patient comfort at Day 1, just after CVC puncture surgery and Day 2, 1 day after CVC puncture. The comfort scale in our study (Table 1) is a visual analogic scale, which has been applied and proved to be a simple and effective method for patients’ comfort or satisfaction assessment [18, 19].
Other secondary outcome measures of our study are as follows: (I) duration of procedure (minutes), defined as the time between the initial cutaneous sterilization and dressing placement; (II) early complications rate. Early complication is defined as the period of time between intraoperative implantation and the first use of catheter, which includes wire advancement difficulties, inadvertent artery puncture, catheter misplacement, pneumothorax or subcutaneous hematoma. All these complications are defined as described in the protocol [17].
Statistical analysis
All the statistical analysis were performed with GraphPad Prism version 8.0 for Windows (GraphPad Software Inc., San Diego, California, USA) and IBM SPSS Statistics for Windows, version 27 (IBMCorp., Armonk, NY., USA). Summary statistics for quantitative variables with normal distribution were expressed with means and standard deviations. Unordered categorical variables were summarized with percentages or ratios. Differences in means of continuous variables were compared with Student’s t-test (two groups). Discontinuous variables were compared using Wilcoxon rank test, and differences in proportions were tested by Chi-Square test. P < 0.05 is considered statistically significant.
Ethics and informed consents
The study was reviewed and approved by the medical ethics committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University (No.2020-KY-053) and followed the provisions of Helsinki Declaration. This study has also been registered on 26/07/2020 in Chinese Clinical Trial Registry (ChiCTR, www.chictr.org.cn) and Chinese Ethics Committee of Registering Clinical Trials (No.ChiCTR2000034986). All patients provided written informed consent to participate in this study and additional consent before surgical procedures. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Baseline characteristics of patients
Total of 248 patients were included in this study and each group had 124 patients. Three patients in IJV group and one patient in AxV/SCV group were lost to follow up at day 2 and day 7.
No significant differences of age, BMI, marriage status and history of diabetes between IJV group and AxV/SCV group at baseline. Tumor side, chemotherapy, breast and lymph node surgery were also without significant difference. Detailed data was showed in Table 2.
Patient comfort of the two groups of patients
Patient comfort of AxV/SCV group was significantly better than that of IJV group on day 1 (P < 0.001), day 2 (P < 0.001) and day 7 (P = 0.023). On day 1, most patients had grade 1 (37/124, 29.8%) or grade 2 (54/124, 43.5%) in IJV group while most patients had grade 0 (83/124, 66.9%) or grade 1 (30/124, 24.2%) in AxV/SCV group. On day 2, about half the patients had grade 2 (52/121, 43.0%) and grade 3 (13/121, 10.7%) in IJV group while only about 30% of the patients of grade 2 (33/123, 26.8%) and grade 3 (5/123, 4.1%) in AxV/SCV group. On day 7, 51.2% cases had grade 0 and 30.1% had grade1 in AxV/SCV group while 35.5% cases had grade 0 and 38.8% cases had grade 1 in IJV group. Only four patients had grade 4 on day 1 and one case on day 2 in IJV group and no patients had grade 4 in AxV/SCV group. No patients had grade 5 on both groups. Detailed data was showed in Table 3 and Fig. 4.
Procedure duration and complications of the two groups of patients
Procedure duration was 27.14 ± 3.29 mins in AxV/SCV group, which was significantly shorter than that 28.92 ± 2.54 mins of IJV group (P < 0.001). There were totally 11 cases with early complications in AxV/SCV group and 2 cases in IJV group (8.87% vs 1.61%, P = 0.019). Among 11 cases with complications in AxV/SCV group, 6 cases had catheter misplacement, 2 cases had subcutaneous hematoma and 3 cases had artery puncture. Only 1 case had subcutaneous hematoma and 1 case had artery puncture in the IJV group. No significant difference of artery puncture or subcutaneous hematoma occurred in these two groups and no severe complications such as pneumothorax or hemothorax were observed. However, there was much more catheter misplacement in the AxV/SCV group than the IJV group (4.84% vs 0%, P = 0.029). Detail was showed in Table 4. Among these 6 cases of catheter misplacement, the catheter tips were located at right internal jugular vein in 2 cases, at right subclavian vein in 2 cases and at left brachiocephalic vein in 2 cases (Fig. 5).
