Abstract
Background
Selection of high-risk subjects for endoscopic screening of esophageal squamous cell carcinoma (ESCC) lacks individual predictive tools based on environmental risk factors.
Methods
We performed a large population-based case-control study of 1418 ESCC cases and 1992 controls in a high-risk area of China. Information on potential risk factors was collected via face-to-face interview using an electronic structured questionnaire. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using unconditional logistic regression models, and predictive nomograms were established accordingly. A weighted analysis was further conducted to introduce age into predictive nomograms due to frequency matching study design.
Results
Most cases were usually exposed to 4 to 6 risk factors, but most controls were usually exposed to 3 to 5 risk factors. The AUCs of male and female predictive nomograms were 0.75 (95%CI: 0.72, 0.77) and 0.76 (95%CI: 0.73, 0.79), respectively. The weighted analysis adding age in the predictive model improved the AUC in both men and women (0.81 (95%CI: 0.79, 0.84) and 0.88 (95%CI: 0.85, 0.90), respectively).
Conclusions
An easy-to-use preclinical predictive tool is provided to select candidate population with high ESCC risk for endoscopic screening. Its usefulness needs to be further evaluated in future screening practice.
Similar content being viewed by others
Background
International Agency for Research on Cancer estimated that the global number of new esophageal cancer cases increased from 456,000 in 2012 to 572,034 in 2018 [1, 2], and esophageal squamous cell carcinoma (ESCC), main histopathologic subtype accounting for about 88% of esophageal cancer, remains the greatest cancer burden in some high-risk areas [3]. The incidence of ESCC varies with more than 10-fold differences across countries, and the regions with highest incidence of ESCC are concentrated in East Asia, Central Asia, the coastal zone along the Great Rift Valley, and the Gaucho Region of South America [4]. Most ESCC patients are diagnosed in late-stage and have a grim prognosis with 5-year overall survival rate of less than 25% [5, 6]. Conversely, early detection and timely treatment can improve the 5-year survival rate of early-stage ESCC to more than 80% [7, 8]. For decreasing the social burden of ESCC, the Chinese government has initiated an endoscopic screening project for esophageal cancer in several high-incidence areas [9]. Although a considerable number of early stage ESCC patients have been identified and treated with improved prognosis via the project [10, 11], less than 0.5% diagnosis rate of ESCC among all endoscopically screened populations implicates a huge waste of medical resources and low compliance in screening due to lack of a relatively accurate selection algorithm of high-risk populations [12]. The current guideline is that those having Condition 1 and any one of Conditions 2–6 should be included in high-risk group and subjected to endoscopic screening: 1) Over 40 years old; 2) From high incidence areas of esophageal cancer; 3) With upper gastrointestinal symptoms; 4) Family history of esophageal cancer; 5) With esophageal diseases; 6) With other risk factors for esophageal cancer (smoking, heavy alcohol drinking, etc.) [13]. Considering the large-scale screening project is usually conducted among asymptomatic residents, a preclinical prediction tool based on easy-to-measure environmental factors can facilitate the selection of high-risk subjects and increase the clinical compliance in field work. Hence, a quantitative prediction model which can easily output individual ESCC risk score will hopefully help risk-stratify population for targeted endoscopic screening [14].
Because more than 95% of esophageal cancer cases are ESCC in China, the current study only focuses on prediction of ESCC risk [3]. We have performed a large population-based case-control study of upper gastrointestinal cancers in Taixing, a high-incidence area in China, and have systematically assessed environmental risk factors of ESCC, including family history of esophageal cancer [15], poor hygiene [16, 17], tobacco and alcohol [18], low socioeconomic status [19], hot tea drinking [20], low BMI and high adult height [21], the interaction of genetic susceptibility and selected exposures [22, 23], and gastric atrophy [24]. However, the combined effects of candidate risk factors have not been systematically explored. Here, we aim at building an easy-to-use predictive nomogram tool of ESCC to select high-risk population based on all candidate environmental risk factors in our questionnaire, which will facilitate the selection of high-risk subjects, improve the diagnosis rate of ESCC among endoscopically screened populations, and save the limited medical resources.
