Abstract
Background
No reliable nomogram has been developed until date for predicting the survival in patients with unresectable pancreatic cancer undergoing treatment with gemcitabine plus nab–paclitaxel (GnP) or FOLFIRINOX.
Methods
This analysis was conducted using clinical data of Japanese patients with unresectable pancreatic cancer undergoing GnP or FOLFIRINOX treatment obtained from a multicenter study (NAPOLEON study). A Cox proportional hazards model was used to identify the independent prognostic factors. A nomogram to predict 6–, 12–, and 18–month survival probabilities was generated, validated by using the concordance index (C–index), and calibrated by the bootstrapping method. And then, we attempted risk stratification for survival by classifying the patients according to the sum of the scores on the nomogram (total nomogram points).
Results
A total of 318 patients were enrolled. A prognostic nomogram was generated using data on the Eastern Cooperative Oncology Group performance status, liver metastasis, serum LDH, serum CRP, and serum CA19–9. The C–indexes of the nomogram were 0.77, 0.72 and 0.70 for 6–, 12–, and 18–month survival, respectively. The calibration plot showed optimal agreement at all points. Risk stratification based on tertiles of the total nomogram points yielded clear separations of the survival curves. The median survival times in the low–, moderate–, and high–risk groups were 15.8, 12.8 and 7.8 months (P<0.05), respectively.
Conclusions
Our nomogram might be a convenient and inexpensive tool to accurately predict survival in Japanese patients with unresectable pancreatic cancer undergoing treatment with GnP or FOLFIRINOX, and will help clinicians in selecting appropriate therapeutic strategies for individualized management.
Similar content being viewed by others
Background
Pancreatic cancer is the seventh leading cause of cancer–related death worldwide, and the fourth leading cause of cancer death in Japan [1, 2]. Although surgical resection is the only curative treatment for pancreatic cancer, only 15% of pancreatic cancer patients are suitable candidates for curative pancreatectomy, because most patients have either distant metastases or locoregional spread, including vascular invasion, even at diagnosis [3]. Palliative chemotherapy is used for patients diagnosed as having unresectable pancreatic cancer. Recently, great strides have been made in palliative chemotherapy for patients with metastatic pancreatic cancer due to development of the gemcitabine plus nab–paclitaxel (GnP) and FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) regimens [4, 5]. However, the overall prognosis of pancreatic cancer remains unsatisfactory. The 5–year survival of patients with pancreatic cancer is a dismal 8% [6]. One of the reasons for the high mortality rate of pancreatic cancer patients may be the absence of a reliable method for prognosis determination and risk stratification. If the prognosis of pancreatic cancer patients could be evaluated more accurately, we could offer better therapeutic strategies and individualized treatments.
The American Joint Committee on Cancer (AJCC) TNM staging system, which is based on the tumor characteristics, and presence/absence of nodal and distant metastases, is currently the mainly used system to predict survival in patients with cancers, including pancreatic cancer [7, 8]. Because patients with unresectable pancreatic cancer would be roughly classifiable as stage III or IV, the AJCC staging system is relatively difficult to discriminate for prediction of survival even in patients with the same AJCC stage [8]. Furthermore, it should be recognized that the AJCC TNM staging system only takes into account three tumor–related factors and not patient–specific factors such as the age, sex, race, and marital status, all of which are known to be associated with the survival in pancreatic cancer patients [7]. Therefore, an individualized, more accurate prognostic system is desirable.
A nomogram is a scoring and visualization tool of a multivariate predictive model, and is accepted as a reliable scale for more accurate survival prediction in individual patients as compared to the AJCC staging system [9,10,11,12]. However, to the best of our knowledge, no reliable nomogram has been developed yet for predicting survival in patients with unresectable pancreatic cancer undergoing treatment with GnP or FOLFIRINOX, which is currently recognized as the standard chemotherapy for these patients. In the present study, we attempted to develop a prognostic nomogram for patients with unresectable pancreatic cancer receiving GnP or FOLFIRINOX treatment, based on the real–world data.
