Abstract
Background
The prognostic roles of three lymph node classifications, number of positive lymph nodes (NPLN), log odds of positive lymph nodes (LODDS), and lymph node ratio (LNR) in lung adenocarcinoma are unclear. We aim to find the classification with the strongest predictive power and combine it with the American Joint Committee on Cancer (AJCC) 8th TNM stage to establish an optimal prognostic nomogram.
Methods
25,005 patients with T1-4N0–2M0 lung adenocarcinoma after surgery between 2004 to 2016 from the Surveillance, Epidemiology, and End Results database were included. The study cohort was divided into training cohort (13,551 patients) and external validation cohort (11,454 patients) according to different geographic region. Univariate and multivariate Cox regression analyses were performed on the training cohort to evaluate the predictive performance of NPLN (Model 1), LODDS (Model 2), LNR (Model 3) or LODDS+LNR (Model 4) respectively for cancer-specific survival and overall survival. Likelihood-ratio χ2 test, Akaike Information Criterion, Harrell concordance index, integrated discrimination improvement (IDI) and net reclassification improvement (NRI) were used to evaluate the predictive performance of the models. Nomograms were established according to the optimal models. They’re put into internal validation using bootstrapping technique and external validation using calibration curves. Nomograms were compared with AJCC 8th TNM stage using decision curve analysis.
Results
NPLN, LODDS and LNR were independent prognostic factors for cancer-specific survival and overall survival. LODDS+LNR (Model 4) demonstrated the highest Likelihood-ratio χ2 test, highest Harrell concordance index, and lowest Akaike Information Criterion, and IDI and NRI values suggested Model 4 had better prediction accuracy than other models. Internal and external validations showed that the nomograms combining TNM stage with LODDS+LNR were convincingly precise. Decision curve analysis suggested the nomograms performed better than AJCC 8th TNM stage in clinical practicability.
Conclusions
We constructed online nomograms for cancer-specific survival and overall survival of lung adenocarcinoma patients after surgery, which may facilitate doctors to provide highly individualized therapy.
Similar content being viewed by others
Background
Lung cancer is identified as the most common cancer and the leading cause of cancer-specific deaths in the United States, accounting for about 135,720 deaths in 2020 [1]. Traditionally, lung cancer is classified as non-small cell lung cancer (NSCLC) and small cell lung cancer. NSCLC constitutes 85% of all types of lung cancer cases, and the most common histological subtypes are lung adenocarcinoma (AC) and lung squamous cell carcinoma (SCC), which accounts for 60 and 15% separately [2]. Although lung AC and SCC are commonly categorized as NSCLC, a large body of experimental evidence support that these are very distinct diseases and have vastly distinct transcriptomic profiles, histopathologic, and clinicopathological features, resulting in different prognostics [3,4,5,6,7]. Hence, some researchers suggested abandoning the concept of NSCLC and adopting a subtype-centered tumor classification to develop more accurate diagnostic, therapeutic, and prognostic procedures [8]. Currently the prognostic researches are mostly on NSCLC [5, 9], while the prognostic models on lung AC are scarce. In addition, the prognosis for patients with NSCLC is currently estimated based on American Joint Committee on Cancer (AJCC) 8th tumor, node, metastasis (TNM) staging system, of which the N classification is based on the lymphatic region involved. This system does not take the number of dissected lymph nodes (NDLN) and number of positive lymph nodes (NPLN) into consideration. All these limitations detract from the accuracy of the prognostic estimates of lung AC.
Although NDLN and NPLN do not contribute to N classification, several studies have confirmed that higher values of NPLN, log odds of positive lymph nodes (LODDS, calculated using the formula: \( \boldsymbol{LODDS}=\mathbf{\log}\frac{\boldsymbol{NPLN}+\mathbf{0.5}\mathbf{0}}{\boldsymbol{NDLN}-\boldsymbol{NPLN}+\mathbf{0.5}} \)) or lymph node ratio (LNR, calculated using the formula: \( \boldsymbol{LNR}=\frac{\boldsymbol{NPLN}}{\boldsymbol{NDLN}} \)) were related to worse prognosis for NSCLC or several other types of malignant tumors [10,11,12,13]. However, of these node classifications, which can obtain the most precise prognosis in lung AC is unclear.
