Abstract
Background
It has been shown that a subgroup of patients with differentiated thyroid cancer (DTC) and medullary thyroid carcinoma (MTC) would progress to advanced stages of thyroid cancer. Therefore, the present study was done to systematically review available evidence in order to investigate efficacy and safety of peptide receptor radionuclide therapy (PRRT) in the patients with advanced radioiodine refractory differentiated thyroid cancer (RR-DTC) and metastatic MTC.
Methods
For this purpose, relevant studies investigated safety and efficacy of PRRT in the patients with advanced RR-DTC and metastatic MTC were identified by searching Medline (Pubmed, Ovid, and Ebsco), Scopus, Embase, Web of Science, and Cochrane Library databases (from database inception to March 24, 2021). The review was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. Searching was done independently by two investigators. Two researchers independently extracted the data and any disagreement was adjudicated by consensus. Quality of the studies was assessed using the tool of case reports/series in systematic reviews.
Results
Among 2284 related papers, 41 papers met the inclusion criteria. A total of 157 patients with RR-DTC were treated with PPRT. Biochemical and objective responses (partial and complete) were observed in 25.3 and 10.5% of patients, respectively. Among 220 patients with metastatic MTC, biochemical and objective responses were observed in 37.2 and 10.6% of the patients, respectively.
Forty-six deaths were reported in 95 patients with advanced RR-DTC. In addition, 63 deaths were observed in 144 patients with metastatic MTC. Major side effects were reported in 124 patients treated with 90Y -based agent. In the patients treated with 177Lu-DOTA-TATE and 111In-Octreotide, mild and transient hematologic or renal complications were reported.
Conclusion
Findings of the study revealed that in the absence of the established treatment for the patients with RR-DTC and metastatic MTC, PRRT could be effective with few adverse events.
Trial registration
PROSPERO registration number: CRD42019125245.
Similar content being viewed by others
Background
Thyroid cancer is the most common endocrine malignancy and its incidence has increased by 4.4% per year during 2007–2011 [1, 2]. Differentiated thyroid cancer (DTC), is the most frequent subtype of thyroid cancer accounting for 85–95% of the cases [3, 4]. Medullary thyroid cancer (MTC) originating from parafollicular or C cells of the thyroid gland accounts for approximately 5% of all thyroid cancer cases [5].
The standard of treatment for most patients with DTC includes thyroidectomy followed by radioiodine treatment. A 10-year overall survival rate of 80–99% has been reported among these patients [6]. However, in spite of highly effective treatment strategies, there is a chance of recurrence in 20% of the subjects. Radioactive iodine plays a major role in diagnosis and treatment of recurrent disease [7]. However, some thyroid cancers are resistant to radioiodine despite the elevated level of thyroglobulin [8]. Radioiodine refractory-DTC (RR-DTC) has shown aggressive clinical behavior and a 10-year survival rate of 10% [9, 10]. Surgery and external beam radiation therapy can be used to manage local disease but not in case of widespread metastases. Moreover, chemotherapeutic agents have shown limited efficacy with considerable side effects [11, 12].
MTC is inherently non-sensitive to radioactive iodine. Hence, its management is more difficult and its prognosis is worse than DTC [7]. The overall survival rate is between 75 and 85% during 10 years for individuals with MTC [13]. In spite of aggressive surgical treatment, there is almost a 50% of chance for persistent or recurrent disease, with deleterious effects on quality of life and the reduced 10-year survival rate by 40% [7, 13]. Reoperation, embolization, and perhaps radiotherapy could improve outcomes [14]. Meanwhile, response to conventional chemotherapy is limited with life-threatening toxicity [7]. Currently, other therapeutic options are scarce and not widely available.
There are few alternative treatments in the patients with advanced RR-DTC. Somatostatin receptor (SSTR) expression on cell surface of neuroendocrine and thyroid tumors regulates cell proliferation [15]. Targeting SSTR with radiotracer in peptide receptor radionuclide therapy (PRRT) can induce tumor cell death. Overexpression of somatostatin receptor subtypes on surface of cells is required for PRRT and therefore, tumor remission can be predicted based on the results of scintigraphy on somatostatin receptor. Thus, PRRT could be a therapeutic option based on scintigraphy results of somatostatin receptor. It has been used previously for treatment of metastatic neuroendocrine tumor and advanced pheochromocytomas and paragangliomas with high efficacy, tolerability, and low toxicity [16, 17].
Accordingly, the present study was conducted to systematically review available evidence in order to investigate efficacy and safety of PRRT in the patients with advanced RR-DTC and metastatic MTC.
Methods
Search strategy and selection criteria
A systematic review was performed on the published works to investigate safety and efficacy of PRRT in the patients with advanced RR-DTC and metastatic MTC, according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [18]. The study was registered before completing formal screening of search results (PROSPERO registration number: CRD42019125245).
