Abstract
Background
Cancer survivors are frequently excluded from clinical research, resulting in their omission from the development of many cancer treatment strategies. Quantifying the prevalence of prior cancer in newly diagnosed cancer patients can inform research and clinical practice. This study aimed to describe the prevalence, characteristics, and trends of prior cancer in newly diagnosed cancer patients in Japan.
Methods
Using Osaka Cancer Registry data, we examined the prevalence, characteristics, and temporal trends of prior cancer in patients who received new diagnoses of lung, stomach, colorectal, female breast, cervical, and corpus uterine cancer between 2004 and 2015. Site-specific prior cancers were examined for a maximum of 15 years before the new cancer was diagnosed. Temporal trends were evaluated using the Cochran-Armitage trend test.
Results
Among 275,720 newly diagnosed cancer patients, 21,784 (7.9%) had prior cancer. The prevalence of prior cancer ranged from 3.3% (breast cancer) to 11.1% (lung cancer). In both sexes, the age-adjusted prevalence of prior cancer had increased in recent years (P values for trend < 0.001), especially in newly diagnosed lung cancer patients. The proportion of smoking-related prior cancers exceeded 50% in patients with newly diagnosed lung, stomach, colorectal, breast, and cervical cancer.
Conclusions
The prevalence of prior cancer in newly diagnosed cancer patients is relatively high, and has increased in recent years. Our findings suggest that a deeper understanding of the prevalence and characteristics of prior cancer in cancer patients is needed to promote more inclusive clinical research and support the expansion of treatment options.
Similar content being viewed by others
Background
Cancer survivors are at risk of developing second primary cancers, and their numbers are increasing worldwide [1,2,3,4,5]. A study from the US Surveillance, Epidemiology, and End Results Program reported that almost 20% of individuals with incident cancers between 2009 and 2013 had prior cancer [6]. The number of patients with second malignant cancers is reportedly increasing, which may be influenced by hereditary and familial risk, antecedent cancer therapy, lifestyle-related factors (e.g., tobacco and alcohol consumption), and environmental factors [7].
Cancer survivors require unique medical and psychosocial support with proactive assessments and follow-up care [4]. Several studies have reported that prior cancer did not adversely affect survival in lung and pancreatic cancer patients [8,9,10,11]. In contrast, another study noted that poorer survival was associated with some prior cancer types (e.g., colorectum, melanoma, and breast), but not others (e.g., esophagus, stomach, and lung) [12].
There is a growing need for evidence-based strategies to improve the quality and effectiveness of care for cancer survivors. However, the frequent exclusion of cancer survivors from clinical studies may undermine the generalizability of findings on the effectiveness and safety of treatment regimens. Therefore, clarifying the types and trends of prior cancers may help to inform the reevaluation of criteria for participation in clinical research and contribute to the improvement of cancer care [13].
Insight into the prevalence and characteristics of prior cancer can inform research and clinical practice, but few studies have used population-based cancer registry data to examine these issues. In addition, most epidemiological evidence for the association between prior cancer and newly diagnosed cancer is derived from studies conducted in the US and Europe, with little evidence from Asian populations. Extensions to the survival time of cancer patients elevates their risk of developing a second cancer, which could eventually manifest as an increase in the prevalence of cancer patients with prior cancer [1]. However, this trend has yet to be explored in Japan. The aim of this study was to provide globally comparable estimates of the prevalence, characteristics, and trends of prior cancer in newly diagnosed cancer patients using a long-term Japanese cancer registry database in order to aid further research and inform healthcare strategies.
Methods
Data source and study design
Data were obtained from the Osaka Cancer Registry (OCR), a population-based cancer registry founded in 1962 for the purpose of registering and monitoring all malignant tumors and benign intracranial tumors throughout Osaka prefecture (the third largest metropolitan area in Japan). The OCR covers a population of 8.8 million people, and allows the identification of prior cancers in individual patients [14].
Data on all cancer patients diagnosed between 1989 and 2015 were extracted for analysis. The various cancers were identified using the corresponding International Classification of Diseases, Tenth Revision (ICD-10) codes. The data also included each patient’s age at diagnosis (hereinafter referred to as diagnostic age), sex, cancer detection method, cancer stage, treatment, month of diagnosis (diagnostic month), year of diagnosis (diagnostic year), inclusion in the registry through death certificate only, living status, and survival time.