Methods
Patients
This was a multicenter retrospective study of patients with unresectable or recurrent pancreatic cancer who underwent treatment with GnP or FOLFIRINOX at any of 14 centers in Kyushu, Japan (NAPOLEON study). We retrospectively reviewed the hospital medical records of the patients for the period between December 2013 and March 2017, and consecutive patients with locally advanced or metastatic pancreatic cancer were included. The following variables of the patients were investigated: the patient demographic characteristics (age, sex and body mass index), Eastern Cooperative Oncology Group performance status (ECOG PS), history of previous therapy (tumor resection and adjuvant chemotherapy), tumor size, tumor location (pancreatic head, body, or tail), tumor histology (adenocarcinoma, or not), sites of metastasis (liver, peritoneum, and/or lung), number of metastatic sites (one, or two or more), presence/absence of ascites, the AJCC TNM stage, and serum albumin, lactate dehydrogenase (LDH), C–reactive protein (CRP), carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) levels, and the first–line chemotherapy regimen used (GnP or FOLFIRINOX). These data were collected by clinicians with expertise in clinical research under the supervision of the statistician and centrally managed. This study was conducted with the approval of the institutional review board of each participating institution, and according to the principles laid down in the Declaration of Helsinki.
Treatment
All patients received either GnP or FOLFIRINOX as the first–line regimen. The GnP group consisted of patients who received nab–paclitaxel at the dose of 125 mg/m2 given as a 30–minute intravenous infusion, followed by GEM at the dose of 1000 mg/m2 given as a 30–minute intravenous infusion on days 1, 8, and 15, every 4 weeks [4]. The FOLFIRINOX group received either the original or the modified regimen. The original FOLFIRINOX regimen consists of a combination of oxaliplatin at the dose of 85 mg/m2 given as a 2–hour intravenous infusion, followed by l–leucovorin at the dose of 200 mg/m2 given as a 2–hour intravenous infusion, with the addition, after 30 minutes, of irinotecan at the dose of 180 mg/m2 given as a 90–minute intravenous infusion, followed by fluorouracil at the dose of 400 mg/m2 given as an intravenous bolus injection, followed by a continuous intravenous fluorouracil infusion at 2400 mg/m2 over a 46–hour period, every 2 weeks. The modified FOLFIRINOX regimen consists of a combination of oxaliplatin at the dose of 85 mg/m2 given as a 2–hour intravenous infusion, followed by l–leucovorin at the dose of 200 mg/m2 given as a 2–hour intravenous infusion, with the addition, after 30 minutes, of irinotecan at the dose of 150 mg/m2 given as a 90–minute intravenous infusion, followed by continuous intravenous fluorouracil infusion at 2400 mg/m2 over a 46–hour period, every 2 weeks [13, 14].
Assessments
The goal of this study was to identify factors influencing the prognosis in pancreatic cancer patients, and then to develop and validate a prognostic nomogram in a relatively large real–world cohort derived from the NAPOLEON study. The overall survival was calculated as the interval from the date of initiation of first–line chemotherapy to the date of death from any cause or the date of the last follow–up. The 8th edition of the AJCC staging system for pancreatic cancer was used [15]. The pancreatic cancer stages are categorized as follows: Stage I A, T1 N0 M0; Stage I B, T2 N0 M0; Stage II A, T3 N0 M0; Stage IIB, T1–3 N1 M0; Stage III, T–Any N2 M0 or T4 N–Any M0; and Stage IV, T– Any N–Any M1.
Statistical analysis and drawing of the nomogram
Missing data were imputed by using the method of multiple imputation with predictive mean matching [16]. The imputation model included variables for tumor size, and serum albumin, LDH, CRP and CA19–9 levels. The Cox proportional hazard model was used to identify the independent prognostic factors for overall survival. Factors showing differences with P values of <0.05 were considered as being statistically significant. Prognostic factors judged to be clinically important and those with P values of <0.05 were selected, and a prognostic nomogram to predict the 6–, 12–, and 18–month survival probabilities was generated on the basis of the final model and validated using the concordance index (C–index) and calibration plot by the bootstrapping method (200 resamplings). The final model was compared with the AJCC TNM staging system to assess discrimination ability for survival prediction based on the time–dependent area under the curve (t–AUC) in a Receiver Operating Characteristic (ROC) curve analysis. And then, we attempted to develop a method of risk stratification for survival according to the tertiles of the total nomogram points and compared the survival times among the risk groups (Fig. 1). The advantage of this nomogram over the AJCC TNM staging system in predicting survival was confirmed to compare the C–indexes of the nomogram and AJCC TNM staging system. The statistical analyses were performed using the software program R ver. 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
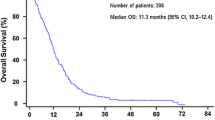
A total of 318 patients were enrolled between December 2013 and March 2017. The baseline characteristics of the 318 patients are shown in Table 1. By the end of the follow–up period, 197 patients (61.9%) had died. Of the 197 deaths, 195 patients died from pancreatic cancer and 2 patients were died from other diseases. The median overall survival was 11.3 months, and the median follow-up period was 11.4 months.
