Abstract
Background
The association of obesity with colorectal cancer (CRC) may vary depending on metabolic status.
Objective
This meta-analysis aimed to investigate the combined impacts of obesity and metabolic status on CRC risk.
Methods
The Scopus, PubMed, and web of sciences databases were systematically searched up to Jun 2021 to find all eligible publications examining CRC risk in individuals with metabolically unhealthy normal-weight (MUHNW), metabolically healthy obesity (MHO), and metabolically unhealthy obesity (MUHO) phenotypes.
Results
A total of 7 cohort studies with a total of 759,066 participants were included in this meta-analysis. Compared with healthy normal-weight people, MUHNW, MHO, and MUHO individuals indicated an increased risk for CRC with a pooled odds ratio of 1.19 (95% CI = 1.09–1.31) in MUHNW, 1.14 (95% CI = 1.06–1.22) in MHO, and 1.24 (95% CI = 1.19–1.29) in MUHO subjects. When analyses were stratified based on gender, associations remained significant for males. However, the elevated risk of CRC associated with MHO and MUHO was not significant in female participants.
Conclusions
The individuals with metabolic abnormality, although at a normal weight, have an increased risk for CRC. Moreover, obesity is associated with CRC irrespective of metabolic status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second one in women; over 1.8 million new cases of CRC were recorded in 2018 [1]. In recent years, the increased incidence of CRC to a high extent is related to epidemiological and nutritional changes as well as the Western lifestyle [2]. A meta-analysis study indicated that the Western dietary pattern increases the risk of CRC. Obesity, which is closely associated with the Western dietary pattern, is also a risk factor for colorectal cancer [3, 4]. The majority of patients with obesity share common metabolic abnormalities, namely hyperglycemia, insulin resistance, abdominal obesity, hyperlipidemia, and hypertension. Metabolic abnormalities have been postulated to explain the role of obesity in the development of CRC [5]. A growing number of evidence from epidemiological studies shows that not all individuals with obesity have metabolic abnormalities, a phenomenon known as metabolically healthy obesity (MHO). Likewise, not all individuals with normal weight are metabolically healthy, a phenomenon known as metabolically unhealthy normal weight (MUNW) [6].. Accordingly, this concept has been recently taken into consideration and different body size phenotypes have been defined based on the metabolic health status [7]. Metabolic phenotypes are the consequence of the interactions between different factors including dietary, lifestyle, environmental factors, genetic factors, and microbial factors [8]. Individuals are classified into the following different metabolic phenotypes including the metabolically healthy normal weight (MHNW), metabolically unhealthy normal weight (MUHNW), metabolically healthy obese (MHO), the prevalence of this phenotype, according to the definitions of obesity and metabolic health, varies from 6 to 38.4% among different populations [9] These people express a favorable metabolic profile, are insulin sensitive, and express an optimal lipid profile, fat distribution and low levels of systemic inflammatory responses [10]. Another phenotypes are metabolically unhealthy obese (MUHO) [11] and metabolically unhealthy and normal-weight (MUNW). The BMI of MUNW individuals is less than 25, but they express metabolic abnormalities such as increased levels of adiposity, insulin resistance, higher susceptibility to type 2 diabetes, and cardiovascular diseases [12]. Kabat et al. (2018) indicated that, compared to metabolically healthy individuals with normal weight, the MUNW phenotype increases the risk of CRC in postmenopausal women [13]. The simultaneous effect of obesity and MetS on CRC has been discussed in previous observational studies and the results were controversial so far [14,15,16]. A recent meta-analysis showed that participants with MHO had a higher risk of cancer (of any type) than those with metabolically healthy normal weight (MHNW) or metabolically healthy non-obesity (included overweight, normal weight, and underweight) [17]. However, this meta-analysis combined different types of cancer in a single analysis. The influence of metabolic obesity phenotypes on the risk of cancer may differ according to the cancer site. Therefore, it may not be appropriate to analyze cancer at different sites as a single exposure. Moreover, MUNW and metabolically unhealthy obesity (MUHO) was not investigated in the prior meta-analysis. Given the considerations mentioned above, we performed a meta-analysis of prospective observational studies to clarify whether MHO, MUHO, or MUNW (compared with MHNW) is associated with CRC risk.