We quantified the prevalence of prior cancer in patients who received new diagnoses of cancer of the following sites between 2004 and 2015: lung, stomach, colorectum, female breast (hereinafter referred to as breast), cervix uteri, and corpus uteri. These sites were selected as they are the target sites for cancer screening programs in Japan, and are associated with high incidence [15]. We then examined the occurrence and types of prior cancers within 15 years before the diagnostic month and year of each newly diagnosed cancer.
Definitions and study subjects
Index cancers were defined as cancers that were newly diagnosed between 2004 and 2015 in patients who met the following criteria: 1) diagnostic age of 15–99 years, 2) pathologically diagnosed cancer for any of the target sites (lung, stomach, colorectum, breast, cervix uteri, and corpus uteri), and 3) survived for three months or more after diagnosis. In Osaka prefecture, the percentage of cases registered from death certificate only fell below 10% from 2004 onward [16]. Therefore, data from 2004 and later were used to identify the index cancer cases. As prior cancers were identified within the 15-year period before each index cancer, data were extracted from 1989 (i.e., 15 years before 2004) onward. Because this study was conducted to provide evidence for the increased inclusion of cancer survivors in clinical trials, we focused on subjects who had survived for three months or more after diagnosis (i.e., patients with relatively longer survival). Patients that were registered in the OCR through death certificate only were excluded from this study because these cases often lack important patient background information, such as diagnosis date and cancer stage.
Cases of multiple cancers are recorded in the OCR in accordance with the guidelines of the International Agency for Research on Cancer and the International Association of Cancer Registries [17]. However, multiple cancers of the same site in an individual patient were combined as the “most common prior cancer” to avoid including the incomplete integration of multiple prior cancers or metastatic cancers [5].
Prior cancers were defined as cancers diagnosed during the 15-year period before the index cancer diagnosis. This 15-year cutoff was set to standardize the retroactive observation period across patients of different ages at the time of index cancer diagnosis and to provide a margin three times that of the 5-year window used in many clinical trials [13, 18]. If two or more prior cancers had the same diagnostic month and year in a patient, the sequence of these cancers was randomly determined using a previously described method [6].
We identified the index and prior cancer sites using the following ICD-10 codes [19]: all sites (C00–C96.x), mouth and pharynx (C00–C14.x), esophagus (C15.x), stomach (C16.x), colon (C18.x), rectum (C19.x–C20.x), liver (C22.x), gallbladder and bile duct (C23.x–C24.x), pancreas (C25.x), larynx (C32.x), lung (C33–C34.x), melanoma of skin (C43.x), other skin (C44.x), mesothelioma (C45.x), breast (C50.x), uterus (C53.x–C55.x), cervix uteri (C53.x), corpus uteri (C54.x), ovary (C56.x), prostate (C61.x), bladder (C67.x), renal and urinary tract (C64.x–C66.x, C68.x), brain and central nervous system (C70.x–C72.x), thyroid (C73.x), Hodgkin lymphoma (C81.x), non-Hodgkin lymphoma (C82.x–C86.x, C96.x), immunoproliferative diseases (C88.x), myeloma (C90.x), lymphoid leukemia (C91.x), acute myeloid leukemia (C92.0), myeloid leukemia (C92.x–C94.x), and unspecified leukemia (C95.x).
Patient and index cancer characteristics
For each patient, we analyzed age group (15–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and 85–99 years), sex (male or female), method of cancer detection (screening and medical check-up, incidental detection during follow-up examination for another disease, and other or unknown; the last category generally involved cancer detection due to the occurrence of subjective symptoms) [20], cancer stage (localized, regional lymph nodes, regional extension, distant metastasis, and other or unknown), treatment (radiotherapy only, chemotherapy only, chemoradiotherapy, surgery only, surgery plus chemotherapy or radiotherapy, and other or unknown), and diagnostic year (2004–2005, 2006–2007, 2008–2009, 2010–2011, 2012–2013, or 2014–2015). Missing values were included in the “unknown” category for each factor.
