Abstract
Background
Neoadjuvant chemoradiation therapy (nCRT) is the standard treatment modality in locally advanced rectal cancer (LARC). Since response to radiotherapy (RT) is dose dependent in rectal cancer, dose escalation may lead to higher complete response rates. The possibility to predict patients who will achieve complete response (CR) is fundamental. Recently, an early tumour regression index (ERI) was introduced to predict pathological CR (pCR) after nCRT in LARC patients.
The primary endpoints will be the increase of CR rate and the evaluation of feasibility of delta radiomics-based predictive MRI guided Radiotherapy (MRgRT) model.
Methods
Patients affected by LARC cT2-3, N0-2 or cT4 for anal sphincter involvement N0-2a, M0 without high risk features will be enrolled in the trial. Neoadjuvant CRT will be administered using MRgRT. The initial RT treatment will consist in delivering 55 Gy in 25 fractions on Gross Tumor Volume (GTV) plus the corresponding mesorectum and 45 Gy in 25 fractions on the drainage nodes. Chemotherapy with 5-fluoracil (5-FU) or oral capecitabine will be administered continuously.
A 0.35 Tesla MRI will be acquired at simulation and every day during MRgRT. At fraction 10, ERI will be calculated: if ERI will be inferior than 13.1, the patient will continue the original treatment; if ERI will be higher than 13.1 the treatment plan will be reoptimized, intensifying the dose to the residual tumor at the 11th fraction to reach 60.1 Gy.
At the end of nCRT instrumental examinations are to be performed in order to restage patients. In case of stable disease or progression, the patient will undergo surgery. In case of major or complete clinical response, conservative approaches may be chosen. Patients will be followed up to evaluate toxicity and quality of life.
The number of cases to be enrolled will be 63: all the patients will be treated at Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome.
Discussion
This clinical trial investigates the impact of RT dose escalation in poor responder LARC patients identified using ERI, with the aim of increasing the probability of CR and consequently an organ preservation benefit in this group of patients.
Trial registration
ClinicalTrials.gov Identifier: NCT04815694 (25/03/2021).
Similar content being viewed by others
Background
Neoadjuvant chemoradiation therapy (nCRT) currently represents the standard treatment modality in locally advanced rectal cancer (LARC) [1], achieving a pathological complete response (pCR) in approximately 11–42% of these patients, regardless of the initial disease stage [2,3,4].
Several studies have shown that patients achieving pCR usually have a better prognosis in terms of local control (LC), metastases-free survival (MFS) and overall survival (OS), representing a sound background for dose escalation approaches, aimed to enhance the therapeutic performance of nCRT [4,5,6]. Furthermore, conservative surgical approaches have recently been investigated in patients showing clinical complete response (cCR) after nCRT [7]. Both local excision (LE) and “Watch and Wait” (W&W) approaches represent to date, feasible options in order to reduce morbidities and toxicities related to unnecessary Total Mesorectal Excision (TME) in the case of successful nCRT [8, 9].
The possibility to predict the patients who will achieve complete response (CR) before surgery or even during nCRT is therefore of utmost importance, paving the way to the most innovative paradigms of a fully personalized medicine.
Several prediction models have been developed to predict CR in LARC patients, providing clinicians with valuable decisional support systems (DSS) for multidisciplinary oncological care personalization, so that patients predicted as “not responding” may take advantage of intensified treatments, while those predicted as “responding” may be addressed to more conservative therapeutic approaches, aiming to achieve organ preservation [10].
Amongst the different published models, a significant number focused on the possibility to predict pCR by analysing medical images, with the large majority of them focusing on Magnetic Resonance Imaging (MRI), as this imaging modality represents the gold standard technique for rectal cancer diagnosis and staging [11, 12]. Different MR based models revealed predictive value of the images acquired before [10, 13], during [14, 15], or after the end of CRT [16,17,18], supporting the possibility to identify imaging based biomarkers of response and modulate the treatment accordingly.
Fiorino and colleagues recently proposed an early regression index (ERI), which compares the Gross tumour volume (GTV) measurements at the time of simulation imaging acquisition and at the 10th fraction, during the second week of treatment, as a potential biomarker for pCR prediction and more recently, also for long-term disease-free survival (DFS) [19, 20].