Methods
In the present meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) statement was followed to write and report data [18]. This study does not contain any studies with human participants or animals carried out by any of the authors.
Search strategy
A systematic literature search was conducted through three major databases including PubMed, Scopus, and Web of Science up to Jun 2021. The systematic search was supplemented by a screening of the reference lists of all eligible studies and reviews. The combination of the following controlled vocabulary term was searched: (Obesity [Mesh] OR “Body Mass Index” [Mesh] OR BMI OR obese OR overweight OR “normal weight” OR non-obese OR non-obese) AND (metabolic OR metabolically OR healthy OR unhealthy OR benign OR Abnormal) AND (Colorectal OR colon OR Rectal OR rectum) AND (Neoplasms [Mesh] OR Neoplasia OR Neoplasm* OR cancer OR carcinoma OR Colorectal Neoplasms OR Colonic Neoplasms OR Rectal Neoplasms OR tumor*). The primary search was not restricted to the language, ethnicity, or geographical region.
Inclusion and exclusion criteria
All relevant studies considered the following criteria were included: 1) studies with prospective design (prospective cohort, nested case-control and case-cohort); 2) examined association of metabolically healthy obese (MHO), metabolically unhealthy normal weight (MUHNW) and metabolically unhealthy obese (MUHO) phenotypes of body size with the risk of CRC; 3) stratified participants according to metabolic status and BMI categories and had one reference group in the MHNW category; 4) reported the definition of being metabolically healthy; 5) studies which reported risk estimates (relative risk (RR) or hazard ratio (HR) or odds ratio (OR)) and the corresponding 95% confidence intervals (CIs) or sufficient data to calculate them. Letters, comments, reviews, short communication, case reports, book chapters, and studies conducted on animals all were excluded.
Data extraction and quality assessment
The required data were extracted independently by two authors according to a standardized form for the following information: the first author’s name, year of publication, country of origin, sex, mean or range of age, sample size, and risk estimates with their 95% CIs, confounding factors adjusted for in the analyzes, and criteria used to define metabolically healthy status. If there was any discrepancy between the two authors the extracted data were compared with the original file. A quality assessment of included studies was carried out by two reviewers. To evaluate the methodological quality of eligible studies, Newcastle-Ottawa Scale (NOS) [19] was applied. The NOS is a star system scoring studies based on selection, comparability, and outcome parameters.
Statistical analysis
In this meta-analysis, estimated pooled ORs with 95% CIs were used to assess the strength of association between metabolic phenotypes of obesity and risk of CRC and the metabolically healthy normal weight (MHNW) was considered as the reference group. Statistical heterogeneity was assessed using the Chi-square test (p < 0.1) and calculation of the I2 statistic. Accordingly, heterogeneity was significant if Q statistic had p < 0.1 or if I2 > 50%. Low heterogeneity (≤ 25%), moderate heterogeneity (> 25 to 50%), and high heterogeneity (> 50%) were also evaluated. Data were combined via the random-effects model (REM) and fixed-effect models when appropriate. To find and attenuate potential sources of heterogeneity subgroup analysis by gender (male/female) was performed. The conclusiveness and robustness of results by excluding each of the studies from the pooled estimate and analyzing the rest of them were evaluated. The publication bias was evaluated through visual inspection of asymmetry, and Egger’s weighted regression test (p-value less than 0.05 considered significant). Study characteristics and data were extracted to RevMan 5 (Review Manager, version 5.3; The Cochrane Collaboration, 2015) and STATA version 17.0 (Stata Corporation, College Station, TX).