In our study, based on the Surveillance, Epidemiology, and End Results (SEER) database, we firstly aimed to comprehensively compare the prognostic efficacy of NPLN, LODDS, and LNR classification for predicting long-term survival outcomes of patients with lung AC. Secondly, according to the result of step one, we intended to establish and validate a model combining the N classification and the selected node number-based or ratio-based staging system, which can make the best use of the information of lymph node region and number of examined and positive nodes to predict the long-term post-operative cancer-specific survival and overall survival for these patients. We present the following article in accordance with the TRIPOD reporting checklist.
Methods
Data source and ethics statement
The SEER database collects data from 18 cancer registries of the National Cancer Institute. SEER database includes data of nearly 34.6% of US population [14]. The data was obtained with the SEER*Stat software (version 8.3.8; RRID: SCR_003293; https://seer.cancer.gov/resources/). Primary cancer histology and site were coded by the 3rd edition of the International Classification of Diseases for Oncology (ICD-O-3). The requirement for informed consent was waived as all SEER data was deidentified before release and contained no personally identifying information of patients. The study was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. No approval by the institutional review board was sought, because SEER is a public database.
Cohort selection
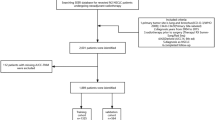
A total of 25,005 patients from SEER database were selected into our study cohort. Inclusion criteria was as followed: (I) patients diagnosed with primary lung tumor between 2004 to 2016 with site codes as C34.0-C34.9; (II) positive pathologic confirmation of histologic type as adenocarcinoma (8140–8147, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, 8572) based on ICD-O-3 His/Behave, malignant; (III) identified as only one primary tumor; (IV) patients who underwent a radical surgery and systematic lymph node dissection with N0–2 disease. Patients were excluded who (I) aged < 18 years; (II) were diagnosed with autopsy/death certificate only; (III) were at N3/M1 disease; (IV) had received preoperative radiotherapy; (V) had received preoperative pathological examination of resected specimens or invasive mediastinal staging; (VI) survived less than 3 months after surgery; (VII) had missing information about NDLN, NPLN, TNM staging as well as survival outcomes; (VIII) had missing information about race, laterality, site, differentiation as well as marital status at diagnosis. The TNM stages of the selected patients from SEER database were updated according to the 8th edition of the AJCC criteria [15].
According to purchased/referred care delivery areas (PRCDA) region, these patients were divided into training cohort (PRCDA = East, Northern plains, and Alaska) and external validation cohort (PRCDA = Pacific coast and Southwest). The prognostic model was subjected to bootstrap internal validation in the training cohort and external validation in the external validation cohort.
Study covariates and outcomes
Information on the baseline demographics of the patients including age at diagnosis, sex, race, PRCDA region, marital status at diagnosis was extracted. Moreover, histopathologic features of tumor including primary site, laterality, differentiation, T classification, and N classification were included. We also extracted therapeutic strategies including type of surgery, radiotherapy, chemotherapy, NDLN, and NPLN. In addition, four continuous variables (NDLN, NPLN, LODDS, and LNR) of training cohort were trichotomized via the X-tile software (version 3.6.1) based on the maximal log-rank χ2 value, representing the greatest group difference in survival outcome [16]. NDLN was grouped into NDLN1 (1 to 10), NDLN2 (11 to 19), and NDLN3 (≥20), while NPLN was divided into NPLN0 (0), NPLN1 (1 to 6), and NPLN2 (7 to 61). Besides, LODDS was categorized into LODDS1 (≥ − 2.26, <− 0.94), LODDS2 (≥ − 0.94, <− 0.41), and LODDS3 (≥ − 0.41, ≤1.52), while LNR was classified into LNR0 (0), LNR1 (> 0, < 0.55), LNR2 (≥ − 0.55, ≤1.00).
In this study, we chose lung cancer-specific survival (CSS) and overall survival (OS) as primary endpoints. CSS was defined as the survival months from diagnosis to lung AC-related death, while OS was defined as the survival months from diagnosis to all-cause death. The information on follow-up and prognosis of the SEER database is updated annually and the latest follow-up data was released in December 31, 2016.