Prior cancer characteristics
For patients with prior cancer, we calculated the number of prior cancers before the index cancer, as well as the diagnostic time interval between the index cancer and most recent prior cancer. In addition, we examined the stage, treatment, and site of each prior cancer. The prior cancer site was identified for the most recent prior cancer. We categorized the following prior cancers as smoking-related cancers based on previous studies [21,22,23,24,25]: mouth, pharynx, larynx, lung, esophagus, stomach, liver, pancreas, kidney, urinary bladder, colorectum, cervix, and acute myeloid leukemia.
Statistical analysis
The main outcome measure was the prevalence of prior cancer in the study subjects. In order to account for the varying age structures of the cancer patient population over time, we examined the trends in the age-adjusted prevalence (measured every two years) of prior cancer for each index cancer site according to sex. First, we calculated the age-specific prior cancer prevalence for each age group according to sex and cancer site. To obtain the expected number of prior cancer cases, we multiplied the age-specific prior cancer prevalence by the number of patients for each age group according to sex in our subjects between 2004 and 2015 (which was set as the reference cancer population). We then totaled the expected number of prior cancer cases from all age groups. Finally, to calculate the age-adjusted prevalence, we divided the total expected number of prior cancer cases by the reference cancer population. We described the temporal trends in prior cancer prevalence from 2004 to 2015 using the Cochran–Armitage trend test [26]. The distributions of the above site-specific measurements were also examined according to sex. The temporal trends in the method of index cancer detection were assessed.
Continuous variables were summarized as median values and interquartile ranges, and categorical variables were summarized as proportions. Proportions were compared using Pearson’s chi-square test. The significance level was set at 5% (two-sided). All analyses were performed using STATA version 14 (Stata corporation, College Station, TX, USA).
Results
Prior cancer prevalence and patient characteristics
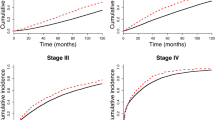
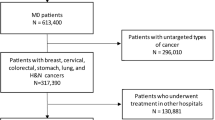
Figure 1 shows the subject selection process. We identified 275,720 index cancer patients that met the inclusion and exclusion criteria. Among these, 21,784 (7.9%) had prior cancer. As shown in Fig. 2, the age-adjusted prevalence of prior cancer had significantly increased over the study period for all index cancers in both male and female patients (all P values < 0.001). The trends in age-adjusted index cancer prevalence and the expected number of index cancer patients with prior cancer are presented in Supplementary Table S1. This prevalence was notably higher in lung cancer patients in both sexes.
Trends in the age-adjusted prevalence of prior cancer between 2004 and 2015. The graphs show the age-adjusted prevalence of prior cancer in a male and b female patients according to index cancer site. P values for trend over the study period were calculated using the Cochran–Armitage test. The percentage of death certificate notification cases (DCN%) according to each diagnostic year was 26.2% in 2004–2005, 19.7% in 2006–2007, 16.5% in 2008–2009, 12.3% in 2010–2011, 9.0% in 2012–2013, and 6.0% in 2014–2015
The characteristics of the patients according to index cancer site are summarized in Table 1. Among all patients, the prevalence of prior cancer was 11.1% in lung cancer patients, 9.5% in stomach cancer patients, 7.5% in colorectal cancer patients, 3.3% in breast cancer patients, 2.7% in cervical cancer patients, and 6.7% in corpus uterine cancer patients. Among older patients aged 65 years or older, the prevalence of prior cancer was 12.1% in lung cancer patients, 10.5% in stomach cancer patients, 8.4% in colorectal cancer patients, 5.2% in breast cancer patients, 5.4% in cervical cancer patients, and 8.5% in corpus uterine cancer patients. Throughout the study period, the proportion of cases detected through screening and medical check-up remained relatively stable, whereas the proportion of cases detected through incidental detection had increased (Supplementary Table S2). Patients whose index cancers were incidentally detected during follow-up examination for another disease had a higher prevalence of prior cancer than other cancer detection methods for all index cancer sites. In addition, the prevalence of prior cancer was also higher in patients whose index cancer was in the localized stage. With the exception of other or unknown treatments, surgery was the most common treatment for lung cancer (16.9%), colorectal cancer (8.2%), breast cancer (4.6%), and cervical cancer (5.2%).