Methods
Study design and participants
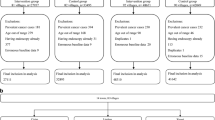
We have delineated in detail the research design of this population-based case-control study in previous reports [18, 24]. In short, we attempted to enroll all newly diagnosed esophageal cancer cases from October 2010 to September 2013 in Taixing (with a population of 1.1million), and the inclusion criteria were 40–85 year-old residents who had lived in Taixing at least 5 years. In the endoscopic units of local four largest governmental hospitals (covering almost 90% of local clinical diagnoses), potential patients were invited to complete a questionnaire by trained interviewers and provided biological samples before treatment, if they were suspected of having upper gastrointestinal tumor by endoscopic doctors. Moreover, we further enrolled missing esophageal cancer patients by cross-linkage with the local Cancer Registry. We finally recruited 1401 suspected esophageal cases from the hospitals’ endoscopy units and 280 reported esophageal cases via the local Cancer Registry during the 3 years. After reviewing the pathological sections and surgical pathology reports for those without pathological sections, 1418 ESCC patients were included in this study. We estimated that about 78.3% of the new incident cases in the research base were recruited in our study based on the statistics of the local Cancer Registry. During the same period, we randomly selected control subjects frequency-matched to ESCC cases by sex and 5-year age groups from the local Total Population Registry. Finally, 1992 eligible controls participated in our study (participation rate: 70.4%).
Exposure assessment
All participants were interviewed face-to-face using electronic questionnaires by trained staff, which contains age, gender, race, marital status, education level, adult height at 20 years old, weight and body shape at 20 years old and 10 years ago, residence history, occupational history, family structure and family socioeconomic status, personal medical history, oral hygiene, family history of selected diseases, smoking history, passive smoking exposure, alcohol and tea drinking history, dietary history 10 years ago and female reproductive history, and so on (as shown in supplementary material).
Body mass index (BMI) (weight in kilograms divided by height in meters squared) was calculated, and subjects were categorized as underweight (< 18.5), normal weight (≥18.5 and < 24), overweight (≥24 and < 28), obesity (≥28) based on Chinese standards. The male and female adult height were converted into four categories according to the published nonlinear relationship (male cutoff values:162, 170 and 174; female cutoff values:152, 156 and 160, respectively, unit: centimeter) [21]. Family wealth score was calculated based on the ownership of valuable home items using a multiple correspondence analysis, and was further categorized as approximate quintiles among control participants [19]. The cumulative missing and filled teeth number, smoking pack-years and daily intensity of alcohol drinking among the exposed were categorized by the median.
Statistical analysis
Analyses were stratified by gender, because of the extreme difference in prevalence and pattern of many environmental factors between men and women [20]. Chi-squared test or Kruskal–Wallis test was performed for testing the difference of the distributions of categorical variables or continuous variables between two groups. Based on all candidate environmental factors (P value < 0.1 in univariate analysis) in our study, we used backward elimination unconditional logistic regression model to estimate odds ratios (ORs) with 95% confidence intervals (CIs) and established a concise predictive model. The post-estimated nomogram for ESCC prediction was built to facilitate the on-site selection of high-risk population. The non-linear dose-response association of total scores with ESCC risk was assessed by restricted cubic spline regression models with five knots and the receiver operating characteristic (ROC) curve of individual score for ESCC risk was plotted to assess the accuracy of nomogram. The area under curve (AUC) was used to summarize the classification accuracy of the predictive model and 95%CI of AUC were estimated by the non-parametric bootstrap. The specificity and sensitivity were evaluated at the optimal cutoff point, which was selected using Youden’s index. Considering the significant difference of age distribution of our controls compared with local population (Table S1) due to the frequency matching case-control study design [25], we further performed a weighted analysis to introduce age as a risk factor into the regression model. All statistical analyses were conducted using Stata 15.1 (StataCorp LP, College Station, TX, USA).
Results
The distributions of candidate environment risk factors for both healthy controls and ESCC cases stratified by gender are summarized in Table 1. Compared with control participants, male and female cases tended to have less education, lower family wealth scores, lower BMI 10 years ago, taller adult height, fewer frequency of tooth brushing per day and were more likely to have a family history of esophageal cancer among their first-degree relatives. However, since smoking, alcohol drinking and habitual tea drinking are uncommon among females, only among males, ESCC cases reported more likely to be cigarette smokers, alcohol drinkers, and hot tea drinkers than controls. Conversely, only among females ESCC cases were slightly older than controls, more likely to be farmers, and had more missing and filled teeth. Among controls, males differed significantly from females regarding several characteristics, i.e., marital status, occupation, education level, BMI, adult height, sum of missing and filled teeth, smoking, alcohol drinking and tea drinking. After summarizing the number of candidate environment risk factors, most cases were exposed to 4 to 6 risk factors, while most controls were exposed to 3 to 5 risk factors.