The original experience was performed on standard staging images acquired on 1.5 Tesla T2-weighted MRI and was later validated on an independent cohort of 52 LARC patients, who had undergone nCRT on a 0.35 T MRI hybrid MRI-LINAC, reporting an area under curve (AUC) of 0.93 in the case of an ERITCP value lower than 13.1 [15].
Since the response to radiotherapy is dose dependent in rectal cancer [21], patients with ERI > 13.1 values could theoretically benefit from more aggressive loco-regional treatments, justifying a dose escalation to the residual tumour by inducing a strong local immune reaction, helping in reducing also the risk of (or postponing) any metastatic spread [22,23,24].
The purpose of “THeragnostic Utilities for Neoplastic DisEases of the Rectum” (THUNDER 2) by MRI guided radiotherapy single centre prospective trial is to apply the ERI, in order to increase dose at 60.1 Gy on the residual primary tumour in the second week of treatment by MRI guided radiation therapy (MRgRT) in patients with a low prediction of pCR, treated with an MRI-LINAC hybrid machine.
Furthermore, such trial will allow to collect a relevant number of images and clinical data, that make it possible to investigate the possibility of improving the ERITCP performance, by integrating such indicator with delta radiomics features, which have already demonstrated promising predictive performance in rectal cancer [14, 25].
Methods/design
Study Design
This is a single center prospective clinical trial. Fig. 1 describes the proposed treatment algorithm.
THUNDER 2: THeragnostic Utilities for Neoplastic DisEases of the Rectum by MRI guided radiotherapy treatment algorithm. LARC: locally advanced rectal cancer; CRT: chemoradiotherapy; GTV: gross tumor volume; ERI: early regression index; PTV: planning target volume; RT: radiation therapy; MR: magnetic resonance.
Study objectives
The study will enroll patients affected by LARC and treated on a hybrid 0.35 T MR-LINAC (MRIdian, ViewRay Inc., Mountain View, CA, USA). The ERI will be calculated at the 10th fraction of RT, in order to support the decision to deliver a RT dose boost on GTV.
The primary aim of this trial is to obtain the 10% increase of CR rate in the patients predicted as “not responders” (ERI > 13.1). Furthermore, the feasibility of delta radiomic-based predictive models in MRgRT will be assessed, in order to integrate the ERI model with omics information and improve its predictive performances [15].
Secondary objectives of the trial are to assess the 3 years LC, MFS, DFS, OS; the R0 resection rate; the tumor regression grade (TRG) 1 and TRG 2 rates [26]; the neoadjuvant rectal (NAR) score; the sphincter preservation rate; the organ preservation rate; the rectal and sexual functions.
Ethics informed consent and safety
The final protocol was approved by the ethics committee of Fondazione Policlinico Universitario “A. Gemelli”, IRCCS of Rome, Italy (ethics committee identifier code 3460). This study is conducted in accordance with the most recent version of the Declaration of Helsinki and with the Italian laws and regulations. Any change to the protocol that may have an impact on the conduct of the study, the potential benefit to the patient, or that may affect patient safety, including changes in study objectives, study design, patient population, sample size, study procedures, or significant administrative aspects, will require a formal amendment to the protocol that will be approved by the Ethics Committee.
The protocol has been written according to the principles of good clinical practice (GCP).
Prior to inclusion in the trial, each patient will personally sign and date a written informed consent. The rationale, benefits and possible side effects will be explained in details to the patients before the voluntary signing of the informed consent. No intervention is carried out prior to the signing of the informed consent. Unexpected serious adverse events will be managed by the medical staff and recorded by the study data manager. All information relating to the study will be stored anonymously and securely.
Statistics
According to our previous internal validation of the ERITCP model on MRIdian images of 43 patients, patients presenting an ERI > 13,1 that have a probability to obtain pCR is 3% [15]. Considering that the primary aim of the trial is to increase the CR rate in patients predicted as “responding” by the ERI to 13%, the calculation of the sample size was carried out using p0 = 3% and p1 = 13%. Using a confidence level of 95% and a power of 80%, a trial size of 42 cases was calculated with a cut-off of at least 4 recovered patients, to accept that a phase III trial should be undertaken [27, 28].