Eligibility criteria
All the original studies containing data related to PRRT were considered eligible to be included in the review study. Exclusion criteria were irrelevant papers (based on screening of titles and abstracts), papers with insufficient data available, duplications, and review papers. All the eligible studies were included to assess efficacy, and/or safety of PRRT.
Study identification
For this systematic review, the Cochrane Central Register of Controlled Trials (Central), Medline (PubMed, Ovid, and Ebsco), Scopus, and Embase databases were searched (from database inception to March 24, 2021). Search terms for English-language publications included: “peptide receptor radionuclide therapy”, “PRRT”, “radionuclide therapy”, “radiolabeled somatostatin analogues”, “thyroid cancer”, “thyroid carcinoma”, “thyroid neoplasm”, “differentiated thyroid cancer”, “differentiated thyroid carcinoma”, “differentiated thyroid neoplasm”, “medullary thyroid cancer”, “medullary thyroid carcinoma”, and “medullary thyroid neoplasm”. Details regarding the search strategy are provided in the Supplementary Table 1.
The first search was done independently by two investigators (ZE and ZM). Also, a complete updated search was performed on all databases available and new studies (if any exist) were identified to assess the details and incorporate findings in this review. The snowballing techniques were used to complete the search by screening reference lists of the included papers for relevant studies. Also, registry of prospective studies with accessible results was searched. Two authors (RM, ZM) independently determined studies that should be evaluated further by scanning the title, abstract, or both based on the inclusion/exclusion criteria, the reviewers were blinded to names of the journals and authors. All the potentially relevant papers as full texts were assessed and any disagreements were resolved by consensus or by arbitration of two experts (MK and MM). In case of duplicates or multiple publications of a primary study, yield of information was enhanced by collating all available data and using the most complete data set aggregated across all the known publications.
Data collection and management
Two reviewers (RM and ZM) independently extracted the data from the included trials and any disagreement was adjudicated by consensus or by arbitration of other reviewers (MK and MM). Published reports were obtained for every study, and standard information was extracted in a spreadsheet. The following data were extracted: author’s name; year of publication; country where the study was performed; number of participants, sex and age of the participants; tumor classification, site of metastases; prior treatments (cumulative radioiodine in RR-DTC); cumulative activity (GBq) of PRRT; response to treatment criteria; time to progression (TTP); follow-up duration; response to treatment; complications (major/minor); mortality rate; and time to death.
Biochemical response was defined in the patients with DTC based on serum thyroglobulin (Tg) level and in the patients with MTC, it was defined based on serum calcitonin and carcino- embryonic antigen (CEA) levels. Different criteria were used to evaluate radiological responses to treatment, namely world health organization (WHO) criteria, response evaluation criteria in solid tumors (RECIST) criteria, and southwest oncology group (SWOG) criteria [19]. Moreover, the European organization for research and treatment of cancer (EORTC) has classified metabolic response to treatment based on the maximum standardized uptake value (SUVmax) [20].
For further analysis, proportions of complete and partial radiologic response were integrated as “objective response”.
Occurrence of adverse events was evaluated using common terminology criteria for adverse events (CTCAE) [21]. Two reviewers (RM and ZM) independently assessed methodological quality of the included studies using the tool of systematic reviews [22], and any disagreement was resolved by consensus.
Results
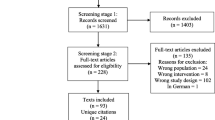
Search on the literature led to identification of 2284 publications, of which 98 papers were reviewed in full text (Fig. 1. shows flow chart of literature search and paper selection). The risk of bias of the included studies was low (Supplementary Table 2). Inter-reviewer’s agreement was “excellent” for the selected papers (Cohen’s test κ = 0.96). Among 41 publications met the inclusion criteria, 12 papers were retrospective in terms of design; 19 papers were prospective studies and remaining 10 papers were case reports. Tables 1 and 2 summarize characteristics of the included studies assessing efficacy of PRRT in the patients with advanced RR-DTC, and metastatic MTC, respectively. Data regarding safety of PRRT are presented in Table 3. Cumulative activity of PRRT ranged between 0.925–83.2 GBq. For 90Y -based agent, most of the studies had used this agent with an administered activity ranging from 0.925 to 5.9 GBq per cycle usually up to 4 cycles. For 177Lu-DOTA-TATE, the administered activity rate was between 5.5–7.7 GBq per cycle usually up to 4 cycles. In terms of follow-up duration, in the patients with advanced RR-DTC, it was between 1 and 99 months after commencement of PRRT (median: 12 months). It was between 1 and 144 months (median: 17 months) in the patients with metastatic MTC. Death was recorded in 109 patients. Time to death varied from 1 to 63 months (median: 11 months). It should be noted that more than one criterion was used to evaluate efficacy of PRRT, and some patients did not complete their full course of treatment.