The candidate variables identified by univariate analysis were all included in the predictive model for males. Namely, for males, the predictive nomogram distinguishing ESCC cases from healthy controls included education, family wealth score, BMI, adult height, tooth brushing times, smoking pack-years, alcohol drinking intensity, tea drinking temperature, and family history of esophageal cancer (Fig. 1a). For each participant, points were assigned for each category of independent ESCC risk factors, then a total score and a corresponding predicted probability of developing ESCC were calculated from the nomogram. The non-linear dose-response association of total scores with ESCC risk is illustrated in Fig. 1b, with a significant monotonous increasing trend. A ROC curve was plotted to estimate the predictive accuracy of the nomogram, and the corresponding AUC (95% CI) was 0.75 (0.72–0.77; Fig. 1c). The ORs (95% CI) and points for all predictive variables are listed in Table S2.
A nomogram to predict ESCC risk in men. a Predictive nomogram of ESCC. Points corresponding to each category of variables are listed in Table 1. b Non-linear dose-response relation between total scores and ESCC risk. The model was adjusted for age and the reference was set as total score of 21.9. c ROC curve of the nomogram model. ESCC, esophageal squamous cell carcinoma; OR, odds ratio; ROC, receiver-operating characteristic; AUC, area under curve
The variable occupation was removed from the predictive model for females, because of its collinearity with education and family wealth score. Thus the nomogram to predict ESCC risk among females included education, family wealth score, BMI, adult height, tooth brushing times, missing and filled teeth number, and family history of esophageal cancer (Fig. 2a, Table S2). The monotonous increase of ESCC risk in association with total scores in women is illustrated in Fig. 2b. The AUC (95% CI) of the nomogram predictive tool for females was 0.76 (0.73–0.79; Fig. 2c).
A nomogram to predict ESCC risk in women. a Predictive nomogram of ESCC. Points corresponding to each category of variables are listed in Table 1. b Non-linear dose-response relation between total scores and ESCC risk. The model was adjusted for age and the reference was set as total score of 24.8. c ROC curve of the nomogram model. ESCC, esophageal squamous cell carcinoma; OR, odds ratio; ROC, receiver-operating characteristic; AUC, area under curve
Weighted analysis
Through the weighted analysis adjusting the age disparity between controls and the general population, risk factors included in the prediction model for males contained age group, education, family wealth score, adult height, frequency of tooth brushing, missing and filled teeth, smoking pack-years, alcohol drinking intensity, tea drinking temperature, and family history of esophageal cancer (Fig. 3a, Table S3). The monotone increasing risk of ESCC with increasing total scores is illustrated in Fig. 3b. The AUC of ROC curve for the prediction model for males was 0.81 (95% CI: 0.79, 0.84; Fig. 3c).
A nomogram to predict ESCC risk in men by a weighted analysis. a Predictive nomogram of ESCC. Points corresponding to each category of variables are listed in Table 1. b Non-linear dose-response relation between total scores and ESCC risk. The reference was set as total score of 21.9. c ROC curve of the nomogram model. ESCC, esophageal squamous cell carcinoma; OR, odds ratio; ROC, receiver-operating characteristic; AUC, area under curve
Analogously, age, education, family wealth score, adult height, tooth brushing frequency, missing and filled teeth number, and family history of esophageal cancer were included in the predictive model for females, with an AUC of 0.88 (95% CI: 0.85–0.90; Fig. 4, Table S3).
A nomogram to predict ESCC risk in women by a weighted analysis. a Predictive nomogram of ESCC. Points corresponding to each category of variables are listed in Table 1. b Non-linear dose-response relation between total scores and ESCC risk. The reference was set as total score of 17.3. c ROC curve of the nomogram model. ESCC, esophageal squamous cell carcinoma; OR, odds ratio; ROC, receiver-operating characteristic; AUC, area under curve
Discussion
Several prospective studies have demonstrated that endoscopic screening for early detection of ESCC could reduce its mortality [26,27,28]. However, among those endoscopically screened, the detection rate of ESCC cases is less than 0.5%, resulting in low cost-efficiency of ESCC screening programs due to lack of accurate risk prediction tools for risk-stratification [11, 12]. Moreover, field experiences show that endoscopic screening with a low true positive rate leads to poor compliance in the preselected population. A quantitative predictive tool providing individual risk assessment could help candidates make reasonable decision on whether or not to undergo endoscopy examination.
