Abstract
Background
To evaluate the prognostic value of the apparent diffusion coefficient (ADC) derived from diffusion-weighted magnetic resonance imaging (MRI) and monitor the early treatment response to induction chemotherapy (IC) with plasma EBV DNA in locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Results
A total of 307 stage III-IVb NPC patients were prospectively enrolled. All patients underwent MRI examinations to calculate ADC and plasma EBV DNA measurements pretreatment and post-IC. The participants’ ADC value of 92.5% (284/307) increased post-IC. A higher percent change in ADC value (ΔADC%high group) post-IC was associated with a higher 5-year OS rate (90.7% vs 74.9%, p < 0.001) than those in the ΔADC%low group. Interestingly, ΔADC% was closely related to the response measured by RECIST 1.1 (p < 0.001) and plasma EBV DNA level (p = 0.037). The AUC significantly increased when post-IC plasma EBV DNA was added to ΔADC% to predict treatment failure. Thus, based on ΔADC% and plasma EBV DNA, we further divided the participants into three new prognostic response phenotypes (early response, intermediate response, and no response) that correlated with disparate risks of death (p = 0.001), disease progression (p < 0.001), distant metastasis (p < 0.001), and locoregional relapse (p < 0.001).
Conclusion
The percentage change in ADC post-IC is indicative of treatment response and clinical outcome. ΔADC% and plasma EBV DNA-based response phenotypes may provide potential utility for early termination of treatment and allow guiding risk-adapted therapeutic strategies for LA-NPC.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a unique epithelial carcinoma occurring in the nasopharynx. NPC is distinguished from other head and neck cancers with regard to its biological behavior, therapeutic strategies, and etiology coexisting with Epstein-Barr virus infection [1, 2]. Intensity-modulated radiotherapy (IMRT) is the major treatment modality for NPC [3]. For locoregionally advanced NPC (LA-NPC), the curative effects of induction chemotherapy (IC) followed by concurrent chemotherapy (CCRT) have been investigated for the past decade [4,5,6]. An increasing number of clinical trials have shown that IC is a relatively safe and effective treatment method for LA-NPC that significantly improves clinical outcomes [7, 8]. Benefiting from the development of radiation techniques and multimodality therapies, the survival rate of NPC has favorably improved. However, approximately 20% of individuals still experience distant or locoregional relapse [9, 10]. Thus, early detection of treatment response would enhance existing therapeutic strategies to better optimize outcomes in individuals with a high risk of treatment failure post primary treatment.
Currently, magnetic resonance imaging (MRI) is the dominating imaging tool for diagnosis, staging, and treatment response evaluation in NPC. Diffusion-weighted MRI generates contrast based on the Brownian movement of water molecules restricted by neighboring structures [11, 12]. Quantitative analysis of the diffusion-weighted MRI signal with the apparent diffusion coefficient (ADC) provides a potential imaging marker related to microvascular circulation, membrane integrity, and cell density for tumor characterization and response assessment [13]. The ADC value has been shown to be associated with treatment response and/or outcomes in many malignant diseases, including esophageal cancer, breast cancer, and colorectal cancer [14,15,16]. Regarding NPC, previous studies demonstrated that ADC increases after IC, and pretreatment ADC or posttreatment changes in ADC are characteristic of treatment response [17,18,19,20]. However, the prognostic value and long-term survival prediction of posttreatment changes in ADC in NPC have not been fully investigated.
Therefore, we conducted a prospective study to investigate whether the posttreatment changes in ADC after IC are independent prognostic markers in LA-NPC and explored the clinical significance between the changes in ADC and plasma EBV DNA. This study may be conducive to identifying different responders to IC and potentially guide treatment decisions.
Methods
All methods were carried out in accordance with SAMPL Guidelines.
Patients
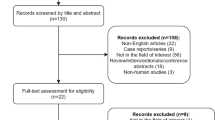
This prospective study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board (IRB) and Clinical Research Committee of the study institute. Patients were required to provide written informed consent before enrolling in the study. From January 2011 to November 2016, we recruited 386 biopsy-confirmed, newly diagnosed NPC patients. Inclusion criteria are listed as follows: (1) age ≥ 18 years old; (2) stage III-IVb disease according to the seventh edition of the International Union Against Cancer/American Joint Committee on Cancer staging system; (3) score of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) performance status grade; (4) underwent IC treatment followed by CCRT; (5) complete pretreatment and post-IC plasma EBV DNA measurement data; and (6) adequate function of blood, liver, and kidneys. The exclusion criteria for patients is listed as follows: (1) a history of previous or synchronous malignant tumors, (2) primary distant metastasis, (3) pregnancy or lactation, (4) unsuitability for MRI, (5) no post-IC or RT EBV DNA measurement, and (6) no post-IC or RT MRI examination and low-quality ADC map. A total of 307 eligible participants were included in the final analysis. The case accrual process is summarized in Fig. 1. This study was approved by the Clinical Research Committee of the study institute.