Selection of prognostic model
To simplify the prognostic models, all the continuous variables were transformed into ranked or categorical variables and displayed as count and percentages. Baseline data of patients stratified by training and external validation cohort was compared by using Mann-Whitney U test or Pearson’s χ2 test for ranked variables or categorical variables respectively. 1-, 3-, and 5-year CSS and OS curves were presented by the Kaplan-Meier (KM) method, and compared using a two-sided log-rank test.
In addition, a 2-step Cox proportional hazards regression analysis was performed to identify the relation between different lymph node classifications and prognosis, and the hazard ratio (HR) and a 95% confidence interval (CI) were presented. Firstly, we performed univariate Cox regression analysis to determine which variates were potential prognostic factors. Variates with statistical significance (P < 0.1) in the univariate Cox regression analysis were then included in multivariate Cox regression analysis. Secondly, we separately put NPLN (Model 1), LODDS (Model 2), LNR (Model 3), LODDS+LNR (Model 4) into four different multivariate Cox regression models using Backward LR method. The predictive performance of these models was assessed according to homogeneity, statistical model fit, discrimination, and accuracy [17]. The likelihood-ratio (LR) χ2 test was applied to evaluate homogeneity between different models. Akaike Information Criterion (AIC) was used to measure statistical model fit. Harrell concordance index (C-index) was used to test the discriminatory ability or accuracy. In addition, comparisons of prediction accuracy between the 4 models were performed by calculating the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI) [18].
Construction and validation of the nomograms
The multivariate Cox regression models for CSS and OS with the optimal predictive performance were transformed into nomograms. Bootstrapping technique was applied on the selected model for internal validation based on 1000 resamples of the training cohort. Calibration for 1-, 3-, and 5-year CSS and OS, which compares the nomogram-predicted survival with the actual survival, was evaluated using calibration curves. The predictions were supposed to fall closely to a 45-degree diagonal line if the model was with high accuracy. Decision curve analysis (DCA) was performed to compare AJCC 8th TNM stage with our nomograms. The nomograms were transformed into convenient online versions.
Statistical analyses were performed using SPSS for windows (version 24.0; RRID: SCR_002865; https://www.ibm.com/products/spss-statistics) and R software (version 4.0.2; RRID: SCR_001905; http://www.r-project.org). All tests were two-sided and P value < 0.05 was considered as statistically significant.
Results
Patients characteristics
Between January 2004 and December 2016, the SEER database collected 241,147 patients diagnosed with lung AC. After employing the selection criteria, the final study cohort included 25,005 patients. 13,551 of them were categorized as training cohort (PRCDA = East, Northern plains, and Alaska) and the external validation cohort (PRCDA = Pacific coast, Southwest) consisted of 11,454 patients. The inclusion and exclusion process were summarized in Additional file Table S1. Baseline demographic and clinicopathological features of all patients stratified by training and external validation cohort were listed in Table 1. The median [interquartile range (IQR)] diagnostic age was 67 years (60–74 years). Most patients were diagnosed in the age of ≥50 years, were diagnosed at N0 disease (74.1%), were Caucasians (81.6%), and received lobectomy (86.0%). Interestingly patients with N0 disease (18,541) were less than patients with NPLN0 disease (18,726), which may be attributed to that part of N classification in SEER database is clinical N classification assessed by imaging manifestations.
Survival analysis
The median (IQR) follow-up times on all selected patients were 65 months (30–105 months) by calculating the interval of time from the median patient entry to the cutoff date for analysis. The CSS and OS of the training cohort patients stratified by different node classifications were shown in Additional file Table S2 and Additional file Table S3. The cumulative 1-, 3-, 5-year CSS rates for LNR0 patients were 96.4% (95% CI, 96.0–96.8%), 85.1 (95% CI, 84.3–85.9%), and 77.0% (95% CI, 76.0–78.0%) respectively, and were 89.6% (95% CI, 88.4–90.8%), 63.3 (95% CI, 61.4–65.4%), and 48.0 (95% CI, 45.8–50.3%) for LNR1 patients respectively, and were 75.6% (95% CI, 72.3–79.1%), 40.7 (95% CI, 36.7–45.1%), and 26.0 (95% CI, 22.3–30.3%) for LNR2 patients respectively. As displayed in Fig. 1, patients with higher value of NPLN, LNR, and LODDS were significantly related with lower CSS and OS rates (log-rank test P < 0.001). In Fig. 2a, Univariate Cox regression analysis demonstrated that age at diagnosis, sex, marital status at diagnosis, laterality, site, differentiation, T classification, N classification, type of surgery, radiotherapy, chemotherapy, NDLN, NPLN, LNR, and LODDS showed significant relationship with CSS (P < 0.1). Similarly, the potential prognostic variates for OS were also displayed in Fig. 2b. Subsequently, using Backward LR method, we separately incorporated NPLN (Model 1), LODDS (Model 2), LNR (Model 3), and LODDS+LNR (Model 4) into four multivariate Cox regression models combined with the same covariates. Multivariate Cox regression analysis (Model 1–3) showed that all the three classifications (NPLN, LODDS, LNR) were independent prognostic variates for CSS and OS in Additional file Table S4 and Additional file Table S5. Model 4 for CSS and OS was demonstrated in Fig. 3a and b.