Efficacy of PRRT in RR-DTC
Overall, 157 patients with advanced RR-DTC were treated with PRRT. Based on biochemical response criteria, from 79 treated patients, 20 cases of partial response (PR), 22 cases of stable disease (SD), and 37 cases of persistent disease (PD) were determined. Out of 91 patients whose radiological response was assessed, 9 cases of PR, 39 cases of SD, and 43 cases of PD were recorded. Metabolic response was evaluated in 48 patients. Six cases of PR, 20 cases of SD, and 22 cases of PD were identified.
In 85 patients treated with 90Y -based agent; 44 patients were assessed based on biochemical response among whom 8 cases of PR, 14 cases of SD, and 22 cases of PD were observed. Seven cases of PR, 23 cases of SD, and 25 cases of PD were identified in 55 patients assessed based on radiological response. Moreover, 2 cases of PR, 5 cases of SD, and 4 cases of PD were reported in 11 patients assessed based on metabolic response.
In 26 patients treated with Lutetium-177 -based agent, 10 cases of PR, and 11 cases of PD showed biochemical response. Considering 20 patients assessed for radiological response, 2 cases of PR, 9 cases of SD, and 9 cases of PD were reported. Out of 9 patients assessed for metabolic response, 1 case of PR, 4 cases of SD, and 4 cases of PD were identified.
Moreover, in 18 patients treated with Indium-111, biochemical response was assessed in 14 patients. Two patients with PR, 8 cases with SD, and 4 cases with PD were reported. Seven SD cases and 9 PD cases were recorded based on radiological response in 16 patients.
Among 157 patients with RR-DTC, biochemical and objective responses (partial and complete) were observed in 25.3 and 10.5% of the patients, respectively.
Efficacy of PRRT in metastatic MTC
In total, 220 patients with metastatic MTC were treated with PRRT. Based on biochemical response to the treatment in 145 patients, 7 cases of complete response (CR), 47 cases of PR, 20 cases of SD, and 71 cases of PD were recognized.
Radiologic response was evaluated among 134 patients. Four cases of CR, 9 cases of PR, 75 cases of SD, and 46 cases of PD were observed. Considering metabolic response among 46 patients, 7 cases of PR, 29 cases of SD, and 10 cases of PD were identified.
Sixty-nine patients were treated by 90Y-DOTATOC, 88 patients were treated with 177Lu-DOTA-TATE, and 12 patients were treated with 111_Indium -based agent. Type of treatment was unknown in other patients.
In 69 patients treated with 90Y-DOTATOC, 1 case of CR, 15 cases of PR, 4 cases of SD, and 35 cases of PD (based on biochemical response criteria in 55 patients) as well as 2 cases of CR, 21 cases of SD and 15 cases of PD (based on radiological response criteria in 38 patients) and 1 case of PR and 1 case of PD (based on metabolic response criteria in 2 patients) were reported. Out of 74 patients treated with 177Lu-DOTA-TATE, 5 cases of CR, 26 cases of PR, 14 cases of SD, and 29 cases of PD were observed based on biochemical response criteria. Moreover, 9 cases of PR, 50 cases of SD, and 26 cases of PD were achieved in 85 patients based on radiological response criteria. Furthermore, SD was found in 3 patients based on metabolic response criteria. In the patients treated with 111_Indium -based agent; 1 case of CR, 2 cases of SD, and 4 cases of PD (in 7 patients assessed based on biochemical response) and also, 2 cases of CR, 4 cases of SD, and 5 cases of PD (in 11 patients assessed based on radiological criteria) were reported.
Overall, in the patients with metastatic MTC, biochemical and objective responses were observed in 37.2 and 10.6% of the patients, respectively.
Safety of PRRT
Safety of PRRT was assessed in 19 studies (totally, 239 patients). Death was observed in 109 patients. In addition, time to death varied from 1 to 63 months.
In 95 patients with advanced RR-DTC, 46 patients died. Time to death ranged from 1 to 63 months from commencement of PRRT. Based on type of PRRT, death occurred in 29/55 patients treated with 90Y -based agent, 6/17 patients treated with 177Lu-DOTA-TATE, and 8/16 patients treated with 111In-Octreotide. Among 44 patients with metastatic MTC, 63 patients died. Time to death ranged from 1 to 26.8 months since initiating the first course of PRRT. Based on the type of PRRT, death occurred in 27/69 patients treated with 90Y-DOTATOC, 31/63 patients treated with 177Lu-DOTA-TATE, and 4/5 patients treated with 111In- Octreotide. Major side effects were reported in 124 patients treated with 90Y -based agent. Fourteen patients developed renal toxicity (2 cases of grade 4, 2 cases of grade 3, 2 cases of grade 2, and 8 cases of grade 1). Furthermore, hematologic toxicity was observed in 64 patients (3 cases developed grade 4 of thrombocytopenia, and 1 patient reported to suffer from grade 4 of anemia). Moreover, in 80 patients treated with 177Lu-DOTA-TATE, mild and transient hematologic and renal complications were reported (4 patients with grade 1 and one case with grade 2 of hematologic toxicity and one patient with grade 2 of renal toxicity). Among 21 patients treated with 111In- Octreotide, one patient developed transient thrombocytopenia (grade1).