Study design
All patients were assessed pretreatment through a complete medical history, physical examination, fiber optic nasopharyngoscopy, chest X-rays, abdominal sonography, electrocardiography, and bone scan or 18 F-FDG positron emission tomography/computed tomography scans, hematology, and biochemical profiles. MRI of the nasopharynx, neck examination, and plasma EBV DNA level measurement using real-time quantitative polymerase chain reactions [21] were conducted before treatment, 1 week after completion of IC, and within 1 week after RT. Response was defined radiologically according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as complete response (CR), partial response (PR), stable disease (SD), or disease progression (PD) [22]. The response was also evaluated biochemically through plasma EBV DNA levels as detectable (> 0 copies/mL) or undetectable (= 0 copies/mL) cases. The patients who exhibited CR/PR and undetectable EBV DNA post IC were considered to be favorable responders.
MRI technique
MRIs were conducted using a 3-T imaging technique (Trio Tim; Siemens, Erlangen, Germany). Information on the MRI procedure is detailed in the supplementary materials.
The ADC value was calculated using ADC = −ln [SI(b)/SI(0)]/b, where SI is the measured signal intensity, b is the b value, and SI(b) and SI(0) are the signal intensities with and without diffusion-sensitizing gradients, respectively. Before treatment and post-IC, the ADC value was assessed for both the primary lesions and metastatic lymph nodes on the ADC map at the level of the maximum tumor diameter to cover most of the lesion, avoiding cystic or necrotic components. Regions of interest (ROIs) were defined by selecting areas with high b value DWI (b = 1000 s/mm2) and relatively low ADC. Regions with high T2 signal, adjacent adipose and fibroglandular tissue, and biopsy clip artifacts were evaded. Tumor ROIs.
were redefined for each treatment time point, referencing lesion location on pretreatment MRI. The longest diameter of primary tumor was determined on the same largest transverse section in pretreatment MRI and post-IC MRI to assess therapeutic response to IC.
Percentage changes in the ADC values (ΔADC%) were calculated as follows: ΔADC% = (post-IC ADC value - pretreatment ADC value)/pretreatment ADC value × 100%. Each MRI image was analyzed by the same radiologists, and the final regions of interest (ROIs) were checked by another radiologist.
Treatment
Two to three cycles of induction chemotherapy were administered to all patients, and the regimens of IC included TPF [cisplatin (60–75 mg/m2, day 1) and docetaxel (60–75 mg/m2, day 1) with 5-fluorouracil (600–750 mg/m2, 96 h of continuous intravenous infusion)] or PF [cisplatin (80–100 mg/m2, day 1) with 5-fluorouracil (800–1000 mg/m2, 96 h of continuous intravenous infusion)]. Concurrent cisplatin (100 mg/m2 every 3 weeks) chemotherapy was conducted every 3 weeks at RT. All of the study participants were treated with IMRT, and a simultaneously integrated boost was mandatory. The design of the IMRT plan and technique are detailed in the supplementary materials [23].
Outcome and follow-up
Overall survival (OS) was the primary study endpoint, which was defined as the time of treatment initiation until death from any cause or last follow-up. Secondary endpoints included progression-free survival (PFS), defined as the time of treatment initiation to the date of the first failure at any site or death from any cause or final follow-up; distant metastasis-free survival (DMFS), defined as the time of treatment initiation to the date of distant relapse or last follow-up; and locoregional relapse-free survival (LRFS), calculated from the time of treatment initiation to locoregional relapse or last follow-up. After treatment, the patients were assessed at least every 3 months for the first 3 years and underwent follow-up examinations every 6 months thereafter or until death.
Statistical analysis
Details of the statistical analysis procedure are presented in the supplementary materials.