Comparison of predictive performance between NPLN, LODDS, and LNR
The predictive performances of Model 1–4 were evaluated and listed in Table S6. In Model 1–3, LNR (Model 3) showed higher LR χ2 test, and lower AIC than NPLN and LODDS (Model 1–2). LNR (Model 3) and LODDS (Model 2) showed similar C-indexes, which were higher than NPLN (Model 1). In other words, LNR exhibited superior predictive potential to NPLN and LODDS. In addition, LODDS+LNR (Model 4) manifested the highest LR χ2 test (2022.4 for nomogram for CSS, 2085.1 for nomogram for OS), highest C-index (0.7241 for nomogram for CSS, 0.6941 for nomogram for OS), and lowest AIC (63,133 for nomogram for CSS, 87,559 for nomogram for OS) in all four models. What’s more, the comparisons between LODDS+LNR (Model 4) and other models using IDI and NRI were shown in Table 2. All the IDI and NRI values were < 0 with most P values < 0.05, suggesting Model 4 had better predicting performance than other models.
Construction and validation of nomograms
Significant independent prognostic factors, including age at diagnosis, sex, marital status at diagnosis, site, differentiation, T, N, LODDS, LNR, type of surgery, and radiotherapy were incorporated into the nomogram for CSS prediction based on Model 4 (Fig. 4a). Likewise, nomogram for OS was also established (Fig. 4b). 1-, 3-, and 5-year CSS and OS can be calculated by these nomograms. As shown in the nomogram for CSS, T classification and differentiation made the largest contribution to this nomogram, followed by LNR, age at diagnosis, site, and other variates. While in the nomogram for OS, age at diagnosis and T classification made the largest contribution, followed by differentiation, site, LNR and other variates. Each factor of these variates corresponded to a score on the point scale. The total score could be calculated by adding these points and by drawing a straight line down from the total score, the estimated CSS or OS rate could be determined.
Internal validation was performed using bootstrapping technique based on 1000 resamples of the study cohort, and the adjusted C-indexes (0.7222 for nomogram for CSS, 0.6920 for nomogram for OS) were presented, indicating a good prediction performance. For the external validation cohort, the C-indexes (0.7302 for nomogram for CSS, 0.7060 for nomogram for OS) were also high. Besides, the calibration plots (Fig. 5) showed the points were close to the 45-degree line, indicating a good coincidence between nomogram-predicted and actual CSS or OS. For both the training cohort and validation cohort, the DCA curves (Fig. 6) for CSS and OS suggested that our nomograms were better than the AJCC 8th TNM staging system. All these results suggested that the nomograms are convincingly accurate and practical.
DCA of AJCC 8th TNM stage and nomogram for 1-, 3-, and 5-year CSS (A, B, C), OS (D, E, F) prediction of the training cohort; DCA of AJCC 8th TNM stage and nomogram for 1-, 3-, and 5-year CSS (G, H, I), OS (J, K, L) prediction of the external validation cohort. DCA, decision curve analysis; AJCC, American Joint Committee on Cancer; TNM, tumor, node, metastasis; CSS, cancer-specific survival; OS, overall survival
Development of webservers for convenient clinical use
Two online dynamic nomograms based on Model 4 were built (https://drboidedwater.shinyapps.io/DynNom-CSS-lungadenocarcinoma/; https://drboidedwater.shinyapps.io/DynNom-OS-lungadenocarcinoma/). The calculation of LODDS and LNR can be troublesome for doctors, while in our nomograms, LODDS and LNR can be calculated automatically using NDLN and NPLN. By putting in the covariates, predicted survival probability and Kaplan-Meier curves can be generated by the webservers.