Discussion
As far as we know, this is the first study to compare patients’ comfort as primary outcome measure between IJV and AxV/SCV puncture. In this study, we found that patients underwent IVAP implantation through AxV/SCV had better comfort than those through IJV on day 1, day 2 and day 7 after puncture. At the same time, the duration time of AxV/SCV puncture was slightly shorter than IJV puncture. Although the risk of complications was higher in patients with AxV/SCV puncture, the totally risk of complications was rather low in both AxV/SCV group and IJV group with ultrasound guidance. In a word, our study provides an alternative better choice of IVAP implantation for breast cancer patients to get better comfort.
As previously described, over the years, an enormous amount of research comparing AxV/SCV and IJV has focused on the complications and safety of these two insertion approaches. However, patient satisfaction and comfort were only mentioned in only a few studies [19, 20]. In this prospective randomized controlled study, we compared patients’ comfort of IVAP implantation as primary outcome measure, and this is the first study to put patient comfort in the forefront. Our study indicated that patients in AxV/SCV group had significantly higher comfort level than those in IJV group. Many IJV group patients were afraid to turn their neck because they felt a foreign body sensation in their neck and this uncomfortable feeling may last for several days after the CVC insertion. Consequently, our study can provide an alternative choice for patients to reach better comfort level.
Our study also compared the procedure duration of CVC puncture between these two approaches and we found that it was significantly shorter in AxV/SCV group than IJV group, although the difference of less than 2 mins was negligible. However, past studies showed that the access time for the first attempt and the average whole procedure time was significantly longer in AxV/SCV group than IJV group [21, 22]. This difference may be explained by the following reasons. Firstly, we used ultrasound guidance and this has been proved to significantly reduce the procedure duration of CVC puncture [23]. Secondly, there are two incisions during procedure in IJV group patients while only one incision in AxV/SCV group. At the same time, subcutaneous tunneling construction is not required during IVAP implantation in AxV/SCV group. Consequently, with the help of ultrasound guidance, the procedure time is slightly shorter in AxV/SCV group than IJV group, and this may be one of the advantages of AxV/SCV puncture.
Comparison of complication incidence between AxV/SCV and IJV approach still remains controversial. Some studies demonstrated higher complications risk such as pneumothorax or symptomatic venous thrombosis in SCV puncture than IJV puncture [22, 24, 25]. However, other research indicated no difference of complication risk between these two different insertion ways [11, 16, 26]. A multicenter randomize trial with 3471 catheters indicated that SCV catheterization had a lower risk of symptomatic thrombosis and bloodstream infection than femoral vein or IJV catheterization but a higher risk of pneumothorax [27]. Another study demonstrated no significant difference of total complication rate between SCV and IJV but there seemed to be more catheter misplacements in SCV catheterization [21], which is consistent with our study. In our study, we found that there are much more catheter misplacements in the AxV/SCV group. In order to decrease the risk of catheter misplacements after IVAP implantation, we can use ultrasound to detect whether the catheter is at the right internal jugular vein or not immediately after the catheter placement. If the catheter is located at the right internal jugular vein, we can withdraw the catheter and replace it again to make sure that the catheter is not at internal jugular vein. However, if the catheter is misplaced at right subclavian vein or left brachiocephalic vein, the ultrasound cannot observe it and we can only observe this after X-ray examination, so these cases should receive operation of catheter replacement again. Generally, in this study, we found that complication rate patients developed was rather low in both IJV group and AxV/SCV group with ultrasound guidance and there were no severe complications cases such as pneumothorax or hemothorax. Hence, our study further confirms the benefits from ultrasound guidance for its safety and convenience and we strongly recommend ultrasound guidance in IVAP implantation. However, further studies to reduce the risk of catheter misplacement during AxV/SCV puncture are of great significance in the future.