Results
Of the entire cohort of 307 participants, the median pretreatment ADC value was 2590 × 10− 6 mm2s− 1 (range: 641 × 10− 6 mm2s− 1 - 4420 × 10− 6 mm2s− 1) (Fig. 2E). After IC, the ADC value of 92.5% (284/307) of the participants increased (Fig. 4). The median post-IC ADC value was 3317 × 10− 6 mm2s− 1 (range: 804 × 10− 6 mm2s− 1-5764 × 10− 6 mm2s− 1) (Fig. 2E), and the median percentage change in the ADC value was 26% (range: − 12-125%). After a median follow-up of 72 months (range: 3–108 months), 16.9% (52/307) of the patients died, and 28.9% (89/307) developed disease progression. The 5-year OS, PFS, DMFS, and LRFS rates were 86.6, 73.6, 85.3, and 86.6%, respectively.
Waterfall plots and box plots showing the association of the percentage change in ADC post induction chemotherapy (IC) with response measured by RECIST 1.1 and plasma EBV DNA levels. (A, D: percentage change in ADC post IC measured by plasma EBV DNA levels post IC; B, G: percentage change in ADC post IC measured by response measured by RECIST 1.1 post IC; C, H: percentage change in ADC post IC measured by plasma EBV DNA levels post radiotherapy; D, I: percentage change in ADC post IC measured by response measured by RECIST 1.1 post radiotherapy; E: The median and range of pretreatment and post IC ADC value)
Prognostic value of pretreatment ADC and percentage change in ADC in LA-NPC
The patients in this study were divided into two groups according to the median pretreatment ADC (ADClow group vs ADChigh group) and ΔADC% (ΔADC%low group vs ΔADC%high group). Patient characteristics in the different groups are shown in Table 1. The Kaplan-Meier survival curves of the groups are shown in Fig. 3. For the pretreatment ADC, the 5-year PFS (78.0% vs 68.2%, p = 0.015; Fig. 3B) rates for the ADClow group were significantly higher than the corresponding rates for the ADChigh group. There were no survival differences in the 5-year OS (87.8% vs 84.5%, p = 0.241, Fig. 3A), DMFS (88.6% vs 82.1%, p = 0.106, Fig. 3C), or 5-year LRFS (89.5% vs 82.7%, p = 0.066, Fig. 3D) rates between the two groups. In terms of ΔADC%, significantly lower survival rates were documented between the ΔADC%low and ΔADC%high groups for all endpoints (OS, 74.9% vs 90.7%, p < 0.001, Fig. 3E; PFS, 61.1% vs 85.0%, p < 0.001, Fig. 3F; DMFS, 74.4% vs 90.5%, p < 0.001, Fig. 3G; LRFS: 80.7% vs 91.6%, p < 0.001, Fig. 3H).
Kaplan-Meier curves of overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional relapse-free survival (LRFS) of patients with LA-NPC stratified by pretreatment ADC (OS: A, PFS: B, DMFS: C, LRFS: D), ΔADC% (OS: E, PFS: F, DMFS: G, LRFS: H), and the ΔADC% and plasma EBV DNA-based response phenotypes (OS: I, PFS: J, DMFS: K, LRFS: L)
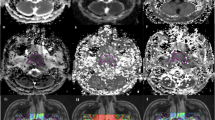
Images of a 47-year-old man with LA-NPC showing intermediate response after induction chemotherapy: (A, D, and G) primary lesion (arrow) in axial T2-weighted MRI with (B, E, and H) corresponding axial diffusion-weighted MRI with b values of 0 and 1000 s/mm2 (C, F, and I), apparent diffusion coefficient (ADC) maps pretreatment, and (J-R) matched sections of the same imaging sequences after induction chemotherapy
A multivariate analysis conducted to adjust for confounding factors demonstrated that ΔADC% was an independent prognostic factor for OS (HR = 0.44, 95% CI = 0.24–0.82, p = 0.009), PFS (HR = 0.35, 95% CI = 0.22–0.56, p < 0.001), DMFS (HR = 0.42, 95% CI = 0.21–0.81, p = 0.010), and LRFS (HR = 0.26, 95% CI = 0.12–0.55, p < 0.001) (Table 2). Receiver operating characteristic (ROC) curves used to assess the prognostic validity demonstrated that the area under the curve (AUC) of ΔADC% was significantly higher than that of pretreatment ADC (0.634 vs 0.534, p = 0.002, Supplementary Fig. 1). Thus, ΔADC% was a better prognostic indicator for the clinical outcomes than pretreatment ADC.