Discussion
The current nodal status of 8th TNM staging system was based on the notion that nodal metastasis takes place first in the nodes closest to the primary tumor and diffuse to more distant nodes sequentially [19]. It categorizes metastasis to ipsilateral peribronchial and/or hilar nodes and intrapulmonary nodes as N1, and classify metastasis into ipsilateral mediastinal and/or subcarinal nodes into N2, without considering number of metastatic lymph node [20]. Surgery quality and the precision of N classification are closely related to the number of lymph node retrieval. If the surgeon cannot achieve sufficient lymph node dissection, the pathological report may lead to N classification migration, leading to insufficient therapy and worse prognosis. Likewise, the accuracy of NPLN staging system is also dependent on number of lymph node dissected and surgery quality.
The widely used N classification and NPLN have been questioned, and LODDS or LNR was believed to be superior to N classification, NPLN or NDLN considered separately for predicting survival outcomes and proposed to be applied to lymph node classification [11, 21, 22]. LODDS and LNR are ratio-based nodal evaluation methods and both include the number of dissected and positive lymph nodes to achieve a better prognostic efficacy which could overcome the limitation of number-based assessment, namely, NDLN and NPLN classification to some extent. Based on those discussions mentioned above, we undertook this study to establish an optimal multivariate Cox regression model to predict survival outcomes of lung AC patients after surgery. We found that NPLN, LODDS and LNR were all independent predictive factors, and LNR was superior to NPLN and LODDS by demonstrating better performance in homogeneity, statistical model fit, discrimination, and accuracy of survival prediction. LODDS was thought to be prognostically superior or equivalent to lymph node ratio in previous lung or digestive cancer studies [10,11,12,13, 17, 23]. Deng et al. studied the predictive performance of LODDS and LNR in patients with node-positive NSCLC and chose 10 as a cutoff value of NDLN [11]. They found that when NDLN < 10, LODDS was slightly superior to LNR and LNR performed better when NDLN ≥10. Zhao et al. performed a single center study on patients with lung AC and supposed that LODDS staging system performs better than LNR [24].
While in our study cohort, LNR (Model 3) showed slightly better prediction performance than LODDS (Model 2), which might be because of the difference of centers, race composition of population, study time window and covariates included in the model. All these studies suggest that putting these two ratio-based classifications into nodal classification for patients with lung AC would be reasonable and feasible.
To the best of our knowledge, this is the first study establishing a novel nomogram combining N, LODDS, and LNR classifications to predict postoperative long-term CSS or OS for lung AC. It is also a multicenter study with the largest population of patients with resectable lung AC. Previous studies concentrate on comparing the N, LODDS, and LNR classifications separately, while considering the importance of accurate evaluation of lung AC for optimizing and implementing of therapeutic methods, we innovatively incorporated LODDS and LNR staging system into one prognostic model (Model 4) to strengthen the TNM stage, which can take full advantage of the valuable pathological evidence retrieved from surgery. As we can see in Table 2, Model 4 exhibited the most powerful prognostic prediction in all 4 models. In this model, patients with higher level of N classification, higher value of LODDS or LNR tend to have worse CSS or OS, suggesting more aggressive therapeutic strategies and close follow-up are needed in this cohort.