The results of the univariate and multivariate analysis are listed in Table 2. The univariate analyses identified higher ECOG PS scores, presence of liver metastasis, more than two sites of metastatic disease, presence of ascites, serum albumin level less than 3.0 g/dL, elevated serum LDH, elevated serum CRP, serum CA19–9 level greater than 370 U/mL, and AJCC TNM stage IV as being significantly associated with shorter overall survival times. Multivariate analysis identified that ECOG PS, presence/absence of liver metastasis, serum LDH, serum CRP, and serum CA19–9 as independent predictors of the overall survival time.
The prognostic nomogram integrating all the significant independent predictors of the overall survival identified by the multivariate analysis is shown in Fig. 2. The values of the C–index (bootstrapping 95% confidence intervals [CIs]) of the prognostic nomogram for overall survival prediction were 0.77 (0.73–0.81), 0.72 (0.67–0.76), and 0.70 (0.65–0.75) for 6–, 12–, and 18–month survival, respectively. These values were statistically significantly higher for all the points examined, as compared to the values for the AJCC TNM staging system (all P values<0.01) (Table 3, Fig. S1).
The calibration plot for the probability of survival at 6, 12, and 18 months showed optimal agreement between the predictions according to the nomogram and the actual observations (Fig. S2). The mean absolute errors between the observed and predicted probabilities were <0.01, 0.03 and 0.04 for 6–, 12–, and 18–month survival, respectively. The errors for 90% of the study population were within 0.01, 0.02 and 0.08, respectively.
Risk stratification by using the tertiles of the total nomogram points yielded clear separations among the survival curves. The median survival times in the low– (total nomogram points <56), moderate– (total nomogram points 56–115), and high– (total nomogram points ≥115) risk groups were 15.8 months (reference), 12.8 months (Hazard ratio [HR], 1.44; 95% CI, 1.03–2.01; P=0.03), and 7.8 months (HR, 3.34; 95% CI, 2.40–4.64; P<0.01), respectively (Fig. 3).
Discussion
In this study, we developed a convenient prognostic nomogram based on five independent prognostic variables (ECOG PS, presence/absence of liver metastasis, serum LDH, serum CRP, and serum CA19–9) which could accurately predict survival in patients with unresectable pancreatic cancer undergoing treatment with GnP or FOLFIRINOX. Currently, the AJCC TNM staging system is the most widely used prognostic tool for patients with cancer, including pancreatic cancer. However, this staging system has a few limitations in regard to the analysis of survival. Importantly, it focuses only on tumor characteristics, while the importance also of patient–related factors in determining the disease outcomes in cancer patients has come to be increasingly recognized in recent years [17] Thus, we were prompted to develop a more accurate prognostic tool, and the nomogram that we have developed is an inexpensive tool based on easily determined variables, including both patient and tumor characteristics; it is expected to be a helpful tool for clinicians engaged in the treatment of unresectable pancreatic cancer patients.
ECOG PS is recognized as one of the most important prognostic factors in patients with a variety of cancers [18, 19], and as in the present study, several previous studies have also reported ECOG PS as an independent prognostic factor in patients with pancreatic cancer [20, 21]. We demonstrated herein that the patient prognosis became poorer as the ECOG PS score increased.
Presence of liver metastasis has been reported as an important predictor of survival in patients with various cancers [4, 22], and the MPACT trial showed that the presence of liver metastasis is an important predictor of survival also in patients with pancreatic cancer [4]. Among the distant metastases, including those to the liver, lung and peritoneum, it is unclear why only the presence of liver metastasis was associated with a poor prognosis in our study. Liver metastasis is associated with activation of hepatic stellate cells, which are key components of the hepatic tumor microenvironment and can acquire chemoresistance [23, 24]. Another possible explanation is that patients with liver metastasis could eventually develop jaundice or hepatic coma with increase in the number of metastatic tumors, which would make it difficult to continue with effective systemic chemotherapy, and potentially result in a fatal outcome.