Association of ΔADC% with response measured by RECIST 1.1 and plasma EBV DNA levels
The participants in the ΔADC%high group were significantly more likely to achieve CR/PR after IC than those in the ΔADC%low group (83.4% vs 63.3%, p < 0.001, Table 1, Fig. 2B and G). Moreover, a significantly higher proportion of the participants in the ΔADC%low group had residual disease after RT than those in the ΔADC%high group (72.7% vs 87.3%, p = 0.001, Fig. 2D and I).
The plasma EBV DNA levels of the participants in the ΔADC%high group were more likely to decrease to undetectable levels both post-IC (70.7% vs 59.3%, p = 0.037, Fig. 2A and F) and post-RT (92.7% vs 97.5%, p = 0.093, Fig. 2C and H) compared with the ΔADC%low group. The ROC curves also showed that the AUC significantly increased when ΔADC% was added to post-IC plasma EBV DNA for predicting treatment failure (Supplementary Fig. 1).
Based on these observations, we defined the patients with three different ΔADC% and plasma EBV DNA-based response phenotypes: early response (ΔADC%high with undetectable EBV DNA post-IC), intermediate response (ΔADC%high with detectable EBV DNA or ΔADC%low with undetectable EBV DNA post-IC), and no response (ΔADC%low with detectable EBV DNA post-IC).
ΔADC% and EBV DNA-based response phenotypes predicted the prognosis of LA-NPC
Additionally, we evaluated the prognostic association of the three response phenotypes with survival outcomes in our study. We observed that prognoses were significantly different among the groups. In particular, the patients in the early response group achieved the most favorable survival among all endpoints (5-year OS = 95.6, 86.7, and 70.1%, respectively, p < 0.001, Fig. 3I; PFS = 90.6, 71.5, and 42.6%, respectively, p < 0.001, Fig. 3J; DMFS = 96.0, 84.6, and 69.4%, respectively, p < 0.001, Fig. 3K; and LRFS = 95.9, 86.2, and 64.3%, respectively, p < 0.001, Fig. 3L).
We investigated potential associations between the ΔADC% and plasma EBV DNA-based response phenotypes and other clinical covariates that may have influenced the outcome, including age, sex, T stage, N stage, smoking, and family history. The response phenotype was an independent prognostic indicator for OS (p = 0.001), PFS (p < 0.001), DMFS (p = 0.001), and LRFS (p < 0.001) (Table 2).
Discussion
In this study, we demonstrated that the percentage change in ADC was a prognostic indicator independent of other clinical factors that could predict and identify responses to induction chemotherapy. The ADC increase in the patients with LA-NPC after induction chemotherapy appeared to be in line with the effective treatment. The patients in the ΔADC%high group were more likely to achieve CR/PR and undetectable EBV DNA after IC than those in the ΔADC%low group. Combining the ΔADC% with plasma EBV DNA after IC resulted in superior prognostic values for clinical outcomes compared to ΔADC% alone.
The establishment of therapeutic decisions is primarily based on the TNM stage. However, the TNM stage reflects only the anatomical invasion of tumors and lacks biological information, as clinical outcomes vary substantially among patients with the same stage. Diffusion-weighted MRI signals using ADC enable noninvasive assessment of tumor microstructure and the intrinsic biological characteristics of tissues and have proven value for monitoring response to therapy and predicting prognosis in many malignancies [13,14,15]. Low ADC values have been shown to indicate viable tissue with high cellularity based on the random displacement of water molecules, with increased ADC posttreatment reflecting increased extracellular space [24, 25].
In 2003, a study demonstrated that changes in diffusion parameters 1 week after initiating treatment using DW-MRI could detect early tumor response to RT in brain malignancies [26]. Later, breast cancer studies also reported that the percent change in ADC for assessing early tumor response to IC than morphological variables [27] and the change in ADC predicted complete pathologic response to IC [15]. A recent multicenter study in ovarian cancer showed that ADC changes are indicative of tumor response. After IC, increased ADC is indicative of improved progression-free survival in relapsed disease, suggesting its potential for effective treatment management.