Results
Findings from the systematic review
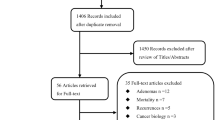
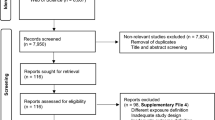
A total of 2590 publications were obtained by the systematic literature search. The flow chart indicating the process of screening of studies is reported in Fig. 1. Finally, 7 cohort studies [13, 20,21,22,23,24,25], with a total sample size of 759,066 participants met eligibility criteria to be included in this meta-analysis. The studies had been published between 2014 and 2020. The sample size of the articles varied from 737 to 408,931 individuals, and the age of participants ranged from 37 to 69 years. The duration of the follow up of the studies varied from 5 to 22 years. Some studies reported multiple effect sizes in their stratified analysis; for such studies, all suitable data were extracted. The outcome was colorectal cancer in all studies except for the study by Moore et al. [23], which assessed specifically colon cancer. Data for the risk of colorectal cancer for individuals with MUHNW, MHO, and MUHO, compared with subjects with MHNW, were reported in 7 studies with 9 data sets [13, 20,21,22,23,24,25] and 6 studies with 8 data sets [13, 20, 21, 23,24,25]. The definition of metabolically unhealthy phenotype was according to the presence of metabolic syndrome [13, 20,21,22], and having elevated blood glucose (> 125 mg/dL) [23], two studies on females [13, 22], two studies assessed the gender-specific association between obesity phenotypes and the risk of CRC [21, 24], and the rest of the studies reported results for a combination of both genders. Three of the studies were from the USA [13, 22, 23], two were from the UK [20, 25] and two were from Korea [21, 24]. The results of all analyzed articles were controlled for the most potential covariates. Following the NOS scale, all publications indicated good quality (Table 1). Detailed characteristics of the included studies are presented in Table 2.
Findings from the meta-analysis
7, 6, and 6 studies were included in the analyses of MUHNW, MHO, and MUHO, respectively. Compared with individuals with MHNW, those with MUHNW (OR = 1.19, 95% CI = 1.09–1.31; Fig. 2) [13, 20,21,22,23,24,25], MHO (OR = 1.14, 95%CI = 1.06–1.22; Fig. 3), or MUHO (OR = 1.24, 95%CI = 1.19–1.29; Fig. 4) phenotypes were significantly at an increased risk of CRC. Low heterogeneity was observed in the analysis of MUHO (I2 = 0%), whereas moderate heterogeneity was evident in other analyses (I2 = 50%). MUHNW (Fig. 5), MHO (Fig. 6), or MUHO (Fig. 7) was associated with an increased risk of CRC in males. By comparison, MUHNW (Fig. 5), but not MHO (Fig. 6) or MUHO (Fig. 7), was associated with a higher risk of CRC in females. Findings showed that the association between metabolic phenotypes with the risk of CRC did not depend on a single study. The pooled effect size ranged from 1.18 (95% CI 1.08–1.27) to 1.32 (95% CI 1.13–1.51) for MUHNW analysis (Supplemental Fig. 1), ranged from 1.11 (95% CI 1.02–1.20) to 1.15 (95% CI 1.10–1.15) for MHO analysis (Supplemental Fig. 2) and ranged from 1.21 (95% CI 1.15–1.28) to 1.23 (95% CI 1.19–1.28) for MUHO analysis, showing the reliability of the results (Supplemental Fig. 3).
Publication bias
No evidence for publication bias was detected based on Egger’s regression test for all analyzes (Fig. 8).
Discussion
Findings showed being metabolically unhealthy can put people at greater risk for CRC despite having normal weight. We also demonstrated that MHO is not a benign condition as individuals with obesity were at greater risk for colorectal cancer regardless of healthy/unhealthy metabolic conditions. After subgroup analysis by gender, associations remained significant for males. However, the elevated risk of CRC associated with MHO and MUHO was not significant in female participants. This review also highlighted limitations and knowledge gaps of the existing literature.
Previous meta-analyses have also refuted that obesity is a benign condition in absence of metabolic disturbance, suggesting both obesity and poor metabolic health can affect the development of chronic conditions including hypertension [26] and chronic kidney disease [12]. In line with this, we additionally observed the adverse effect of obesity on the development of CRC may be partially offset by metabolic health. By contrast, some studies are indicating an unhealthy metabolic profile completely outweighs the impact of obesity on the risks and progression of certain diseases. The greater risk of cardiovascular disease [27], liver, stomach, prostate, and bladder cancers [20] have been only observed in MUHO but not MHO.