There are still some limitations in this study. Firstly, our study was not blinded to patients and may influence patient comfort to some extent. Also, we did not compare late complications in our study. Lastly, this was a single center research and the sample size was not large enough; hence, it is necessary to decrease the risk of catheter misplacement in the future, and to perform a prospective multicenter survey to compare patient comfort between AxV/SCV puncture and IJV puncture with a larger sample size.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CVC:
-
Central Venous Catheters
- IVAP:
-
Implantable venous access port
- IJV:
-
Internal Jugular Vein
- SCV:
-
Subclavian Vein
- AxV:
-
Axillary Vein
- DVT:
-
Deep venous thrombosis
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85.
Mincey BA, Palmieri FM, Perez EA. Adjuvant therapy for breast cancer: recommendations for management based on consensus review and recent clinical trials. ONCOLOGIST/4789. 2002;7(3):246–50.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast Cancer. N Engl J Med. 2018;379(2):111–21.
Wiegering V, Schmid S, Andres O, Wirth C, Wiegering A, Meyer T, et al. Thrombosis as a complication of central venous access in pediatric patients with malignancies: a 5-year single-center experience. BMC Hematol. 2014;14(1):18.
Tewari M, Shukla HS. Administration of cancer chemotherapeutic drugs through the enhanced peripheral veins by creating a radiocephalic fistula. Am J Surg. 2007;194(2):240–2.
Jan HC, Chou SJ, Chen TH, Lee CI, Chen TK, Lou MA. Management and prevention of complications of subcutaneous intravenous infusion port. SURG ONCOL/3514. 2012;21(1):7–13.
Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58(6):323–46.
Galloway S, Bodenham A. Ultrasound imaging of the axillary vein--anatomical basis for central venous access. Br J Anaesth. 2003;90(5):589–95.
Sharma A, Bodenham AR, Mallick A. Ultrasound-guided infraclavicular axillary vein cannulation for central venous access. Br J Anaesth. 2004;93(2):188–92.
O'Leary R, Ahmed SM, McLure H, Oram J, Mallick A, Bhambra B, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762–8.
Troianos CA, Hartman GS, Glas KE, Skubas NJ, Eberhardt RT, Walker JD, et al. Councils on intraoperative E, vascular ultrasound of the American Society of E: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291–318.
Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105–17.
Peris A, Zagli G, Bonizzoli M, Cianchi G, Ciapetti M, Spina R, et al. Implantation of 3951 long-term central venous catheters: performances, risk analysis, and patient comfort after ultrasound-guidance introduction. ANESTH ANALG/3827. 2010;111(5):1194–201.
Matsushima H, Adachi T, Iwata T, Hamada T, Moriuchi H, Yamashita M, et al. Analysis of the outcomes in central venous access port implantation performed by residents via the internal jugular vein and subclavian vein. J SURG EDUC/195. 2017;74(3):443–9.
Nagasawa Y, Shimizu T, Sonoda H, Mekata E, Wakabayashi M, Ohta H, et al. A comparison of outcomes and complications of totally implantable access port through the internal jugular vein versus the subclavian vein. INT SURG/05. 2014;99(2):182–8.
Chen Y-B, Bao H-S, Deng H-R, Hu T-T, Wen B-L, Yi C-Y, et al. Comparison of comfort and complications in breast cancer patients of implantable venous access port (IVAP) with ultrasound guided internal jugular vein (IJV) and axillary vein/subclavian vein (AxV/SCV) puncture: a randomized controlled study protocol. Annals of Palliative Medicine. 2020;9(6):4323–31.
Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. CRIT CARE/495. 2018;22(1):120.
Babu KG, Suresh Babu MC, Lokanatha D, Bhat GR. Outcomes, cost comparison, and patient satisfaction during long-term central venous access in cancer patients: experience from a tertiary Care Cancer Institute in South India. Indian J Med Paediatr Oncol. 2016;37(4):232–8.
Shafique MN, Akhtar SH, Mahnoor M, Hussain M. Hemodialysis internal jugular vein versus subclavian vein catheters: complications, patients’ comfort, tolerance and cost-effectiveness. PAK J MED SCI/0544. 2019;35(1):124–8.
Shin HJ, Na HS, Koh WU, Ro YJ, Lee JM, Choi YJ, et al. Complications in internal jugular vs subclavian ultrasound-guided central venous catheterization: a comparative randomized trial. Intensive Care Med. 2019;45(7):968–76.
Macdonald S, Watt AJ, McNally D, Edwards RD, Moss JG. Comparison of technical success and outcome of tunneled catheters inserted via the jugular and subclavian approaches. J VASC INTERV RADIOL/257. 2000;11(2 Pt 1):225–31.
Orsi F, Grasso RF, Arnaldi P, Bonifacio C, Biffi R, De Braud F, et al. Ultrasound guided versus direct vein puncture in central venous port placement. J VASC ACCESS/1535. 2000;1(2):73–7.
Vinson DR, Ballard DW, Hance LG, Stevenson MD, Clague VA, Rauchwerger AS, et al. Pneumothorax is a rare complication of thoracic central venous catheterization in community EDs. Am J Emerg Med. 2015;33(1):60–6.
Bjorkander M, Bentzer P, Schott U, Broman ME, Kander T. Mechanical complications of central venous catheter insertions: a retrospective multicenter study of incidence and risks. Acta Anaesthesiol Scand. 2019;63(1):61–8.
Shinde PD, Jasapara A, Bansode K, Bunage R, Mulay A, Shetty VL. A comparative study of safety and efficacy of ultrasound-guided infra-clavicular axillary vein cannulation versus ultrasound-guided internal jugular vein cannulation in adult cardiac surgical patients. Ann Card Anaesth. 2019;22(2):177–86.
Parienti J-J, Mongardon N, Mégarbane B, Mira J-P, Kalfon P, Gros A, et al. Intravascular complications of central venous catheterization by insertion site. NEW ENGL J MED/59558. 2015;373(13):1220–9.
Acknowledgements
The authors wish to thank all the patients who participated in this study and their families for their support of this study.
Funding
This study was supported by National Natural Science Foundation of China (81172524), Sun Yat-sen Clinical Research Cultivation Program of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (SYS-Q-202105) and Medical Research Foundation of Guangdong Province (A2021280).
Author information
Authors and Affiliations
Contributions
YB, HS, HR and JN designed the study. HS and HR performed CVC puncture for all patients. TT, FT, Z H, BW and FX involved in patient recruitment and follow-up work. All authors wrote and revised the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No.2020-KY-053) and was registered on 26/07/2020 in Chinese Clinical Trial Registry (ChiCTR, www.chictr.org.cn) and Chinese Ethics Committee of Registering Clinical Trials (No.ChiCTR2000034986). All patients provided written informed consent to participate in this study and additional consent before surgical procedures.
Consent for publication
Not Applicable.
Competing interests
All authors declare no conflict interest. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y.B., Bao, H.S., Hu, T.T. et al. Comparison of comfort and complications of Implantable Venous Access Port (IVAP) with ultrasound guided Internal Jugular Vein (IJV) and Axillary Vein/Subclavian Vein (AxV/SCV) puncture in breast cancer patients: a randomized controlled study. BMC Cancer 22, 248 (2022). https://doi.org/10.1186/s12885-022-09228-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09228-6