Previous studies of NPC found that pretreatment ADC was a valuable marker for the prediction of the response to IC and local failure [17, 19]. Regarding the change in ADC, Chen et al. showed that a large increase in ADC was associated with good treatment response after IC in a cohort study of 31 patients with LA-NPC [18]. Zhang et al. and Hong et al. also found that percentage increases in ADC after IC were higher for responders than for nonresponders [20, 28]. In our study, post-IC percentage changes in ADC correlated with response evaluated by RECIST 1.1, which was in line with the results of previous studies. However, the relationship between the percentage change in ADC and clinical outcomes was not explored. The correlation between post-IC percentage changes in ADC and long-term survival outcomes in NPC remains unknown. Hence, we further analyzed the association of post-IC percentage change in ADC with clinical outcomes in a large cohort of patients with NPC from the endemic region and demonstrated that post-IC percentage change in ADC was an independent prognostic factor for overall survival, progression-free survival, distant metastasis-free survival, and locoregional relapse-free survival. The post-IC percentage change in ADC was observed to be closely related to the plasma EBV DNA change, a biological marker that has been widely used to predict the treatment response and prognosis of NPC [29, 30]. Consistent with these observations, we further divided the patients in this study into three different response phenotypes: early response, intermediate response, and no response. Notably, our results confirmed that the aforementioned phenotypic groups were associated with disparate risks of death, disease progression, and locoregional and distant relapse. Thus, prognostic response phenotypes could provide directions for future clinical trial designs. For patients in the early response groups, the treatment intensity could be reduced to avoid unnecessary toxicities. Therefore, we suggested reducing the radiation dose of RT, by either substituting concurrent chemotherapy with EGFR inhibitors or sparing concurrent chemotherapy with RT. Subsequently, for patients in intermediate groups, we proposed exploring the integration of EGFR inhibitors and immune checkpoint inhibitor therapy with CCRT. Finally, the combination of a second antitumor drug, such as paclitaxel, to enhance the radiosensitivity of CCRT, the inclusion of adjuvant chemotherapy, or the addition of immunotherapy for patients in the no response group can target residual resistant disease.
This study had limitations. First, the participants were collected from only a single center, and the results may not be easily generalized to other centers as a result of interinstitutional differences in MRI techniques. Second, no standard method was established to determine the ADC value. Thus, the applicability of the results in more challenging cohorts remains to be investigated. Prospective multicenter studies are required to validate the results of our study.
Conclusion
ADC measurements provide a noninvasive method of detecting early microstructural changes that occur in response to LA-NPC treatment. When there was residual measurable disease and plasma EBV DNA, ADC changes were greater in responders than in nonresponders. We identified patients with different radiobiological responses with disparate relapse risks among LA-NPC treated with IC followed by CCRT. These response phenotypes may allow different treatment intensities for optimal tumor control. As the initial treatment is important for LA-NPC, determining the appropriate treatment is crucial. The capacity to predict response may enable early intervention of treatment in nonresponding patients, avoid supererogatory toxicity, and allow early changes in therapeutic strategy.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the privacy of the patients but are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ΔADC%:
-
Percentage change in ADC value
- CCRT:
-
Concurrent chemoradiotherapy
- CR:
-
Complete response
- DMFS:
-
Distant metastasis-free survival
- EBV DNA:
-
Epstein-Barr virus deoxyribonucleic acid
- IC:
-
Induction chemotherapy
- IMRT:
-
Intensity modulated radiation therapy
- LA-NPC:
-
Locoregionally advanced nasopharyngeal carcinoma
- LRFS:
-
Locoregional relapse-free survival
- MRI:
-
Magnetic resonance imaging
- NPC:
-
Nasopharyngeal carcinoma
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PD:
-
Disease progression
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- ROC:
-
Receiver-operating characteristic
- ROIs:
-
Regions of interest
- SD:
-
Stable disease
- SI:
-
Signal intensity
References
Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–8.
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–24.
Lee AW, Ma BB, Ng WT, Chan AT. Management of Nasopharyngeal Carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–64.
Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242–9.
Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;91:952–60.
Chen YP, Tang LL, Yang Q, et al. Induction chemotherapy plus concurrent Chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018;24:1824–33.
Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–20.
Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and Cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–35.
Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22:4604–12.
Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network Meta-analysis. J Clin Oncol. 2017;35:498–505.
Wesbey GE, Moseley ME, Ehman RL. Translational molecular self-diffusion in magnetic resonance imaging. II. Measurement of the self-diffusion coefficient. Investig Radiol. 1984;19:491–8.
Le Bihan D, Turner R, Moonen CT, Pekar J. Imaging of diffusion and microcirculation with gradient sensitization: design, strategy, and significance. J Magn Reson Imaging. 1991;1:7–28.
Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR imaging in the head and neck. Radiology. 2012;263:19–32.
Vollenbrock SE, Voncken FEM, Bartels LW, et al. Diffusion-weighted MRI with ADC mapping for response prediction and assessment of oesophageal cancer: a systematic review. Radiother Oncol. 2020;142:17–26. https://doi.org/10.1016/j.radonc.2019.07.006.