Discussion
Herein, a comprehensive systematic review was done to investigate efficacy and safety of PRRT in management of advanced RR-DTC and metastatic MTC. The results suggested that PRRT could maintain disease stability with few adverse events. In short-term, toxicity is mild and transient. In addition, long-term toxicity is rare and with low grade. To the best of our knowledge, no similar systematic review or meta-analysis has been done previously to investigate efficacy and safety of PRRT in RR-DTC and metastatic MTC.
There are few recommended treatments for the patients with RR-DTC and therapeutic options are associated with certain limitations in case of the patients with metastatic DTC. The choice of treatment depends on bulk of the tumor. Simple observation, multi-targeted, or mutation-selected kinase inhibitors (MKI), and traditional cytotoxic chemotherapy are the available options [12, 62]. Despite approval of doxorubicin by the food and drug administration (FDA), treatment with cytotoxic agents has shown disappointing results [63]. Therefore, benefit-risk ratio must be carefully evaluated before starting treatment [62].
For majority of the patients with MTC, primary surgery is curative at early stages. However, local and distant metastases after surgery are the major causes of mortality [14]. Resurgery, chemotherapy, external beam radiation therapy, and biological agents, such as RET and MEK inhibitors have yielded disappointing and limited results. Although, treatment with tyrosine kinase inhibitors (TKIs) (Vandetanib and Cabozantinib) improves progression-free survival (PFS), severe adverse events could limit the use of them. There is no curative treatment for these patients, and all the available treatment modalities have been shown to have certain limitations and complications [6].
In the 1990s, the role of SSTR in regulation and proliferation of normal thyroid cells and tumoral tissues was reported that led to introduction of peptide receptor imaging and PRRT in management of metastatic MTC and advanced RR-DTC [15]. Type of SSTRs expression could have an effect on survival rate of these patients [64]. From 5 subtypes of SSTR described in human cells, SSRT2 is expressed in MTC [7]. However, SSRT2 expression has not been identified in papillary or follicular thyroid cancer, and it is irregularly expressed in Hurthle cell adenoma and Hurthle cell carcinoma [65].
Generally, PRRT is able to deliver a high dose of radiation to intracellular components of cancer cells, and induce tumor shrinkage [7]. Currently, PRRT is considered as a safe and effective treatment modality for metastatic inoperable well-differentiated neuroendocrine tumors and advanced pheochromocytomas and paragangliomas [16, 17].
The most frequently used radionuclides in PRRT are 90Y and Lutetium-177. They have different physical characteristics, namely different emission ranges. This results in various maximum tissue penetrations ranging from 3 mm for Lutetium-177 to 12 mm for 90Y. Since, 90Y has the highest energy and maximum tissue penetration; it is a preferable radionuclide for tumors with large size and poor vascularization. On the other hand, Lutetium-177 emits intermediate-energy suitable for small-sized tumors. Few studies had used 111In-Octreotide, with tissue penetration ranging from 0.2 to 10 mm (Table 1) [7]. Krenning et al., for the first time reported treatment of the patients with advanced DTC with 111In-Octreotide analogs. One patient, who received total cumulative activity of at least 20 GBq showed disease stabilization [40]. In a pilot study conducted in Netherlands, 9 patients with advanced RR-DTC were treated with high, fixed doses of 111In -Octreotide. Six months after the last therapy, 4 patients had SD, and 5 patients showed PD. Mean Tg value was higher in PD cases than patients with SD. They concluded low Tg value could have a positive effect on the outcome [41].
Görges et al., in a study regarding the first cases of treatment with 90Y-DOTATOC in 3 patients with advanced RR-DTC and pulmonary metastasis showed deceleration in short-term disease progression [26]. In the last report on treatment with 90Y-DOTATOC in RR-DTC, median survival was found to be 21 months from initiating the first course of PRRT with only minor and transient hematological toxicity in some patients [15]. Recently, 177Lu-DOTA-TATE has been used more than 90Y but, number of patients treated with this somatostatin analog was limited.