As for the NDLN, a larger NDLN is closely associated with more-accurate node staging and better long-term survival outcomes and there is no final conclusion on optimal NDLN [25,26,27]. Liang et al. reported 16 as the cutoff value for assessing the quality of LN examination and prognostic prediction for patients with declared node-negative NSCLC [26]. While Dai et al. suggested the optimal NDLN correlated with tumor size, and the optimal NDLN for patients with T1a, T1b, T1c, and T2a NSCLC was 8, 9, 10, and 11 respectively [28]. In our study, the X-tile software trichotomized NDLN into NDLN1 (1 to 10), NDLN2 (11 to 19), and NDLN3 (≥20), and NDLN turned out to be independent prognostic factor in Model 1 for CSS. Based on comprehensive evaluation on these models, we consider patients with lung AC could benefit more from NDLN ≥11 than NDLN < 11. Our study revealed that patients who underwent radiotherapy were associated with a significantly worse CSS or OS. This may be attributed to that patients with radiotherapy were in higher TNM stage and have poor prognostic outcomes essentially. Which cohort could benefit from radiotherapy still needs further research. Another interesting finding in our study was that the long-term CSS and OS of patients with a married status at diagnosis had no significant difference from patients with a single status (never married), while the patients with other marital status at diagnosis (including divorced, separated, unmarried/domestic partner, and widow) had worse long-term CSS and OS (Additional file Table S4, Additional file Table S5, and Fig. 3). Zhang’s group demonstrated that married status was an independent protective factor for both CSS and OS for soft tissue sarcoma, and widowed patients had the highest death risk among the unmarried groups [29]. A propensity-adjusted study found that patients with NSCLC who were married had better CSS compared to unmarried ones, and among the unmarried ones, patients who were single had worse CSS than those who were divorced or widowed [30]. The different conclusion concerning influence of single status on prognosis may be due to the different TNM stage and histology of lung cancer between our studies. However, the impact of the subtypes of other marital status on long-term survival of lung AC patients need further investment.
In the end, two convincing nomograms based on Model 4 for CSS and OS were established with relatively high C-indexes, and they’re internally validated using bootstrapping technique and externally validated using calibration curves. Well-corresponded calibration plots were exhibited for the prediction of CSS and OS using the nomograms. Most importantly, a generalizable conclusion could be drawn based on our multicenter and large cohort study. The online nomograms, consisted of a few easily obtained prognostic factors, can help doctors in evaluation of death risks, patient counseling, and decision-making. In other words, patients with worse survival outcomes estimated by the nomograms may need more aggressive treatments, including chemotherapy and radiotherapy.
Our study had several limitations. First, SEER database is lacked of some potential prognostic factors, such as smoking history, sequence of surgery and chemotherapy, specific chemotherapy regimens, tyrosine kinase inhibitors treatment, immune checkpoint inhibitors treatment etc. Second, as this is a population study, we are unable to use uniform counting methods, thus may resulting into underestimation when the lymph nodes are adhesive to each other or difficult to be separated from the dissected tissues and overestimation when fragmentation of nodal tissues happens. Third, there is no recording of recurrence-free survival in the SEER database.
Conclusions
We confirmed the value of combination of LODDS with LNR was superior to NPLN, LODDS, and LNR separately in predicting survival outcomes in lung AC patients who received surgery. Online dynamic nomograms including 8th TNM stage complemented by LODDS and LNR to evaluate CSS and OS were constructed. The well-performed nomograms may facilitate doctors to provide smarter, individualized therapy for lung AC patients.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the SEER*Stat software (version 8.3.8; RRID:SCR_003293; https://seer.cancer.gov/resources/).
Abbreviations
- AC:
-
Adenocarcinoma
- AIC:
-
Akaike information criterion
- AJCC:
-
American Joint Committee on Cancer
- CI:
-
Confidence interval
- C-index:
-
Harrell concordance index
- CSS:
-
Cancer-specific survival
- DCA:
-
Decision curve analysis
- HR:
-
Hazard ratio
- ICD-O-3:
-
3rd edition of the International Classification of Diseases for Oncology
- IDI:
-
Integrated discrimination improvement
- IQR:
-
Interquartile range
- KM:
-
Kaplan-Meier
- LNR:
-
Lymph node ratio
- LODDS:
-
Log odds of positive lymph nodes
- LR:
-
Likelihood ratio
- NDLN:
-
Number of dissected lymph nodes
- NPLN:
-
Number of positive lymph nodes
- NRI:
-
Net reclassification improvement
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PRCDA:
-
Purchased/referred care delivery areas
- SEER:
-
Surveillance, Epidemiology, and End Results
- TNM:
-
Tumor, node, metastasis
References
National Cancer Institute [Internet]. Surveillance, Epidemiology, and End Results Program: cancer of the lung and bronchus: cancer stat facts; 2020 [cited 2021 Jan 2]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html.
Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung Cancer: a review. JAMA. 2019;322(8):764–74. https://doi.org/10.1001/jama.2019.11058.
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu Y, et al. RNA-Seq profiling of circular RNA in human lung adenocarcinoma and squamous cell carcinoma. Mol Cancer. 2019;18(1):134. https://doi.org/10.1186/s12943-019-1061-8.
Wang BY, Huang JY, Chen HC, Lin CH, Lin SH, Hung WH, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146(1):43–52. https://doi.org/10.1007/s00432-019-03079-8.
Wu Y, Han C, Wang Z, Gong L, Liu J, Chong Y, et al. An externally-validated dynamic nomogram based on Clinicopathological characteristics for evaluating the risk of lymph node metastasis in small-size non-small cell lung Cancer. Front Oncol. 2020;10:1322. https://doi.org/10.3389/fonc.2020.01322.
Deng HY, Zeng M, Li G, Alai G, Luo J, Liu LX, et al. Lung adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched analysis. World J Surg. 2019;43(3):955–62. https://doi.org/10.1007/s00268-018-4848-7.
Meng F, Zhang L, Ren Y, Ma Q. The genomic alterations of lung adenocarcinoma and lung squamous cell carcinoma can explain the differences of their overall survival rates. J Cell Physiol. 2019;234(7):10918–25. https://doi.org/10.1002/jcp.27917.
Relli V, Trerotola M, Guerra E, Alberti S. Abandoning the notion of non-small cell lung Cancer. Trends Mol Med. 2019;25(7):585–94. https://doi.org/10.1016/j.molmed.2019.04.012.
Dziedzic D, Piotr R, Langfort R, Orlowski T. Polish lung Cancer study G. log odds of positive lymph nodes as a novel prognostic indicator in NSCLC staging. Surg Oncol. 2017;26(1):80–5. https://doi.org/10.1016/j.suronc.2017.01.004.
Baqar AR, Wilkins S, Wang W, Oliva K, McMurrick P. Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer. 2020;20(1):762. https://doi.org/10.1186/s12885-020-07260-y.
Deng W, Xu T, Wang Y, Xu Y, Yang P, Gomez D, et al. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer. 2018;122:60–6. https://doi.org/10.1016/j.lungcan.2018.05.016.
Jiang S, Zhao L, Xie C, Su H, Yan Y. Prognostic Performance of Different Lymph Node Staging Systems in Patients With Small Bowel Neuroendocrine Tumors. Front Endocrinol (Lausanne). 2020;11:402. https://doi.org/10.3389/fendo.2020.00402.
Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides G, Fields RC, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262(6):991–8. https://doi.org/10.1097/SLA.0000000000001040.
Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer. 2014;120(Suppl 23):3755–7. https://doi.org/10.1002/cncr.29049.
Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American joint committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):138–55. https://doi.org/10.3322/caac.21390.
Wang Y, Wang H, Wang W, Liu W, Liu N, Liu S, et al. Prognostic value of platelet recovery degree before and after achieving minimal residual disease negative complete remission in acute myeloid leukemia patients. BMC Cancer. 2020;20(1):732. https://doi.org/10.1186/s12885-020-07222-4.
Yu Y, Zhang P, Yao R, Wang J, Wang P, Xue X, et al. Prognostic value of log odds of positive lymph nodes in node-positive lung squamous cell carcinoma patients after surgery: a SEER population-based study. Transl Lung Cancer Res. 2020;9(4):1285–301. https://doi.org/10.21037/tlcr-20-193.
Cook NR. Comments on 'Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond' by M. J. Pencina et al., statistics in medicine. Stat Med. 2008;27(2):191–5. https://doi.org/10.1002/sim.2987.
Lababede O, Meziane MA. The eighth edition of TNM staging of lung Cancer: reference chart and diagrams. Oncologist. 2018;23(7):844–8. https://doi.org/10.1634/theoncologist.2017-0659.
Taylor MD, LaPar DJ, Thomas CJ, Persinger M, Stelow EB, Kozower BD, et al. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann Thorac Surg. 2013;96(4):1163–70. https://doi.org/10.1016/j.athoracsur.2013.04.031.