Partridge SC, Zhang Z, Newitt DC, et al. Diffusion-weighted MRI findings predict pathologic response in Neoadjuvant treatment of breast Cancer: the ACRIN 6698 multicenter trial. Radiology. 2018;289:618–27.
Koh DM, Scurr E, Collins D, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–8.
Zhang Y, Liu X, Zhang Y, et al. Prognostic value of the primary lesion apparent diffusion coefficient (ADC) in nasopharyngeal carcinoma: a retrospective study of 541 cases. Sci Rep. 2015;5:12242.
Chen Y, Liu X, Zheng D, et al. Diffusion-weighted magnetic resonance imaging for early response assessment of chemoradiotherapy in patients with nasopharyngeal carcinoma. Magn Reson Imaging. 2014;32:630–7.
Zheng D, Chen Y, Chen Y, et al. Early assessment of induction chemotherapy response of nasopharyngeal carcinoma by pretreatment diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2013;37:673–80.
Zhang GY, Wang YJ, Liu JP, et al. Pretreatment diffusion-weighted MRI can predict the response to Neoadjuvant chemotherapy in patients with nasopharyngeal carcinoma. Biomed Res Int. 2015;2015:307943.
An X, Wang FH, Ding PR, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117:3750–7.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–8.
Hill DK, Heindl A, Zormpas-Petridis K, et al. Non-invasive prostate Cancer characterization with diffusion-weighted MRI: insight from in silico studies of a transgenic mouse model. Front Oncol. 2017;7:290.
Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224:177–83.
Mardor Y, Pfeffer R, Spiegelmann R, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094–100.
Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22:104–13.
Hong J, Yao Y, Zhang Y, et al. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2013;149:707–13.
Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614–9.
Liu LT, Tang LQ, Chen QY, et al. The prognostic value of plasma Epstein-Barr viral DNA and tumor response to Neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–9.
Acknowledgments
Not applicable.
Funding
This study was funded by grants from the National Key R&D Program of China (2017YFC0908500, 2017YFC1309003), the National Natural Science Foundation of China (No. 81425018, No. 81672868, No.81802775, No. 82073003, No.82002852, No. 82003267), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the Sun Yat-sen University Clinical Research 5010 Program (No. 2019023), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Natural Science Foundation of Guangdong Province (No.2017A030312003, No.2018A0303131004), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (No. 2018B030306001), the Health & Medical Collaborative Innovation Project of Guangzhou City (No.201803040003), Pearl River S&T Nova Program of Guangzhou (No. 201806010135), the Planned Science and Technology Project of Guangdong Province (2019B020230002), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2014BAI09B10), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Study concepts: Hai-Qiang Mai and Chuan-Miao Xie. Study design: Hai-Qiang Mai, Li-Ting Liu and Chuan-Miao Xie, Data acquisition: Hai-Qiang Mai,Li-Ting Liu, Shan-Shan Guo, Hui Li, Chao-Lin and Rui Sun. Quality control of data and algorithms: Hai-Qiang Mai, Li-Ting Liu, Shan-Shan Guo, Hui Li, Chao-Lin and Rui Sun. Data analysis and interpretation: Li-Ting Liu, Chuan-Miao Xie. Statistical analysis: Li-Ting Liu and Shan-Shan Guo. Manuscript preparation: Li-Ting Liu, Shan-Shan Guo, Hui Li, Chao-Lin, Rui Sun and Qiu-Yan Chen, Lin-Quan Tang. Manuscript editing: Hai-Qiang Mai, Li-Ting Liu, Shan-Shan Guo, Hui Li, Chao-Lin, Rui Sun, and Yu-Jing Liang. Manuscript review: Li-Ting Liu, Shan-Shan Guo, Hui Li, Chao Lin, Rui Sun, Qiu-Yan Chen, Yu-Jing Liang, Qing-Nan Tang, Xue-Song Sun, Lin-Quan Tang, Chuan-Miao Xie, and Hai-Qiang Mai. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Review Board (IRB) and Clinical Research Committee of Sun Yat-sen University Cancer Center. Patients were required to provide written informed consent before enrolling in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, LT., Guo, SS., Li, H. et al. Percent change in apparent diffusion coefficient and plasma EBV DNA after induction chemotherapy identifies distinct prognostic response phenotypes in advanced nasopharyngeal carcinoma. BMC Cancer 21, 1320 (2021). https://doi.org/10.1186/s12885-021-09063-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-09063-1