Considering that 84% of LARC patients undergoing nCRT do not achieve pCR, the total number of patients to be enrolled for the clinical protocol is 50 (42: 0.84). Moreover, considering a drop-out rate of 20%, the total number of patients to be enrolled is therefore 63. All the patients will be treated at Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome.
Clinical patients data will be prospectively collected using BOA (Beyond Ontology Awareness), a software used for standardized clinical data collection for research purposes in our institution [29]. Patients data will be integrated in large data warehouse, property of Fondazione Policlinico Universitario Agostino Gemelli IRCCS of Rome.
MR images and contours will then be exported to the MODDICOM [30] advanced image analysis platform and radiomic features will be extracted and analyzed in terms of absolute values and delta ones (calculated as the ratio between the simulation value and the single fractions ones).
First order histogram, morphological, textural and fractal features will be analyzed first, following the methodology proposed, previously described by our group in Boldrini et al. [14, 25, 31].
The most significant features predicting CR will then be selected, using AUC and Mann–Whitney test.
Tumour clinical (i.e. cT, cN) and geometrical features (i.e. volume, surface, volume/surface ratio) will be finally added to setup a multivariate logistic model (based on a generalized linear model) to predict clinical and pathological CR.
Model performance will be evaluated by receiver operating characteristic (ROC) curve and internal bootstrapping for calibration errors detection (TRIPOD classification 1b) [32].
Stratification
0.35 T MR images will be acquired at simulation and daily, throughout the whole MRgRT treatment and ERI will be calculated at fraction 10.
If ERI will be < 13.1, the patient will continue the prescribed treatment; otherwise, for ERI values > 13.1, treatment plan will be personalized and reoptimized considering the residual tumor at fraction 10 as a new target volume, where the dose will be intensified, reaching 60.1 Gy. The study workflow is described in Fig. 1.
Patient selection
Besides general and patient’s specific criteria, a staging MRI of adequate technical quality is mandatory. The inclusion and exclusion criteria are presented in Table 1.
Radiotherapy setting
Patients who meet the enrollment criteria will undergo pre-operative CRT treatment that will last 5 weeks.
Patients will be treated on a 0.35 T MRI-LINAC (MRIdian, ViewRay Inc) hybrid machine.
The Fluxboard device (FluxboardTM, MacroMedics, The Netherlands) will be used to immobilize patients in the supine position in a personalized and comfortable setting.
The initial 25-s (sec) acquisition will be carried out to verify the irradiation field. Subsequently, for contouring and planning purposes, a high definition 175 s-sequence will be obtained. After about 20 min, a simulation CT scan will be acquired to provide electronic densities for planning purposes with the same immobilization systems previously used. In the end, a co-registration of the CT images with the 175 s MR images will be performed [33].
The GTV will be contoured on the simulation MRI acquired at the beginning of treatment and on the MRI at 10th fraction and the ERITCP will then be calculated. If ERITCP will be lower than 13.1, the patient will continue the original treatment with a total dose of 55 Gy on PTV2.
If ERITCP will be higher than 13.1, treatment plan will be reoptimized considering GTV3 (at 10th fraction) as a further therapy volume and the dose will be intensified up to 60.1 Gy on PTV3, thanks to the online adaptive approach.
The CTV1 includes the primary rectal tumor, total mesorectum and selected lymphatic drainage stations, and/which will be delineated manually according to the guidelines proposed by Valentini et al. [34] by a radiation oncologist with specific expertise in the treatment of lower GI malignancies.
Planning target volume (PTV) 1 will correspond to the CTV1 + 0.5 cm in all directions.
The CTV2 includes the primary rectal tumour plus the corresponding mesorectum. PTV2 is the CTV2 + 0.5 cm in all directions [33].
In case of the need for treatment intensification, the boost volume (PTV3) will be defined as the GTV delineated on MRI acquired at the fraction 10 (at 22 Gy) with an added isotropic margin of 0.3 cm.
The normal tissue volumes (i.e. organs at risk – OARs) to be contoured are bladder, small bowel, sigmoid colon, anal canal, femoral heads and iliac bones.