An elevated serum LDH level in pancreatic cancer patients has been recognized as an indicator of tumor aggressiveness, tumor burden, and poor outcome [25], and has also been associated with chemoresistance to several anticancer–drugs, including paclitaxel and gemcitabine [26]. These phenomena might be explained by tumor hypoxia, which promotes the growth of immature and highly permeable blood vessels that drive the abnormal growth and metastatic behavior of pancreatic cancer and facilitate the passage of tumor cells into the circulation [27]. Actually, serum level of LDH significantly increases in hypoxic condition, and serves as an indirect marker of tumor hypoxia [25]. For these reasons, the results of our study, consistent with previous reports, also suggested that an elevated serum LDH level might be associated with a poor prognosis [25, 28].
An elevated serum CRP level has also been demonstrated to be an independent prognostic factor in patients with various types of cancers [29]. Proinflammatory cytokines, such as interleukin–6 (IL–6), interleukin–1, and tumor necrosis factor–alpha, are secreted by monocytes or macrophages under inflammatory conditions and cancer [30]. Serum concentrations of IL–6 and CRP are known to be positively correlated with each other, and recent evidence suggests that IL–6 also affects the rate of cancer progression [31]. Furthermore, there is also evidence to suggest that these inflammatory cytokines play important roles in the genesis of cancer–associated cachexia, which shortens the survival time in patients with advanced pancreatic cancer [32, 33].
Serum CA19–9 is the only biomarker that the National Comprehensive Cancer Network guidelines for pancreatic cancer suggest is useful as a prognostic marker in patients receiving chemotherapy [34]. One prospective study has reported the possible usefulness of serum CA19–9 as a prognostic biomarker in patients with advanced pancreatic cancer [35], and another prospective study showed that a decrease of the serum CA19–9 level during chemotherapy is predictive of a longer survival time in patients with advanced pancreatic cancer [36].
Our prognostic nomogram was created based on the above theoretical background of the above–mentioned independent prognostic factors. Then, we verified the nomogram by determining the values of the C–index and t–AUC, and constructing a calibration plot and Kaplan–Meier curves for the three risk categories. The values of the C–index of the nomogram for 6–, 12–, and 18–month survival were all more than 0.7, indicating a good match between the predicted and actual survival. Calibration and validation using the bootstrapping method also indicated satisfactory performance of the nomogram. In addition, total nomogram points can also be useful for predicting the survival, and Kaplan–Meier curves constructed using tertiles of the total nomogram points showed clear separations among the survival curves. Moreover, our nomogram provided better predictive performance for overall survival as compared to the AJCC TNM staging system using t–AUC. Notably, our nomogram was not only based on the real–world data of patients treated with GnP or FOLFIRINOX, but also constructed using conventional variables which can easily be obtained at any medical institution in daily practice. Compared with previous nomogram in pancreatic cancer, our nomogram was created using larger cohort of 14 institutions, which could improve the accuracy of the model. In addition, our nomogram can predict prognosis not only at 6–month but also at 12– and 18–month [37]. Thus, this nomogram can be helpful to clinicians for making appropriate clinical decisions in daily practice. Furthermore, another benefit of this prognostic nomogram includes the possibility of selecting patients who are fit for clinical trials.
This study had several limitations. Firstly, it was a non–randomized, retrospective study, which could introduce selection bias, with a smaller number of patients as compared to previous studies [38]. Thus, we were unable to include several patient data, such as weight loss, quality of life, and screening status before the diagnosis, which were not fully documented in the hospital records. To compensate the small number of cases, we selected the bootstrapping method which is statistically established as adjusting the optimism of the model [39], and there are several studies which also adopted this method [10-12 ] . We also consider it’s necessary to extend the number of patients and validate the results including other regimens, so we are going to collect patients’ data prospectively and analyze in the future. The second limitation was that the study lacked cross–validation so as that it would be difficult to generalize our results to other cohorts. However, we developed the nomogram using a spatiotemporally heterogeneous population recruited from multiple centers, which could contribute to improving the validity of this model. The third limitation was that our study focused on specific race and geographical location. Since this study was focused only on Japanese, it might be difficult to apply the results to other race or geographical regions. However, there are some similarities between our results and those of other regions. Previous studies from other regions reported that CA19–9, performance states and liver metastases are associated with survival as we showed [20, 37, 40]. Prognostic factors such as performance states and tumor marker were also identified in other cancers [41, 42]. Although it is a matter of speculation, these factors might be common regardless of race, regions or type of cancers. Finally, some patients were only clinically diagnosed as having pancreatic cancer, without histological confirmation. These indicate that some patients in the real–world situation have no choice, but to receive systemic chemotherapy without histological evidence for various reasons, including those related to the patients themselves and/or to the facilities that they seek treatment at.