In the patients with metastatic MTC, limited experience with PRRT treatment has been reported. Results of a study on the patients with metastatic MTC suggested that treatment with 90Y-DOTATOC is associated with a long-term survival benefit. However, treatment response was independent of pre-treatment scintigraphy results [45]. Recently, Beukhof et al., reported 17 years of experiences with 177Lu-octreotate treatment. They concluded that this treatment could be considered as a treatment in the patients with high uptake on 111In-DTPA-Octreotide scan (uptake grade 3) and positive SSTR2a expression in tumor histology [54]. Budiawan et al., found that the patients with RR-DTC having good response had less undergone other treatment modalities prior to PRRT than non-responders. In addition, they introduced lung metastasis as a poor prognostic factor for survival after PRRT [6].
However, PRRT is not free from adverse effects and minor complications,such as nausea, asthenia, and elevation in liver enzyme level are observed in up to 16.7% of patients, while major complications,such as nephrotoxicity and hematologic adverse events are rare and transient [23, 24]. Proximal tubular reabsorption of radio peptide and its interstitial retention lead to glomerular fibrosis [40], which is markedly observed after treatment with 90Y-DOTATOC. Hence, kidney protection is mandatory along with co-administration of positively-charged amino acids,such as L-lysine and/or L-arginine competitively inhibiting proximal tubular reabsorption of the radio peptide, or prolonged infusion over 10 h to 2 days after administration of radio peptide. Despite kidney protection, loss of renal function may become clinically evident years after PRRT, especially after administration of 90Y- DOTATOC. Sporadic reported cases of delayed renal failure have received activities greater than 7.4 GBq /m2 in very few cycles, without kidney protection [61]. Cumulative and per-cycle renal uptake dose, age, hypertension, diabetes and previous chemotherapy with nephrotoxic agents could accelerate the decrease in renal function after PRRT [66]. Considering these risk factors, one can modify treatment plan or change choice of radio peptide based on burden of tumors. Hematologic side effects generally are mild and temporary,such as reduction in count of lymphocytes and platelets [57].
Our systematic review demonstrated that treatment with PRRT not only could lead to minor complications in approximately 10% of cases but also it can cause very rare and transient major complications.
This systematic review benefited from a comprehensive search conducted by two independent investigators, no time limits, independent reviews by two reviewers, and no publication bias. However, the main limitation of the present study was low quality of the available evidence. However, other underlying problems and limitations included retrospective nature of the studies, a selection bias, the amount of radioactivity administered (1–83 GBq), non-uniform response criteria, huge difference in follow-up periods (1–99 months),and the limited number of patients per report. Also, our search was restricted to English -language papers.
This systematic review investigated efficacy and safety of PRRT in treatment of RR-DTC and metastatic MTC. Given paucity of evidence, it is recommended to perform further multi-center randomized controlled clinical trials.
Conclusions
According to findings of our study, due to lack of various treatment modalities, PRRT could be an option for treatment of advanced RR-DTC, as well as metastatic MTC, with few adverse events.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- RR-DTC:
-
Radioiodine-refractory differentiated thyroid cancer
- MTC:
-
Medullary thyroid cancer
- PFS:
-
Progression-free survival
- SSTR:
-
Somatostatin receptor
- PRRT:
-
Peptide receptor radionuclide therapy
- TTP:
-
Time to progression
- Tg:
-
Thyroglobulin
- CEA:
-
Carcino embryogenic antigen
- WHO:
-
World health organization
- RECIST:
-
Response evaluation criteria in solid tumors
- SWOG:
-
Southwest oncology group
- EORTC:
-
European organization for research and treatment of cancer
- RAI:
-
Radioactive iodine
- CR:
-
Complete response
- SD:
-
Stable disease
- PR:
-
Partial response
- PD:
-
Persistent disease
- TKI:
-
Tyrosine kinase inhibitors
- MKI:
-
Mutation-selected kinase inhibitors
- FDA:
-
Food and drug administration
References
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al (eds). SEER Cancer Statistics Review, 1975-2011. Bethesda: National Cancer Institute; 2014. https://seer.cancer.gov/archive/csr/1975_2011/.
Atlanta G. Cancer facts and figures; 2015.
Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. https://doi.org/10.1056/NEJM199801293380506.
Deshpande HA, Gettinger SN, Sosa JA. Novel chemotherapy options for advanced thyroid tumors: small molecules offer great hope. Curr Opin Oncol. 2008;20(1):19–24. https://doi.org/10.1097/CCO.0b013e3282f28373.
Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998;83(12):2638–48. https://doi.org/10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1.
Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90) yttrium and (177) lutetium labeled somatostatin analogs: toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. 2013;4(1):39–52.
Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nuclear Med. 2016;46(3):215–24. https://doi.org/10.1053/j.semnuclmed.2016.01.010.