The exact mechanism linking obesity and poor metabolic health to CRC remains unclear, although several possibilities and potential pathways have been proposed. Obesity is positively associated with increased APC mutations, reported as gatekeepers in the early stages of the colorectal adenoma-carcinoma sequence [28, 29]. However, poor metabolic health is associated with insulin resistance and consequently activation of insulin-like growth factor-I and epidermal growth factor receptor (EGFR) [30, 31]. EGFR is involved in K-ras mutation, an essential component for the development and progression of CRC to the advanced stages [32]. Considering these points, it is probable that obesity triggers the early stages of adenoma initiation and development to CRC, while metabolic abnormalities may be responsible in both the early and late stages of CRC progression, but maybe more involved in the late stages. The lower risk of CRC in MHO individuals compared to MUHO can be justified by previous studies which have indicated although MHO individuals accumulate high body fat, they display a better insulin sensitivity, lower inflammatory biomarkers, and high adiponectin levels [33]. Genetic susceptibility, histological characteristics, and geographical locations may partly determine the metabolic health in MHO individuals [34]. Some studies have focused on how dietary intakes could affect CRC initiation and progression by considering different obesity phenotypes. A previous study has shown that adherence to Mediterranean diet or Dietary Approaches to Stop Hypertension (DASH) style diet - which are in accordance with “healthy” pattern and can justify the positive effect in reducing the risk of CRC [35] - was not associated with MHO phenotypes in men > 45 years and premenopausal women [36]. Another study reported that higher pro-inflammatory diet was associated with higher odds of unhealthy phenotype in overweight/obese individuals [37] and a meta-analysis of 40 studies indicated that inflammatory diets such as western-style and alcohol-consumption patterns were associated with an increased risk of CRC, whereas, the healthy dietary pattern was associated with a decreased risk of CRC [37] These results suggest that there is no relationship between metabolically healthy/unhealthy obese individuals and healthy dietary intakes, so they are at a greater risk for CRC.
Concerning subgroup analysis, the greater risk for CRC observed in males compared with females can be justified through several genetic and epigenetic factors [38]. For instance, one explanation may be related to the hormonal status as estrogen and its receptors have shown protective effects in the initiation and progression of CRC [39]. In support of this hypothesis, the results of the Women’s Health Initiative study demonstrated that hormonal replacement therapy can mitigate the risk of colon and rectal cancer by, respectively, 30 and 43% [40]. Apart from estrogen, both insulin and insulin-like growth factor axis may also act differently by sex in CRC carcinogenesis [41].
Obesity was measured based on BMI which is not a very valid indicator of body composition compared to the dual-energy X-ray absorptiometry (DEXA) as a gold standard. However, if the participants with MHO or MUHO had higher lean mass than that of normal-weight participants, the observed associations for the risk of CRC would have been attenuated toward the null. Moreover, the cut-points used to define obesity were different in the included studies. However, this was done to capture ethnicity differences as some ethnic groups have shown a higher risk of weight-related diseases at lower BMI values. Additionally, studies used different guidelines to distinguish metabolic healthy and unhealthy individuals such as ATP III, and so on. Even in studies that used ATP III, some of them only relied on one or a few criteria of metabolic syndrome to determine metabolically abnormal individuals, whereas others considered all the six criteria provided by ATP III. Therefore, it might be difficult to make direct comparisons among these studies. Although reported findings were all conditioned on certain confounders, covariates have widely differed across studies. Measurements were also done at a baseline time point, which cannot capture body weight and metabolic change throughout the study. In previous studies, about 30 to 50% of MHO transitioned to a metabolically unhealthy state, whereas 25 to 30% of MUHO recovered their metabolic health [42,43,44,45,46]. However, the majority of included studies did not reflect the longitudinal change in participants’ body weight and laboratory findings during follow-up. As data regarding trajectories of either BMI or metabolic health were not available, included studies could not properly distinguish between contributing/confounding roles of metabolic status. Another limitation is that we restricted the systematic review and meta-analysis to the use of cohort studies, which are prone to recall and selection bias. The current review is strengthened by applying the most robust approach of meta-analysis for evidence synthesizing and using an established questionnaire of NOS to critically appraise the quality of the evidence. The other strength is the large pooled sample size that can ensure statistical power of findings.