In conclusion, our prognostic nomogram might be a convenient and inexpensive tool for accurate prediction of the prognosis in Japanese patients with unresectable pancreatic cancer undergoing treatment with GnP or FOLFIRINOX, and will help clinicians in selecting appropriate therapeutic strategies for individualized management.
Availability of data and materials
All data generated or analyzed in this article are stored in a secured research database. They are not publicly available, however, are available through the corresponding author on reasonable request.
Abbreviations
- GnP:
-
gemcitabine plus nab–paclitaxel
- C–index:
-
concordance index
- AJCC:
-
American Joint Committee on Cancer
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- LDH:
-
lactate dehydrogenase
- CRP:
-
C–reactive protein
- CEA:
-
carcinoembryonic antigen
- CA19–9:
-
carbohydrate antigen 19–9
- t–AUC:
-
time–dependent area under the curve
- ROC:
-
receiver operating characteristic
- CI:
-
confidence interval
- HR:
-
hazard ratio
- IL–6:
-
interleukin–6
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Japanese journal of clinical oncology. 2015;45(9):884–91.
Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143(1):75–83 discussion 83.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23(10):1872–80.
Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, et al. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269(5):944–50.
Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical Nomogram for Predicting Survival Outcomes in Early Mucinous Breast Cancer. PLoS One. 2016;11(10):e0164921.
Li Y, Ju J, Liu X, Gao T, Wang Z, Ni Q, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in patients with major salivary gland cancer: a population-based study. Oncotarget. 2017;8(15):24469–82.
Zhang ZY, Luo QF, Yin XW, Dai ZL, Basnet S, Ge HY. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer. 2016;16(1):658.
Dong F, Shen Y, Gao F, Shi X, Xu T, Wang X, et al. Nomograms to Predict Individual Prognosis of Patients with Primary Small Cell Carcinoma of the Bladder. J Cancer. 2018;9(7):1152–64.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–25.
Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81(6):1017–23.
Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual. Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25(4):845–7.
Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol. 2014;14:75.
Hanahan D, Weinberg RA. Hallmarks of cancer. the next generation. Cell. 2011;144(5):646–74.
Suzuki Y, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, et al. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J Gastroenterol. 2019;54(3):281–90.
Lim ST, Hee SW, Quek R, Tao M. Performance status is the single most important prognostic factor in lymphoma patients aged greater than 75 overriding other prognostic factors such as histology. Leuk Lymphoma. 2008;49(1):149–51.
Fernández A, Salgado M, García A, Buxò E, Vera R, Adeva J, et al. Prognostic factors for survival with nab-paclitaxel plus gemcitabine in metastatic pancreatic cancer in real-life practice: the ANICE-PaC study. BMC cancer. 2018;18(1):1185-1185.
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC cancer. 2008;8:82.
Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC cancer. 2018;18(1):78.
Ruan Q, Wang H, Burke LJ, Bridle KR, Li X, Zhao CX, et al. Therapeutic modulators of hepatic stellate cells for hepatocellular carcinoma. Int J Cancer. 2020.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18(5):549–60.
Yu SL, Xu LT, Qi Q, Geng YW, Chen H, Meng ZQ, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194.
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33.
Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110(1):172–82.
Gan J, Wang W, Yang Z, Pan J, Zheng L, Yin L. Prognostic value of pretreatment serum lactate dehydrogenase level in pancreatic cancer patients: A meta-analysis of 18 observational studies. Medicine (Baltimore). 2018;97(46):e13151.
Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–70.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–12.
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88.
Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89(5):1681–4.
Vainer N, Dehlendorff C, Johansen JS. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget. 2018;9(51):29820–41.
Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17(5.5):603-5.
Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9(2):132–8.
Halm U, Schumann T, Schiefke I, Witzigmann H, Mössner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82(5):1013–6.