Silberstein EB. The problem of the patient with thyroglobulin elevation but negative iodine scintigraphy: the TENIS syndrome. Semin Nucl Med. 2011;41(2):113–20. https://doi.org/10.1053/j.semnuclmed.2010.10.002.
Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–11.
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–9. https://doi.org/10.1210/jc.2005-2838.
Santini F, Bottici V, Elisei R, Montanelli L, Mazzeo S, Basolo F, et al. Cytotoxic effects of carboplatinum and epirubicin in the setting of an elevated serum thyrotropin for advanced poorly differentiated thyroid cancer. J Clin Endocrinol Metab. 2002;87(9):4160–5. https://doi.org/10.1210/jc.2001-011151.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. https://doi.org/10.1089/thy.2015.0020.
Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13(5):539–47. https://doi.org/10.1634/theoncologist.2007-0239.
Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4(1):22–32. https://doi.org/10.1038/ncpendmet0717.
Czepczynski R, Matysiak-Grzes M, Gryczynska M, Baczyk M, Wyszomirska A, Stajgis M, et al. Peptide receptor radionuclide therapy of differentiated thyroid cancer: efficacy and toxicity. Arch Immunol Ther Exp. 2015;63(2):147–54. https://doi.org/10.1007/s00005-014-0318-6.
Satapathy S, Mittal BR, Bhansali A. Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis. Clin Endocrinol. 2019;91(6):718–27. https://doi.org/10.1111/cen.14106.
Gulenchyn K, Yao X, Asa S, Singh S, Law C. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol. 2012;24(4):294–308. https://doi.org/10.1016/j.clon.2011.12.003.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Julka PK, Doval DC, Gupta S, Rath GK. Response assessment in solid tumours: a comparison of WHO, SWOG and RECIST guidelines. Br J Radiol. 2008;81(966):444–9. https://doi.org/10.1259/bjr/32785946.
Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):55–66. https://doi.org/10.1007/s00259-017-3687-3.
Institute NC. Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute Enterprise Vocabulary Services website. 2010.
Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–3. https://doi.org/10.1136/bmjebm-2017-110853.
Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, et al. Differentiated thyroid cancer: a new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid. 2014;24(4):715–26. https://doi.org/10.1089/thy.2013.0225.
Iten F, Muller B, Schindler C, Rasch H, Rochlitz C, Oertli D, et al. (90) yttrium-DOTA -TOC response is associated with survival benefit in iodine-refractory thyroid Cancer long-term results of a phase 2 clinical trial. Cancer. 2009;115(10):2052–62. https://doi.org/10.1002/cncr.24272.
Gabriel M, Froehlich F, Decristoforo C, Ensinger C, Donnemiller E, von Guggenberg E, et al. 99mTc-EDDA/HYNIC-TOC and (18) F-FDG in thyroid cancer patients with negative (131) I whole-body scans. Eur J Nucl Med Mol Imaging. 2004;31(3):330–41. https://doi.org/10.1007/s00259-003-1376-x.
Gorges R, Kahaly G, Muller-Brand J, Macke H, Roser HW, Bockisch A. Radionuclide-labeled somatostatin analogues for diagnostic and therapeutic purposes in nonmedullary thyroid cancer. Thyroid. 2001;11(7):647–59. https://doi.org/10.1089/105072501750362718.
Waldherr C, Schumacher T, Pless M, Crazzolara A, Maecke HR, Nitzsche EU, et al. Radiopeptide transmitted internal irradiation of non-iodophil thyroid cancer and conventionally untreatable medullary thyroid cancer using. Nucl Med Commun. 2001;22(6):673–8. https://doi.org/10.1097/00006231-200106000-00011.
Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32(2):148–55. https://doi.org/10.1053/snuc.2002.31565.
Traub-Weidinger T, Raderer M, Uffmann M, Angelberger P, Kurtaran A, Leimer M, et al. Improved quality of life in patients treated with peptide radionuclides. World J Nucl Med. 2011;10(2):115–21. https://doi.org/10.4103/1450-1147.89779.
Basu S, Parghane RV, Naik C. Clinical efficacy of (177) Lu-DOTATATE peptide receptor radionuclide therapy in thyroglobulin-elevated negative iodine scintigraphy: a "not-so-promising" result compared to GEP-NETs. World J Nucl Med. 2020;19(3):205–10. https://doi.org/10.4103/wjnm.WJNM_21_19.
Cinkir HY, Elboga U. An alternative therapy option in metastatic thyroid Cancer: peptide receptor radionuclide therapy. J Istanb Fac Med. 2020;83(4):339–44.
Roll W, Riemann B, Schäfers M, Stegger L, Vrachimis A. 177Lu-DOTATATE therapy in radioiodine-refractory differentiated thyroid Cancer: a single center experience. Clin Nucl Med. 2018;43(10):e346–e51. https://doi.org/10.1097/RLU.0000000000002219.