Yan Y, Lin J, Zhang M, Liu H, Zhou Q, Chen R, et al. A novel staging system to forecast the Cancer-specific survival of patients with resected gallbladder Cancer. Front Oncol. 2020;10:1281. https://doi.org/10.3389/fonc.2020.01281.
Zhou YY, Du XJ, Zhang CH, Aparicio T, Zaanan A, Afchain P, et al. Comparison of three lymph node staging schemes for predicting the outcome in patients with small bowel adenocarcinoma: a population-based cohort and international multicentre cohort study. EBioMedicine. 2019;41:276–85. https://doi.org/10.1016/j.ebiom.2019.02.043.
Nomori H, Horio H, Naruke T, Orikasa H, Yamazaki K, Suemasu K. Use of technetium-99m tin colloid for sentinel lymph node identification in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;124(3):486–92. https://doi.org/10.1067/mtc.2002.124496.
Zhao Y, Li G, Zheng D, Jia M, Dai W, Sun Y, et al. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;153(3):702–9 e1. https://doi.org/10.1016/j.jtcvs.2016.11.053.
Becker DJ, Levy BP, Gold HT, Sherman SE, Makarov DV, Schreiber D, et al. Influence of extent of lymph node evaluation on survival for pathologically lymph node negative non-small cell lung Cancer. Am J Clin Oncol. 2018;41(8):820–5. https://doi.org/10.1097/COC.0000000000000379.
Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung Cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. 2017;35(11):1162–70. https://doi.org/10.1200/JCO.2016.67.5140.
Yendamuri S, Dhillon SS, Groman A, Dy G, Dexter E, Picone A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg. 2018;156(1):394–402. https://doi.org/10.1016/j.jtcvs.2018.03.113.
Dai J, Liu M, Yang Y, Li Q, Song N, Rocco G, et al. Optimal lymph node examination and adjuvant chemotherapy for stage I lung Cancer. J Thorac Oncol. 2019;14(7):1277–85. https://doi.org/10.1016/j.jtho.2019.03.027.
Zhang SL, Wang WR, Liu ZJ, Wang ZM. Marital status and survival in patients with soft tissue sarcoma: a population-based, propensity-matched study. Cancer Med. 2019;8(2):465–79. https://doi.org/10.1002/cam4.1802.
Chen Z, Yin K, Zheng D, Gu J, Luo J, Wang S, et al. Marital status independently predicts non-small cell lung cancer survival: a propensity-adjusted SEER database analysis. J Cancer Res Clin Oncol. 2020;146(1):67–74. https://doi.org/10.1007/s00432-019-03084-x.
Acknowledgements
The authors would like to thank all patients and staff who have participated in the SEER program.
Funding
This study was funded by Development Fund for the Department of Anesthesiology of Shanghai Pulmonary Hospital, National Nature Science Foundation of China (NO. 81300102, 81770244) and Science Foundation of Shanghai Municipal Commission of Health and Family Planning (NO. 201740225).
Author information
Authors and Affiliations
Contributions
SW and ML conceived and designed the analysis, collected the data, performed the analysis and wrote the paper. YY conceived and designed the analysis, contributed data or analysis tools, performed the analysis. WX and XL interpreted the data and wrote the paper. YZ conceived and designed the analysis and interpreted the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. No approval by the institutional review board was sought, and no individual patient consent was required, because SEER is a public database and the data are de-identified. The authors confirm that they are accountable for all aspects of the work (if applied, including full data access, integrity of the data and the accuracy of the data analysis) in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for publication
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Selection procedure of study cohort. Table S2. 1-, 3-, and 5-year cancer-specific survival rate of subgroups of different classifications in training cohort. Table S3. 1-, 3-, and 5-year overall survival rate of subgroups of different classifications in training cohort. Table S4. Multivariable Cox regression analysis (Model 1–3) of prognostic predictors for CSS in training cohort. Table S5. Multivariable Cox regression analysis (Model 1–3) of prognostic predictors for OS in training cohort. Table S6. Comparison of predictive performance of different classifications in training cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, S., Yu, Y., Xu, W. et al. Dynamic nomograms combining N classification with ratio-based nodal classifications to predict long-term survival for patients with lung adenocarcinoma after surgery: a SEER population-based study. BMC Cancer 21, 653 (2021). https://doi.org/10.1186/s12885-021-08410-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08410-6