All the patients will start a long-course radiotherapy treatment consisting of 25 fractions, with a total prescription dose of 55 Gy in fractions of 2.2 Gy to PTV2 and 45 Gy in fractions of 1.8 Gy to PTV1, following a simultaneous integrated boost (SIB) protocol [33].
ERITCP will be calculated at fraction 10: a treatment intensification protocol will be administered in patients showing ERITCP > 13.1, while patients showing ERITCP ≤ 13.1 will continue the original treatment.
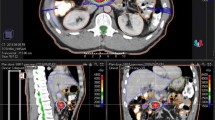
Treatment intensification will consist in a local dose boost to PTV3, where a fractional dose of 2.54 Gy will be delivered from fraction 11 to the end of treatment. PTV1 and PTV2 will continue the original prescription dose. At the end of the intensified treatment, PTV3 will have received a total physical dose of 60.1 Gy, which corresponds to 74.6 Gy in biologically effective dose (BED) and 62.2 in equivalent dose, assuming an alpha/beta equal to 10 [35]. Fig. 2 shows an example of dose escalation.
Example of dose escalation in accordance with the ERI index. Figure 2A shows the simulation plan according to the SIB 2 protocol. Figure 2B represents the dose escalation obtained at the tenth treatment fraction, where the red colourwash isodose line represents the V95% of the 60.1 Gy prescribed to PTV3. The orange colourwash isodose line represents the V95% of 55 Gy prescribed to PTV2, while the yellow colourwash isodose line represents the V95% of 45 Gy prescribed to PTV1
An “inverse planning” approach using computerized optimization will be used (Intensity Modulated Radiation Therapy – IMRT) and the prescription dose will be normalized to the target mean according to ICRU 83 [36].
The reported doses for each PTV will include the prescription dose, the maximum point dose, the % target volume receiving > 105% and > 110% of its prescribed dose, the % target volume receiving ≥ 95% of the prescribed dose, and the mean dose to the PTV’s. Doses to the OARs will also be recorded.
Concomitant chemotherapy (CHT) with 5-FU (225 mg/mq/day in continuous infusion) or oral capecitabine (1650 mg/mq/day chronomodulated) will be administered with no scheduled interruptions.
The patient will be positioned at the isocenter prior to each daily fraction. To improve the reproducibility, an internal protocol will be used to achieve stable conditions of bladder filling: patients will be instructed to drink 500 cc of water 30 min before simulation and before each treatment session.
In the case of dose escalation up to 60.1 Gy, the GTV3 will be recontoured and the plan reoptimized daily, while the patient is on the treatment couch, using an adaptive online RT approach in order to ensure target coverage and OARs sparing [37].
A cine-MRI gating protocol will be performed on PTV2 or PTV3, setting a 5% region of interest (ROI) value in a 3 mm boundary from the CTV2 or CTV3, therefore ensuring that the target volumes are always in the expected position. Delay time value is set at 0 s, so that the movement of the ROI outside the boundary will immediately trigger the beam off.
Response and resectability evaluation
The clinical response will be evaluated with pelvis MRI and body CT or 18F-FDG PET-CT, repeated 8–10 weeks after the end of nCRT. Surgery which will consist of total or partial mesorectal excision, which will be performed 10–12 weeks after the end of CRT in case of partial, stable, or progression disease.
In case of major or complete clinical response at restaging imaging, endoscopic examination should be performed. In case of cCR, a watch and wait (W&W) or local excision (LE) approach could be followed according to the multidisciplinary tumor board (MDT) decision. The administration of adjuvant CHT will be discussed in the framework of MDT, based on clinical and pathological high-risk features.
The follow up surveillance will be scheduled depending on the chosen conservative or surgical approach as reported in Table 2 and 3, respectively. Clinical, instrumental, toxicity, and quality of life (QoL) assessment [38,39,40] will be performed during follow-up.