Olivan-Sasot P, Falgas-Lacueva M, Garcia-Sanchez J, Vera-Pinto V, Olivas-Arroyo C, Bello-Arques P. Use of (177) Lu-dotatate in the treatment of iodine refractory thyroid carcinomas. Rev Esp Med Nucl Imagen Mol. 2017;36(2):116–9. https://doi.org/10.1016/j.remn.2016.08.001.
Elboğa U, Özkaya M, Sayiner ZA, Çelen YZ. Lu-177 labelled peptide treatment for radioiodine refractory differentiated thyroid carcinoma. BMJ Case Rep. 2016;8;2016:bcr2015213627. https://doi.org/10.1136/bcr-2015-213627.
Jois B, Asopa R, Basu S. Somatostatin receptor imaging in non–131I-avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with 177Lu-DOTATATE: low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of Chromogranin a level–positive neuroendocrine differentiation. Clin Nucl Med. 2014;39(6):505–10. https://doi.org/10.1097/RLU.0000000000000429.
Teunissen JJ, Kwekkeboom DJ, Kooij PP, Bakker WH, Krenning EP. Peptide receptor radionuclide therapy for non-radioiodine-avid differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl 1):107s–14s.
Parihar AS, Sood A, Kumar R, Bhusari P, Shukla J, Mittal BR. Novel use of (177) Lu-DOTA-RGD2 in treatment of (68) Ga-DOTA-RGD2-avid lesions in papillary thyroid cancer with TENIS. Eur J Nucl Med Mol Imaging. 2018;45(10):1836–7. https://doi.org/10.1007/s00259-018-4036-x.
Campennì A, Pignata SA, Baldari S. Can peptide receptor radionuclide therapy (PRRT) be useful in radioiodine-refractory differentiated thyroid cancer? Endocrine. 2015;50(2):516–8. https://doi.org/10.1007/s12020-014-0491-8.
Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [in-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32(2):110–22. https://doi.org/10.1053/snuc/2002.31025.
Krenning E, De Jong M, Kooij P, Breeman W, Bakker W, De Herder W, et al. Radiolabelled somatostatin analogue (s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999;10(suppl_2):S23–S9.
Stokkel MP, Verkooijen RB, Bouwsma H, Smit JW. Six month follow-up after 111In-DTPA-octreotide therapy in patients with progressive radioiodine non-responsive thyroid cancer: a pilot study. Nucl Med Commun. 2004;25(7):683–90. https://doi.org/10.1097/01.mnm.0000130244.14444.5e.
Scalorbi F, Filice F, Sollini M, Menga M. Peptide receptor radionuclide therapy (PRRT) in metastatic thyroid tumors: an opportunity after traditional treatment failure. Clin Transl Imaging. 2017;5(Suppl 1):S100.
Öksüz M, Winter L, Pfannenberg C, Reischl G, Müssig K, Bares R, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with 90Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of 68Ga-DOTATOC? 2014;95(3):289–300.
Bertagna F, Giubbini R, Savelli G, Pizzocaro C, Rodella C, Biasiotto G, et al. A patient with medullary thyroid carcinoma and right ventricular cardiac metastasis treated by (90) Y-Dotatoc. Hell J Nucl Med. 2009;12(2):161–4.
Iten F, Muller B, Schindler C, Rochlitz C, Oertli D, Macke HR, et al. Response to (90) yttrium-DOTA -TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res. 2007;13(22):6696–702. https://doi.org/10.1158/1078-0432.CCR-07-0935.
Bodei L, Handkiewicz-Junak D, Grana C, Mazzetta C, Rocca P, Bartolomei M, et al. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother Radiopharm. 2004;19(1):65–71. https://doi.org/10.1089/108497804773391694.
Gao ZR, Biersack HJ, Ezziddin S, Logvinski T, An R. The role of combined imaging in metastatic medullary thyroid carcinoma: In-111-DTPA-octreotide and I-131/I-123-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130(11):649–56. https://doi.org/10.1007/s00432-004-0588-1.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26(11):1439–47. https://doi.org/10.1007/s002590050476.
Bilgic S, Saǧer MS, Beytur MF, Nazari A, Uslu Beşli RL, Asa S, et al. The effectiveness of 177Lu-DOTATATE in patients with metastatic medullary thyroid cancer. Eur J Nucl Med Mol Imaging. 2020;47(SUPPL 1):S27–S8.
Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, et al. Clinical utility of Lu-177-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck-J Sci Spec Head Neck. 2020;42(3):401–16. https://doi.org/10.1002/hed.26024.