Fornaro L, Leone F, Vienot A, Casadei-Gardini A, Vivaldi C, Lièvre A, et al. Validated Nomogram Predicting 6-Month Survival in Pancreatic Cancer Patients Receiving First-Line 5-Fluorouracil, Oxaliplatin, and Irinotecan. Clin Colorectal Cancer. 2019;18(4):e394–401.
Song W, Miao DL, Chen L. Nomogram for predicting survival in patients with pancreatic cancer. Onco Targets Ther. 2018;11:539–45.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Vernerey D, Huguet F, Vienot A, Goldstein D, Paget-Bailly S, Van Laethem JL, et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br J Cancer. 2016;115(3):281–9.
Miyoshi Y, Noguchi K, Yanagisawa M, Taguri M, Morita S, Ikeda I, et al. Nomogram for overall survival of Japanese patients with bone-metastatic prostate cancer. BMC Cancer. 2015;15:338.
Ichikawa W, Uehara K, Minamimura K, Tanaka C, Takii Y, Miyauchi H, et al. A nomogram for predicting overall survival (OS) in Japanese patients (pts) with advanced colorectal cancer (aCRC) treated with irinotecan (IRI)-based regimens. Annals of Oncology. 2016;27:vi192.
Acknowledgement
We thank all of the patients and their families, and all of the investigators at the 14 institutions that participated in the NAPOLEON study. We would also like to thank the Fukuoka Medical Oncology Group – Kyushu Yamaguchi Total Oncology Group (FMOG–KYTOG) and the Saga Study Group of Liver Disease (SASLD) for their cooperation. We are indebted to Dr. Y. Okabe of the Kurume University Hospital, Dr. Y. Kawaguchi of the Saga Medical Centre Koseikan, and Dr. M. Uenomachi of the Hamanomachi Hospital for their assistance in the data collection or discussion. We also thank International Medical Information Center (Shinjuku–ku, Tokyo, Japan) for providing medical writing support.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
TS1, TO, TS2, MA, TM and KM contributed to the study conception and design. Data acquisition was performed by TS1, MK, TO, TS2, FK, YU, JN, AK, SO, MF, SA, AM, HT, TH, KN, YI and KM. Quality control of data, data analysis and interpretation were performed by TS1, TO, TS2 and KM. Statistical analysis was performed by TS1, TO, TS2 and MS. The first draft of the manuscript was written by TS1, TO, TS2, TM and KM. All authors commented on previous of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted to the ethical guideline of the Declaration of Helsinki and was centrally approved by the Institutional review board of Saga Medical Center Koseikan (study ID 17-09-01-02), and also approved by the Institutional review boards or ethics committee of following institutions: Imari Arita Kyoritsu Hospital, Japanese Red Cross Kumamoto Hospital, Kagoshima City Hospital, Oita University Hospital, Kagoshima University Hospital, Kurume University Hospital, Japan Community Healthcare Organization Kyushu Hospital, Saiseikai Sendai Hospital, Nagasaki University Hospital, Hamanomachi Hospital, Sasebo Kyosai Hospital, Karatsu Red Cross Hospital and Fukuoka Wajiro Hospital. Because this study was a retrospective observational study carried out in Japan, informed consent was obtained using the opt-in/opt-out approach according to each participating institution’s policy.
Consent for publication
Not applicable
Competing interests
M.S. received a personal fee from Sysmex Corporation; S.A. received personal fees from Taiho Pharmaceutical, Novartis Pharma, Chugai, Bristol–Myers Squibb, Daiichi–Sankyo, and AstraZeneca; A.M. received fees from Eli Lilly, Chugai and Takeda; T.S. received fees from Taiho Pharmaceutical, Chugai and Takeda; T.O. received a fee from Chugai. The other authors have no competing interests or financial disclosures to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The C–indices were statistically significantly higher for all the points examined, as compared to those for the AJCC TNM staging system.
Additional file 2: Figure S2.
The calibration plot for the probability of survival at 6–, 12–, and 18–months showed optimal agreement between the predictions according to the nomogram and the actual observations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shibuki, T., Mizuta, T., Shimokawa, M. et al. Prognostic nomogram for patients with unresectable pancreatic cancer treated with gemcitabine plus nab–paclitaxel or FOLFIRINOX: A post–hoc analysis of a multicenter retrospective study in Japan (NAPOLEON study). BMC Cancer 22, 19 (2022). https://doi.org/10.1186/s12885-021-09139-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-09139-y