Discussion
One of the main goals of nCRT treatment for LARC patients is to achieve CR, which corresponds to improved survival outcomes [41]. Many preoperative strategies are reported in literature with the aim of increasing the CR rate. Studies on intensification of preoperative CHT have been conducted showing no particular benefit, except in the German CAO/ARO/AIO-04 study, where a DFS benefit was demonstrated with the addition of oxaliplatin to neoadjuvant and adjuvant CHT regimens [42]. Based on the assumption that the response of tumor also occurs after the end of RT treatment, it has also been showed that the rate of pCR is correlated with the prolongation of the interval between nCRT and surgery [5]. Furthermore, RT dose escalation correlates with the CR rate after nCRT. Appelt et al. demonstrated a significant correlation between dose and tumour regression when doses in the range of 50.4 to 70 Gy are achieved [41].
Radiotherapy doses ≥ 60 Gy correlate with a pCR rate of 20.4% and low rates of acute G3 toxicity (10.3%), consisting mostly of gastro-intestinal complaints, dermatitis, leukopenia/neutropenia, pain, and high rates of resectability (89.5%) in particular, are reported in the literature [21]. The increase in toxicity does not depend on an increase in dose, although it is expected that more advanced RT techniques may contribute to increased tolerability to treatment, due to a higher dose conformation that allows a more efficient OAR sparing [33, 43, 44]. The choice of achieving a dose of 60.1 Gy on the GTV was made according to the reported evidence in the literature and the dosimetric possibility of performing a SIB3 approach. Dose escalation starting from the eleventh treatment fraction with a prescribed dose per fraction of 2.54 Gy on the GTV was found to be satisfactory from a dosimetric point of view, in order to maintain adequate coverage and dose fall-off between the three PTVs obtained.
Different technologies may be applied in order to boost the dose on macroscopic disease, such as contact therapy (CRX) used for distal, small lesions in well-selected patients, enhancing sphincter preservation rates or the addition of brachytherapy boost to nCRT that leads to an increase in pCR, even if not to a corresponding increase in late outcomes [45].
In the framework of personalized treatments, it is necessary to exploit the benefits of available technologies to safely deliver the RT dose while sparing OARs.
MRgRT offers the opportunity to take advantage of MR imaging combined with innovative gating solutions. Furthermore, it is possible to optimize the treatment plan online on a daily basis, taking into account all the possible anatomical modifications, while the patient is still on the treatment couch [33, 37]. This may have a significant impact especially from the perspective of dose escalation, thanks to an efficient management of the different degrees of filling and position of the OARs surrounding the targets volumes (i.e. –bowel, bladder). Daily MR imaging also allows the response to treatment to be monitored and toxicities to be early intercepted/detected and even potentially avoided [46]. Considering the available therapeutic opportunities, it is therefore crucial to stratify treatment options based on disease and patient characteristics.
Indeed, LARC patients should be divided into two categories, which are the intermediate- and high-risk patients, based on the presence of extramural vascular invasion, involvement of mesorectal fascia, massive nodal involvement or enlarged lateral lymph nodes [47, 48].
These features suggest a high potential risk of developing distant metastases and systemic disease control becomes the priority, requiring systemic therapy intensification as demonstrated by the recent results of the RAPIDO trial [49]. Furthermore, results from the recent Prodige 23 trial, which enrolled patients with cT3 and cT4M0 tumors even with high-risk features, confirmed this evidence. The authors proposed an approach based on the inclusion of early chemotherapy in the treatment workflow (total neoadjuvant therapy—TNT) that has been shown to significantly improve 3y-DFS [50].
In contrast, for the group of intermediate-risk patients, LC is the key treatment endpoint. A predictive model able to detect patients who will achieve complete response versus those who are bad responders, may be of significant help to design a trial of RT dose escalation in this subset of patients.
In recent studies, researchers focused on changes in tumor volume on MR images acquired during treatment [51], observing in particular that the ERI index based on disease volumes on simulation and in the second week of treatment MR images, [20] correlates with pCR after nCRT for rectal cancer with high sensitivity and negative predictive value. This index was also validated in a following external cohort of patients using low tesla MR images, reporting promising results also in other tumor sites [15, 52].
To our knowledge, this is the first trial where an image based predictive model is used in clinical workflow to support the decision for a RT intensification on residual disease volume.