Conclusions
Individuals with metabolic abnormality, although at a normal weight, have an increased risk for CRC. Moreover, obesity is associated with CRC irrespective of metabolic status. Since the relationship between metabolic phenotypes of obesity and cancer risk has not been extensively investigated by systematic reviews and meta-analyses, the current study offers novel insights into the joint effect of obesity and metabolic abnormality on colorectal cancer risk, which could potentially inform public health practice to keep metabolic healthy, even with normal weight. To uncover the etiological characteristics of metabolic phenotypes an important step forward may be to include different and alternative definitions/criteria of metabolic status for comparison purposes.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- CRC:
-
Colorectal cancer
- MUHNW:
-
Metabolically unhealthy normal-weight
- MHO:
-
Metabolically healthy obesity
- MUHO:
-
Metabolically unhealthy obesity
- MetS:
-
Metabolic syndrome
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR:
-
Relative risk
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- CIs:
-
Confidence intervals
- NOS:
-
Newcastle-Ottawa Scale
- REM:
-
Random-effects model
- EGFR:
-
Epidermal growth factor receptor
- DEXA:
-
Dual-energy X-ray absorptiometry
References
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64.
Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–43 e10.
Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47.
Jochem C, Leitzmann M. Obesity and colorectal cancer. Obesity Cancer. 2016:17–41.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32.
Perez-Martinez P, Alcala-Diaz JF, Delgado-Lista J, Garcia-Rios A, Gomez-Delgado F, Marin-Hinojosa C, et al. Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur J Clin Investig. 2014;44(11):1053–64.
Eshtiaghi R, Keihani S, Hosseinpanah F, Barzin M, Azizi F. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: the Tehran lipid and glucose study. Int J Obes. 2015;39(3):514–9.
Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–8.
Haghighatdoost F, Amini M, Aminorroaya A, Abyar M, Feizi A. Different metabolic/obesity phenotypes are differentially associated with development of prediabetes in adults: results from a 14-year cohort study. World J Diabetes. 2019;10(6):350.
Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129(10):3978–89.
Kabat G, Wu WY, Bea J, Chen C, Qi L, Stefanick M, et al. Metabolic phenotypes of obesity: frequency, correlates and change over time in a cohort of postmenopausal women. Int J Obes. 2017;41(1):170–7.
Alizadeh S, Esmaeili H, Alizadeh M, Daneshzad E, Sharifi L, Radfar H, et al. Metabolic phenotypes of obese, overweight, and normal weight individuals and risk of chronic kidney disease: a systematic review and meta-analysis. Arch Endocrinol Metab. 2019;63(4):427–37.
Kabat GC, Kim MY, Stefanick M, Ho GY, Lane DS, Odegaard AO, et al. Metabolic obesity phenotypes and risk of colorectal cancer in postmenopausal women. Int J Cancer. 2018;143(3):543–51.
Bitzur R, Brenner R, Maor E, Antebi M, Ziv-Baran T, Segev S, et al. Metabolic syndrome, obesity, and the risk of cancer development. Eur J Internal Med. 2016;34:89–93. https://doi.org/10.1016/j.ejim.2016.08.019. Epub 2016 Aug 18. PMID: 27545645.
Croft B, Reed M, Patrick C, Kovacevich N, Voutsadakis IA. Diabetes, obesity, and the metabolic syndrome as prognostic factors in stages I to III colorectal cancer patients. J Gastrointestinal Cancer. 2019;50(2):221–9.
Saetang J, Sangkhathat S. Diets link metabolic syndrome and colorectal cancer development. Oncol Rep. 2017;37(3):1312–20.