Recently, Sheikh et al. generated a risk scoring system summing up exposures like smoking, hot tea drinking, fruit intake, vegetable intake, tooth loss, un-piped water, and indoor air pollution and presented a significant dose-dependent relationship between ESCC risk and combined environmental risk factors based on the Golestan Cohort Study in Iran [29], but it was not suitable for preselection of high-risk population, because the individual ESCC risk probability was not evaluated. A nomogram for predicting the risk of mixed premalignant lesions containing reflux esophagitis, inflammatory lesions, dysplasia, and so on, showed an AUC of 0.749 (0.711–0.788) based on information on age, sex, education, occupation, income, labor intensity and mining exposure collected from an esophageal endoscopic screening project in China [12], but the etiology of ESCC is substantially different from esophagitis and mixed premalignant lesions [30, 31]. As a risk prediction method, nomogram has shown promising value in clinical prognosis prediction [32, 33]. Here, to our best knowledge, we first established an easy-to-use prediction tool via nomogram to optimize the preselection of candidate high-risk population for ESCC endoscopic screening programs.
The relationships between all identified environmental risk factors and ESCC risk have been well discussed in our previous articles [15, 17,18,19,20,21]. Although several predictive variables may not directly cause ESCC, they might be surrogate variables and their predictive values are notable [34, 35]. For primary prevention of ESCC, promoting good oral hygiene and alcohol abstinence should be the most cost-efficient and easy-to-apply community intervention measures. Moreover, based on our results, we will develop a mobile App to automatically analyze and report the individual ESCC risk when the information of multiple environmental risk factors is collected. If asymptomatic residents receive a high score of ESCC development probability, they are advised to avoid risk factors, and also can choose to undergo a prophylactic endoscopic examination.
Our study has several advantages. To decrease selection bias, we attempted to enroll all newly-diagnosed ESCC cases and randomly selected frequency matching control participants from the total registry of residents of the study area. We interviewed most ESCC cases before they were aware of their diagnoses, which would partly reduce potential report bias and recall bias. Moreover, our study had a relatively large sample size, independent pathophysiological confirmation of all cases, relatively high response rates for both cases and controls, and the systematic collection of information on environmental risk factors.
There are also some limitations to our nomogram-based model. First, the study was conducted in a high-risk area of ESCC in China, which would weaken the generalization of our risk prediction tool to other areas. Second, despite our best efforts to collect candidate risk factors of ESCC, the questionnaire interview hardly covered all information of ESCC etiological factors. Third, although the AUCs of ESCC prediction nomogram in both sexes were slightly over 0.8, the predictive tool was not generated to deliver an accurate diagnosis but to optimize the preselection of eligible high-risk population of ESCC for endoscopic screening which is better than available approaches at present. Finally, the age distribution of the frequency matching controls was different from that of local residents, but we performed a weighted analysis to overcome this limitation.
Conclusions
We established an easy-to-use preclinical prediction tool for both sexes to select candidate population with high ESCC risk for subsequent endoscopy screening, which optimizes the implementation of endoscopic screening projects and promotes early prevention of ESCC. The diagnostic accuracy and cost-effectiveness of our predictive tool need to be further validated in prospective studies and reappraised in ESCC screening programs.
Availability of data and materials
All data that support the findings of this study are available from the corresponding authors for a reasonable request.
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- BMI:
-
Body mass index
- OR:
-
Odds ratios
- CI:
-
Confidence intervals
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–7. https://doi.org/10.1136/gutjnl-2014-308124.
Abnet CC, Arnold M, Wei W-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–73. https://doi.org/10.1053/j.gastro.2017.08.023.
Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31(6):1141–6. https://doi.org/10.1111/jgh.13289.
Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136(8):1921–30. https://doi.org/10.1002/ijc.29227.
Chiu PWY, Uedo N, Singh R, Gotoda T, Ng EKW, Yao K, et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. 2019;68(2):186–97. https://doi.org/10.1136/gutjnl-2018-317111.
Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–47. https://doi.org/10.1016/S1470-2045(13)70436-4.
Bai Y, Li ZS. Evolution of gastrointestinal endoscopy in the mainland of China. Chin Med J. 2009;122(19):2220–3.
Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal Cancer in China. J Clin Oncol. 2015;33(17):1951–7. https://doi.org/10.1200/JCO.2014.58.0423.