New exploratory studies are currently on-going to explore the potentialities of integrating ERI index with delta radiomic features: one of the main limitations of this indicator is in fact that it does not take into consideration the textural characteristics within the tumour, but only volumetric information. It is reasonable to suppose that the integration of radiomic features, describing the variation of heterogeneity within the tumor during the treatment, can effectively integrate the volumetric information, improving the predictive performance, as demonstrated in some recent reviews dealing this topic [53, 54].
The THUNDER 2 trial is designed to increase LC rates in the arm of patients with poor prognosis by applying the ERI index, thereby offering an innovative perspective on the management of LARC patients undergoing MRgRT. The aim of this study is therefore to further evaluate prospectively the predictive power of the ERI index alone and when combined with the analysis of delta radiomics features extracted from MR images during the course of RT treatment, to assess the "strength" of the two different models alone and in combination in a fully personalized and translational framework.
Availability of data and materials
The datasets used analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 5-FU:
-
5- Fluorouracil
- ADC:
-
Apparent diffusion coefficient
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under curve
- BOA:
-
Beyond ontology awareness
- cCR:
-
Clinical complete response
- CHT:
-
Chemotherapy
- CR:
-
Complete response
- CRT:
-
Chemoradiation therapy
- CT:
-
Computed tomography
- CTCAE:
-
Common terminology criteria for adverse events
- CTV:
-
Clinical target volume
- DFS:
-
Disease free survival
- DRE:
-
Digital rectal examination
- DSS:
-
Decisional support system
- DWI:
-
Diffusion-weighted imaging
- EMVI:
-
Extramural venous invasion
- ERI:
-
Early regression index
- ERITCP:
-
Tumor control probability based early regression index
- FSFI:
-
Female sexual function index
- GTV:
-
Gross tumor volume
- ICRU:
-
International commission on radiation units and measurements
- IIEF:
-
International Index of Erectile Function
- IMRT:
-
Intensity modulated radiation therapy
- KBO:
-
Knowledge-based oncology
- LARC:
-
Locally andvanced rectal cancer
- LC:
-
Local control
- LE:
-
Local excision
- LINAC:
-
Linear accelerator
- MDT:
-
Multidisciplinary tumor board
- MFS:
-
Metastases-free survival
- MR:
-
Magnetic resonance
- MRgRT:
-
Magnetic resonance guided radiation therapy
- MRI:
-
Magnetic resonance imaging
- MSKCC BFI:
-
Memorial Sloan-Kettering Cancer Center Bowel Function Instrument
- NAR:
-
Neoadjuvant rectal
- nCRT:
-
Neoadjuvant chemoradiation therapy
- OARs:
-
Organs at risk
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- PTV:
-
Planning target volume
- QoL:
-
Quality of life
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- RT:
-
Radiation therapy
- SIB:
-
Simultaneous integrated boost
- SUV:
-
Standardized uptake value
- TRG:
-
Tumor regression grade
- US:
-
Ultrasound
- W&W:
-
Watch and wait
References
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Belluco C, De Paoli A, Canzonieri V, Sigon R, Fornasarig M, Buonadonna A, et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Ann Surg Oncol. 2011;18:3686–93.
Tamas K, Walenkamp AME, de Vries EGE, van Vugt MATM, Beets-Tan RG, van Etten B, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41:671–9.
Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107.
Gambacorta MA, Masciocchi C, Chiloiro G, Meldolesi E, Macchia G, van Soest J, et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol. 2021;154:154–60.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L-J, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
van der Valk MJM, Hilling DE, Bastiaannet E, Kranenbarg EM-K, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. The Lancet. 2018;391:2537–45.
Maas M, Beets-Tan RGH, Lambregts DMJ, Lammering G, Nelemans PJ, Engelen SME, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28.
Cusumano D, Dinapoli N, Boldrini L, Chiloiro G, Gatta R, Masciocchi C, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med. 2018;123:286–95.
Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730–9.
Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–75.
Dinapoli N, Barbaro B, Gatta R, Chiloiro G, Casà C, Masciocchi C, et al. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int J Radiat Oncol Biol Phys. 2018;102:765–74.