Makis W, McCann K, McEwan AJ. Medullary thyroid carcinoma (MTC) treated with 177Lu-DOTATATE PRRT: a report of two cases. Clin Nucl Med. 2015;40(5):408–12. https://doi.org/10.1097/RLU.0000000000000706.
Vaisman F, Rosado de Castro PH, Lopes FP, Kendler DB, Pessoa CH, Bulzico DA, et al. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clin Nucl Med. 2015;40(2):123–7. https://doi.org/10.1097/RLU.0000000000000628.
Soydal Ç, Peker A, Özkan E, Küçük ÖN, Kir MK. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with lu-177 DOTATATE. Turkish J Med Sci. 2016;46(2):409–13. https://doi.org/10.3906/sag-1412-11.
Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen MF, de Rijke YB, Hofland LJ, et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer. 2019;19(1):325. https://doi.org/10.1186/s12885-019-5540-5.
Mathew D, Hephzibah J, Shanthly N, Oommen R. Overview of peptide receptor radionuclide therapy with 177Lutetium-DOTATATE in our institution: 4 years' experience. Indian J Nucl Med. 2018;33(5):S65.
Pasieka JL, McEwan AJB, Rorstad O. The palliative role of 131I-MIBG and 111In-octreotide therapy in patients with metastatic progressive neuroendocrine neoplasms. Surgery. 2004;136(6):1218–26. https://doi.org/10.1016/j.surg.2004.06.050.
Caplin M, Mielcarek W, Buscombe J, Jones A, Croasdale P, Cooper M, et al. Toxicity of high-activity 111In-Octreotide therapy in patients with disseminated neuroendocrine tumours. Nucl Med Commun. 2000;21(1):97–102. https://doi.org/10.1097/00006231-200001000-00016.
Buscombe JR, Caplin ME, Hilson AJW. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med. 2003;44(1):1–6.
Hayes AR, Crawford A, Al Riyami K, Tang C, Wild D, Khoo B, et al. Metastatic medullary thyroid cancer (MTC): is there a role for peptide receptor radionuclide therapy (PRRT)? Neuroendocrinology. 2019;108:273.
Puranik A, Baum RP, Kulkarni H, Singh A, Rangarajan V, Agrawal A, et al. Peptide receptor radionuclide therapy using 177Lu and 90Y-DOTATATE in metastatic treatment-refractory medullary thyroid cancer. Neuroendocrinology. 2019;108:228.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90 Y-DOTATOC and 177 Lu-DOTATATE: the role of associated risk factors. 2008;35(10):1847–56.
Weitzman SP, Sherman SI. Novel drug treatments of progressive radioiodine-refractory differentiated thyroid Cancer. Endocrinol Metab Clin N Am. 2019;48(1):253–68. https://doi.org/10.1016/j.ecl.2018.10.009.
Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol. 2010;22(6):464–8. https://doi.org/10.1016/j.clon.2010.03.014.
de Vries LH, Lodewijk L, Willems SM, Dreijerink KMA, de Keizer B, van Diest PJ, et al. SSTR2A expression in medullary thyroid carcinoma is correlated with longer survival. Endocrine. 2018;62(3):639–47. https://doi.org/10.1007/s12020-018-1706-1.
Forssell-Aronsson EB, Nilsson O, Bejegard SA, Kolby L, Bernhardt P, Molne J, et al. 111In-DTPA-D-Phe1-octreotide binding and somatostatin receptor subtypes in thyroid tumors. J Nucl Med. 2000;41(4):636–42.
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90) Y-DOTA (0),Tyr (3)-octreotide and (177) Lu-DOTA (0), Tyr (3)-octreotate. J Nucl Med. 2005;46(Suppl 1):83s–91s.
Acknowledgements
None.
Funding
For this study no funding was received.
Author information
Authors and Affiliations
Contributions
MK had the original idea of this work. MK, MM, RM and ZM designed and conceived the protocol. ZE and ZM designed the search strategies. MK, ZM and RM performed the data extraction and wrote the manuscript. All authors critically revised the draft of the manuscript and approved its final version.
Author’s information
Affiliations
Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran
Zohreh Maghsoomi, Zahra Emami, Ramin Malboosbaf & Mohammad E. Khamseh
Research Center for Prevention of Cardiovascular Disease, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran
Mojtaba Malek
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
Medline (Pubmed, Ovid and Ebsco), Scopus, Embase, Web of Science and the Cochrane Library database (Last Updated March 24, 2021).
Additional file 2: Supplemental Table 2.
Risk of bias assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maghsoomi, Z., Emami, Z., Malboosbaf, R. et al. Efficacy and safety of peptide receptor radionuclide therapy in advanced radioiodine-refractory differentiated thyroid cancer and metastatic medullary thyroid cancer: a systematic review. BMC Cancer 21, 579 (2021). https://doi.org/10.1186/s12885-021-08257-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08257-x