He Z, Liu Z, Liu M, Guo C, Xu R, Li F, et al. Efficacy of endoscopic screening for esophageal cancer in China (ESECC): design and preliminary results of a population-based randomised controlled trial. Gut. 2019;68(2):198–206. https://doi.org/10.1136/gutjnl-2017-315520.
Xing J, Min L, Zhang H, Li P, Li W, Lv F, et al. A nomogram for endoscopic screening in a high esophageal squamous cell cancer risk area: results from a population-based study. Cancer Manag Res. 2019;11:431–42. https://doi.org/10.2147/CMAR.S167311.
National Health Commission of the People's Republic of China. Esophageal Cancer Diagnosis and Treatment Specifications (2018 Edition, in Chinese). Chin J Digest Med Imaged (in Chinese). 2019;9(4):158–92. https://doi.org/10.3877/cma.j.issn.2095-2015.2019.04.005.
Murphy G, McCormack V, Abedi-Ardekani B, Arnold M, Camargo MC, Dar NA, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28(9):2086–93. https://doi.org/10.1093/annonc/mdx279.
Chen T, Cheng H, Chen X, Yuan Z, Yang X, Zhuang M, et al. Family history of esophageal cancer increases the risk of esophageal squamous cell carcinoma. Sci Rep. 2015;5(1):16038. https://doi.org/10.1038/srep16038.
Chen X, Winckler B, Lu M, Cheng H, Yuan Z, Yang Y, et al. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS One. 2015;10(12):e0143603. https://doi.org/10.1371/journal.pone.0143603.
Chen X, Yuan Z, Lu M, Zhang Y, Jin L, Ye W. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma-a population-based case-control study in China. Int J Cancer. 2016;140(3):626–35. https://doi.org/10.1002/ijc.30484.
Yang X, Chen X, Zhuang M, Yuan Z, Nie S, Lu M, et al. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: a population-based case-control study in China. Sci Rep. 2017;7(1):17249. https://doi.org/10.1038/s41598-017-17617-2.
Gao P, Yang X, Suo C, Yuan Z, Cheng H, Zhang Y, et al. Socioeconomic status is inversely associated with esophageal squamous cell carcinoma risk: results from a population-based case-control study in China. Oncotarget. 2018;9(6):6911–23. https://doi.org/10.18632/oncotarget.24003.
Yang X, Ni Y, Yuan Z, Chen H, Plymoth A, Jin L, et al. Very hot tea drinking increases esophageal squamous cell carcinoma risk in a high-risk area of China: a population-based case-control study. Clin Epidemiol. 2018;10:1307–20. https://doi.org/10.2147/CLEP.S171615.
Yang X, Zhang T, Yin X, Yuan Z, Chen H, Plymoth A, et al. Adult height, body mass index change, and body shape change in relation to esophageal squamous cell carcinoma risk: a population-based case-control study in China. Cancer Med. 2019;8(12):5769–78. https://doi.org/10.1002/cam4.2444.
Suo C, Yang Y, Yuan Z, Zhang T, Yang X, Qing T, et al. Alcohol intake interacts with functional genetic polymorphisms of aldehyde dehydrogenases (ALDH2) and alcohol dehydrogenase (ADH) to increase esophageal squamous cell Cancer risk. J Thorac Oncol. 2019;14(4):712–25. https://doi.org/10.1016/j.jtho.2018.12.023.
Suo C, Qing T, Liu Z, Yang X, Yuan Z, Yang YJ, et al. Differential cumulative risk of genetic polymorphisms in familial and nonfamilial esophageal squamous cell carcinoma. Cancer Epidemiol Biomark Prev. 2019;28(12):2014–21. https://doi.org/10.1158/1055-9965.EPI-19-0484.
Ekheden I, Yang X, Chen H, Chen X, Yuan Z, Jin L, et al. Associations between gastric atrophy and its interaction with poor Oral health and the risk for esophageal squamous cell carcinoma in a high-risk region of China: a population-based case-control study. Am J Epidemiol. 2020;189(9):931–41. https://doi.org/10.1093/aje/kwz283.
Salim A, Delcoigne B, Villaflores K, Koh WP, Yuan JM, van Dam RM, et al. Comparisons of risk prediction methods using nested case-control data. Stat Med. 2017;36(3):455–65. https://doi.org/10.1002/sim.7143.
di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the esophagus: screening, diagnosis, and therapy. Gastroenterology. 2018;154(2):421–36. https://doi.org/10.1053/j.gastro.2017.07.041.