Boldrini L, Cusumano D, Chiloiro G, Casà C, Masciocchi C, Lenkowicz J, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med. 2019;124:145–53.
Cusumano D, Boldrini L, Yadav P, Yu G, Musurunu B, Chiloiro G, et al. External Validation of Early Regression Index (ERITCP) as Predictor of Pathologic Complete Response in Rectal Cancer Using Magnetic Resonance-Guided Radiation Therapy. International Journal of Radiation Oncology, Biology, Physics. 2020;0.
Chiloiro G, Rodriguez-Carnero P, Lenkowicz J, Casà C, Masciocchi C, Boldrini L, et al. Delta Radiomics Can Predict Distant Metastasis in Locally Advanced Rectal Cancer: The Challenge to Personalize the Cure. Front Oncol. 2020;10:595012.
Jeon SH, Song C, Chie EK, Kim B, Kim YH, Chang W, et al. Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat Oncol. 2019;14:43.
Chen H, Shi L, Nguyen KNB, Monjazeb AM, Matsukuma KE, Loehfelm TW, et al. MRI Radiomics for Prediction of Tumor Response and Downstaging in Rectal Cancer Patients after Preoperative Chemoradiation. Adv Radiat Oncol. 2020;5:1286–95.
Fiorino C, Passoni P, Palmisano A, Gumina C, Cattaneo GM, Broggi S, et al. Accurate outcome prediction after neo-adjuvant radio-chemotherapy for rectal cancer based on a TCP-based early regression index. Clin Transl Radiat Oncol. 2019;19:12–6.
Fiorino C, Gumina C, Passoni P, Palmisano A, Broggi S, Cattaneo GM, et al. A TCP-based early regression index predicts the pathological response in neo-adjuvant radio-chemotherapy of rectal cancer. Radiother Oncol. 2018;128:564–8.
Burbach JPM, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113:1–9.
Zhang M, Li X, Guan B, Guan G, Lin X, Wu X, et al. Dose escalation of preoperative short-course radiotherapy followed by neoadjuvant chemotherapy in locally advanced rectal cancer: protocol for an open-label, single-centre, phase I clinical trial. BMJ Open. 2019;9:e025944.
Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clin Immunol. 2013;148:44–55.
Scheithauer H, Belka C, Lauber K, Gaipl US. Immunological aspects of radiotherapy. Radiat Oncol. 2014;9:185.
Cusumano D, Boldrini L, Yadav P, Yu G, Musurunu B, Chiloiro G, et al. Delta radiomics for rectal cancer response prediction using low field magnetic resonance guided radiotherapy: an external validation. Phys Med. 2021;84:186–91.
Dhadda AS, Dickinson P, Zaitoun AM, Gandhi N, Bessell EM. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur J Cancer. 2011;47:1138–45.
A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66.
Stallard N. Sample size determination for phase II clinical trials based on Bayesian decision theory. Biometrics. 1998;54:279–94.
Meldolesi E, van Soest J, Damiani A, Dekker A, Alitto AR, Campitelli M, et al. Standardized data collection to build prediction models in oncology: a prototype for rectal cancer. Future Oncol. 2016;12:119–36.
Dinapoli N, Alitto AR, Vallati M, Gatta R, Autorino R, Boldrini L, et al. Moddicom: a complete and easily accessible library for prognostic evaluations relying on image features. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:771–4.
Cusumano D, Meijer G, Lenkowicz J, Chiloiro G, Boldrini L, Masciocchi C, et al. A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer. Radiol Med. 2021;126:421–9.
Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4:627–35.
Chiloiro G, Boldrini L, Meldolesi E, Re A, Cellini F, Cusumano D, et al. MR-guided radiotherapy in rectal cancer: First clinical experience of an innovative technology. Clin Transl Radiat Oncol. 2019;18:80–6.
Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. 2016;120:195–201.
Joiner MC, Bentzen SM. Fractionation: The linear-quadratic approach. In: In: Basic Clinical Radiobiology. 5th ed. CRC Press; 2018.
Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol. 2012;188:97–9.
Placidi L, Romano A, Chiloiro G, Cusumano D, Boldrini L, Cellini F, et al. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Technical Innovations & Patient Support in Radiation Oncology. 2020;15:15–21.