Lin CJ, Chang YC, Cheng TY, Lo K, Liu S, Yeh T. The association between metabolically healthy obesity and risk of cancer: A systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21(10):e13049. https://doi.org/10.1111/obr.13049. Epub 2020 Jun 1. PMID: 32476278.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):1–5.
Cao Z, Zheng X, Yang H, Li S, Xu F, Yang X, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123(8):1336–44.
Cho YK, Lee J, Kim HS, Park JY, Lee WJ, Kim YJ, et al. Metabolic health is a determining factor for incident colorectal cancer in the obese population: a nationwide population-based cohort study. Cancer Med. 2021;10(1):220–9. https://doi.org/10.1002/cam4.3607. Epub 2020 Nov 20. PMID: 33216467; PMCID: PMC7826459.
Liang X, Margolis KL, Hendryx M, Rohan TE, Groessl EJ, Thomson CA, et al. Metabolic phenotype and risk of colorectal cancer in normal-weight postmenopausal women. Cancer Epidemiol Prev Biomarkers. 2017;26(2):155–61.
Moore LL, Chadid S, Singer MR, Kreger BE, Denis GV. Metabolic health reduces risk of obesity-related cancer in Framingham study adults. Cancer Epidemiol Prev Biomarkers. 2014;23(10):2057–65.
Shin CM, Han K, Lee DH, Choi YJ, Kim N, Park YS, et al. Association Among Obesity, Metabolic Health, and the Risk for Colorectal Cancer in the General Population in Korea Using the National Health Insurance Service–National Sample Cohort. Dis Colon Rectum. 2017;60(11):1192–200.
Murphy N, Cross AJ, Abubakar M, Jenab M, Aleksandrova K, Boutron-Ruault MC, et al. A nested case-control study of metabolically defined body size phenotypes and risk of colorectal Cancer in the European prospective investigation into Cancer and nutrition (EPIC). PLoS Med. 2016;13(4):e1001988. https://doi.org/10.1371/journal.pmed.1001988. PMID: 27046222; PMCID: PMC4821615.
Mirzababaei A, Mozaffari H, Shab-Bidar S, Milajerdi A, Djafarian K. Risk of hypertension among different metabolic phenotypes: a systematic review and meta-analysis of prospective cohort studies. J Hum Hypertens. 2019;33(5):365–77.
Mirzababaei A, Djafarian K, Mozafari H, Shab-Bidar S. The long-term prognosis of heart diseases for different metabolic phenotypes: a systematic review and meta-analysis of prospective cohort studies. Endocrine. 2019;63(3):439–62.
Pfalzer AC, Kamanu FK, Parnell LD, Tai AK, Liu Z, Mason JB, et al. Interactions between the colonic transcriptome, metabolome, and microbiome in mouse models of obesity-induced intestinal cancer. Physiol Genomics. 2016;48(8):545–53.
Yaoita T, Sasaki Y, Yokozawa J, Sato T, Kanno N, Sakuta K, et al. Treatment with anti-interleukin-6 receptor antibody ameliorates intestinal polyposis in ApcMin/+ mice under high-fat diet conditions. Tohoku J Exp Med. 2015;235(2):127–34.
Aleksandrova K, Nimptsch K, Pischon T. Influence of obesity and related metabolic alterations on colorectal cancer risk. Curr Nutr Rep. 2013;2(1):1–9.
Hu YP, Patil SB, Panasiewicz M, Li W, Hauser J, Humphrey LE, et al. Heterogeneity of receptor function in colon carcinoma cells determined by cross-talk between type I insulin-like growth factor receptor and epidermal growth factor receptor. Cancer Res. 2008;68(19):8004–13.
Markman B, Javier Ramos F, Capdevila J, Tabernero J. EGFR and KRAS in colorectal cancer. Adv Clin Chem. 2010;51:72.
Aguilar-Salinas CA, García EG, Robles L, Riano D, Ruiz-Gomez DG, García-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93(10):4075–9.
Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14(8):585–91.
Tangestani H, Salari-Moghaddam A, Ghalandari H, Emamat H. Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern reduces the risk of colorectal cancer: a systematic review and meta-analysis. Clin Nutr. 2020;39(10):2975–81.
Park YM, Steck SE, Fung TT, Zhang J, Hazlett LJ, Han K, et al. Mediterranean diet, dietary approaches to stop hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin Nutr. 2017;36(5):1301–9.
Soltani S, Moslehi N, Hosseini-Esfahani F, Vafa M. The association between empirical dietary inflammatory pattern and metabolic phenotypes in overweight/obese adults. Int. J Endocrinol Metab. 2018;16(2):e60048. https://doi.org/10.5812/ijem.60048. PMID: 30008758; PMCID: PMC6035353.
Kim S-E, Paik HY, Yoon H, Lee JE, Kim N, Sung M-K. Sex-and gender-specific disparities in colorectal cancer risk. World J Gastroenterol: WJG. 2015;21(17):5167.
Edvardsson K, Ström A, Jonsson P, Gustafsson J-Å, Williams C. Estrogen receptor β induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol Endocrinol. 2011;25(6):969–79.
Hartz A, He T, Ross JJ. Risk factors for colon cancer in 150,912 postmenopausal women. Cancer Causes Control. 2012;23(10):1599–605.
Yamaji T, Iwasaki M, Sasazuki S, Tsugane S. Gender difference in the association of insulin and the insulin-like growth factor axis with colorectal neoplasia. Int J Obes. 2012;36(3):440–7.
Lee S-H, Yang HK, Ha H-S, Lee JH, Kwon H-S, Park Y-M, et al. Changes in metabolic health status over time and risk of developing type 2 diabetes: a prospective cohort study. Medicine. 2015;94(40):e1705. https://doi.org/10.1097/MD.0000000000001705. PMID: 26448024; PMCID: PMC4616763.
Cho YK, Kang YM, Yoo JH, Lee J, Park J-Y, Lee WJ, et al. Implications of the dynamic nature of metabolic health status and obesity on risk of incident cardiovascular events and mortality: a nationwide population-based cohort study. Metabolism. 2019;97:50–6.
Cho YK, Lee J, Kim HS, Park J-Y, Lee WJ, Kim Y-J, et al. Impact of transition in metabolic health and obesity on the incident chronic kidney disease: a nationwide cohort study. J Clin Endocrinol Metab. 2020;105(3):e148–e57.
Hamer M, Bell JA, Sabia S, Batty GD, Kivimäki M. Stability of metabolically healthy obesity over 8 years: the English longitudinal study of ageing. Eur J Endocrinol. 2015;173(5):703–8.
Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65(1):101–2.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
PJ, supervised and critically reviewed the manuscript for important intellectual content, carried out the analyses, checked all the study processes, and reviewed and revised the manuscript. GG, carried out search processes and drafted the manuscript, SA, designed the study, HM and TR, collected and extracted data FM, BR, ML, and NG, drafted the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since the design of this study (meta-analysis), it is exempt from ethics approval.
Consent for publication
Not Applicable.
Competing interests
No conflict of interest was declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1
: Sensitivity analysis in studies investigating the association of MUHNW phenotype, compared with individuals with MHNW, with odds of CRC.
Additional file 2: Supplemental Figure 2
: Sensitivity analysis in studies investigating the association of MHO phenotype, compared with individuals with MHNW, with odds of CRC.
Additional file 3: Supplemental Figure 3
: Sensitivity analysis in studies investigating the association of MUHO phenotype, compared with individuals with MHNW, with odds of CRC.
Additional file 4: Supplemental Table 1
. The risk of bias of included studies.
Additional file 5: Supplemental Table 2
. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Goodarzi, G., Mozaffari, H., Raeisi, T. et al. Metabolic phenotypes and risk of colorectal cancer: a systematic review and meta-analysis of cohort studies. BMC Cancer 22, 89 (2022). https://doi.org/10.1186/s12885-021-09149-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-09149-w