Liu M, He Z, Guo C, Xu R, Li F, Ning T, et al. Effectiveness of intensive endoscopic screening for esophageal Cancer in China: a community-based study. Am J Epidemiol. 2019;188(4):776–84. https://doi.org/10.1093/aje/kwy291.
Morimoto M, Nishiyama K, Nakamura S, Suzuki O, Kawaguchi Y, Nakajima A, et al. Significance of endoscopic screening and endoscopic resection for esophageal cancer in patients with hypopharyngeal cancer. Jpn J Clin Oncol. 2010;40(10):938–43. https://doi.org/10.1093/jjco/hyq068.
Sheikh M, Poustchi H, Pourshams A, Etemadi A, Islami F, Khoshnia M, et al. Individual and combined effects of environmental risk factors for esophageal Cancer based on results from the Golestan cohort study. Gastroenterology. 2019;156(5):1416–27. https://doi.org/10.1053/j.gastro.2018.12.024.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–96. https://doi.org/10.1016/S0140-6736(17)31462-9.
Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and eastern countries. World J Gastroenterol. 2015;21(26):7933–43. https://doi.org/10.3748/wjg.v21.i26.7933.
Lo SN, Ma J, Scolyer RA, Haydu LE, Stretch JR, Saw RPM, et al. Improved risk prediction calculator for sentinel node positivity in patients with melanoma: the melanoma institute Australia Nomogram. J Clin Oncol. 2020;38(24):2719–27. https://doi.org/10.1200/JCO.19.02362.
Serenari M, Han KH, Ravaioli F, Kim SU, Cucchetti A, Han DH, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73(4):855–62. https://doi.org/10.1016/j.jhep.2020.04.032.
Courtin E, Kim S, Song S, Yu W, Muennig P. Can social policies improve health? A systematic review and meta-analysis of 38 randomized trials. Milbank Q. 2020;98(2):297–371. https://doi.org/10.1111/1468-0009.12451.
Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang L, Bian Z, et al. Socioeconomic Status in Relation to Risks of Major Gastrointestinal Cancers in Chinese Adults: A Prospective Study of 0.5 Million People. Cancer Epidemiol Biomarkers Prev. 2020;29(4):823–31. https://doi.org/10.1158/1055-9965.EPI-19-0585.
Acknowledgements
The authors would like to acknowledge the interviewers and technicians at Fudan University Taizhou Institute of Health Sciences, for their invaluable contribution to data collection and sample preparation, the staff at the Taixing Center for Disease Control and Prevention for their help in organization of field work, and the staff at Taixing People’s Hospital for their assistance with sample collection.
Funding
This work was supported by National Natural Science Foundation of China (81973116, 82073637, 91846302 and 81573229), National Key Research and Development program of China (2017YFC0907002 and 2017YFC0907003), International S&T Cooperation Program of China (2015DFE32790), European Research Council (682663), and Shandong Provincial Natural Science Foundation (grant number: ZR2020QH302). The funding body had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
XRY: collected research datasets, analyzed data and drafted the manuscript; CS and JRY: analyzed data. TCZ, XLY, JYM, ZYY and HC: participated in data collection and cleaning; ML, XDC, WMY and LJ initiated, organized and supervised the study; ML, XDC and WMY: critically revised the manuscript; ML and XDC: provided technical support. All authors interpreted the results and revised the final version of the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Boards of the School of Life Sciences, Fudan University (date: February 19, 2009), Qilu Hospital, Shandong University (date: March 8, 2010), and Stockholm Ethical Vetting Board (2018/357–31). The study was carried out in accordance with the approved protocol, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary material.
The questionnaire of upper gastrointestinal disease in Taixing, China.
Additional file 2: Table S1.
The age distribution of local residents in Taixing city and the weight of controls in our data analysis.
Additional file 3: Table S2.
OR with 95%CIs and nomogram points of candidate ESCC risk factors.
Additional file 4: Table S3.
OR with 95%CIs and nomogram points of candidate ESCC risk factors by a weighted analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, X., Suo, C., Zhang, T. et al. A nomogram for screening esophageal squamous cell carcinoma based on environmental risk factors in a high-incidence area of China: a population-based case-control study. BMC Cancer 21, 343 (2021). https://doi.org/10.1186/s12885-021-08053-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08053-7