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30.
Temple LK, Bacik J, Savatta SG, Gottesman L, Paty PB, Weiser MR, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–65.
Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74–80.
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89.
Boldrini L, Intven M, Bassetti M, Valentini V, Gani C. MR-Guided Radiotherapy for Rectal Cancer: Current Perspective on Organ Preservation. Front Oncol. 2021;11:619852.
Gani C, Boldrini L, Valentini V. Online MR guided radiotherapy for rectal cancer. New opportunities Clin Transl Radiat Oncol. 2019;18:66–7.
Appelt AL, Vogelius IR, Pløen J, Rafaelsen SR, Lindebjerg J, Havelund BM, et al. Long term results of a randomized trial in locally advanced rectal cancer: No benefit from adding a brachytherapy boost. Int J Radiat Oncol Biol Phys. 2014;90:110–8.
Boldrini L, Chiloiro G, Pesce A, Romano A, Teodoli S, Placidi L, et al. Hybrid MRI guided radiotherapy in locally advanced cervical cancer: Case report of an innovative personalized therapeutic approach. Clin Transl Radiat Oncol. 2020;20:27–9.
Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327–34.
Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798–804.
Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM-K, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42.
Conroy T, Bosset J-F, Etienne P-L, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15.
Palmisano A, Esposito A, Di Chiara A, Ambrosi A, Passoni P, Slim N, et al. Could early tumour volume changes assessed on morphological MRI predict the response to chemoradiation therapy in locally-advanced rectal cancer? Clin Radiol. 2018;73:555–63.
Cusumano D, Catucci F, Romano A, Boldrini L, Piras A, Broggi S, et al. Evaluation of an Early Regression Index (ERITCP) as Predictor of Pathological Complete Response in Cervical Cancer: A Pilot-Study. Appl Sci. 2020;10:8001.
Mazzei MA, Nardone V, Di Giacomo L, Bagnacci G, Gentili F, Tini P, et al. The role of delta radiomics in gastric cancer. Quant Imaging Med Surg. 2018;8:719–21.
Cusumano D, Boldrini L, Dhont J, Fiorino C, Green O, Güngör G, et al. Artificial Intelligence in magnetic Resonance guided Radiotherapy: Medical and physical considerations on state of art and future perspectives. Phys Med. 2021;85:175–91.
Acknowledgements
Not applicable
Funding
The study will be funded by ViewRay Inc (Mountain View, CA, USA). The sponsor has positively evaluated the design of the study and will grant 36 months funding. This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
The Principal Investigator will be GC. The trial will be managed by GC for the clinical part and by DC for the physical one. AR, LB and EM will support the study following the patients in the clinical therapeutic workflows. LPl and MN will support DC for the elaboration of the radiotherapy treatment plans. The whole trial will be supervised by BB, MAG and VV. RM, LSo, AC, SA, RP, CC, RR, LPe and LSa will be included in the development of the clinical trial. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS on 19 November 2020 (ethics committee identifier code 3460). Prior to inclusion in the trial, each patient will personally sign and date a written informed consent.
Consent for publication
All patients recruited in the study will sign informed consent regarding publishing their data.
Competing interests
Dr. Luca Boldrini and Dr. Davide Cusumano have active research agreements with ViewRay Inc (Mountain View, CA, USA). and received speaker honoraria for scientific presentations and travel reimbursements. Prof. Vincenzo Valentini has received departmental research grants from Varian Medical Systems, ViewRay Inc., Elekta, Merck-Serono, Roche. Prof. Riccardo Manfredi received speaker honoraria from Bracco and Bayer. Dr. Lisa Salvatore received speaker, advisory board and consultancy honoraria from Amgen, Merck, Bayer, Servier, AstraZeneca, Pierre-Fabre. Dr. Lorenzo Placidi reports a consulting agreement and research grants with ViewRay, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiloiro, G., Cusumano, D., Boldrini, L. et al. THUNDER 2: THeragnostic Utilities for Neoplastic DisEases of the Rectum by MRI guided radiotherapy. BMC Cancer 22, 67 (2022). https://doi.org/10.1186/s12885-021-09158-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-09158-9