Prior cancer characteristics
The characteristics of male and female patients with prior cancer are shown in Tables 2 and 3, respectively. Approximately 90% of these patients had only one prior cancer regardless of sex. The proportions of male patients with two prior cancers were 10.4% for lung cancer, 8.7% for stomach cancer, and 8.9% for colorectal cancer. In female patients, these proportions were 7.2% for lung cancer, 5.6% for stomach cancer, 6.1% for colorectal cancer, 5.0% for breast cancer, 7.3% for cervical cancer, and 5.4% for corpus uterine cancer. The cumulative proportions of the most recent prior cancers diagnosed within 5 years before the index cancer diagnosis were 69.4% (male) and 65.0% (female) for lung cancer, 75.0% (male) and 67.4% (female) for stomach cancer, 75.5% (male) and 65.3% (female) for colorectal cancer, 65.2% (female) for breast cancer, 69.0% (female) for cervical cancer, and 54.7% (female) for corpus uterine cancer. The most common and least common prior cancer stages were localized and distant metastasis, respectively. Surgery only was the most common treatment for prior cancer across all index cancer sites in both sexes.
The most common sites of the most recent prior cancers were analyzed according to index cancer site in male patients (Table 2) and female patients (Table 3). In male patients, smoking-related cancers accounted for approximately 70% of prior cancers. In female patients, smoking-related prior cancers were more common in index breast cancer patients and less common in index corpus uterine cancer patients than patients with the other index cancers (Fig. 3). The proportions of smoking-related prior cancers in male patients were significantly higher than in female patients for index lung, stomach, and colorectal cancer (Fig. 3). Supplementary Table S3 presents the temporal trends in the proportion of smoking-related index cancers, and Supplementary Table S4 summarizes the temporal trends in the proportion of smoking-related prior cancers among index cancer patients.
Proportions of smoking-related prior cancers according to index cancer site. Smoking-related prior cancers included cancers of the mouth, pharynx, larynx, lung, esophagus, stomach, liver, pancreas, kidney, urinary bladder, colorectum, cervix, and acute myeloid leukemia. Pearson’s chi-square test was used to compare the proportions of smoking-related prior cancers between the sexes. Error bars represent 95% confidence intervals
Discussion
In this analysis of population-based cancer registry data from a major metropolitan area in Japan, we ascertained the prevalence, characteristics, and trends of prior cancer in patients newly diagnosed with one of five major cancer types. Even after accounting for the changes in age structure, the prevalence of prior cancer was found to have increased over time for all index cancer sites. These trends may be attributable to earlier cancer detection (increase in localized cancer rates from 35.2% in 2004 to 48.4% in 2015). The screening and medical check-up method can result in earlier detection, but the use of this method was relatively stable throughout the study period. In contrast, cancers detected using the incidental detection method (where cancer is detected during follow-up examination for another disease) had increased over the study period. This may have contributed to the earlier detection of cancers, which subsequently led to the rising prevalence of prior cancer in recent years. The increasing trends in the prevalence of prior cancer may also be attributable to longer survival (increase in relative survival in all cancers from 46.6% in 2004 to 59.2% in 2010) and expanded cancer registry coverage (decrease in the percentages of cases with death certificate notification or death certificate only) in more recently diagnosed cancer patients [15, 27, 28].
Among our subjects, patients with newly diagnosed lung cancer had the highest prevalence of prior cancer among the assessed index cancers. These differences in the prevalences of prior cancer may be indicative of underlying or shared risk factors (e.g., lifestyle habits such as smoking), and require further investigation.
Our analysis showed that the prevalence of prior cancer increased with age for all index cancers, with adolescents and young adults (aged 15–39 years) having a lower prevalence than older patients. However, our estimated prevalences of prior cancer in patients aged 65 years or older were lower than those reported in a previous study conducted in the US (lung: 18.7%, stomach: 17.8%, colorectum: 15.3%, breast: 7.4%, cervix: 13.6%, and corpus uteri: 13.6%) [6]. This disparity may be influenced by an inherent difference in the age-standardized cancer incidence rates for all sites between the US (393.2 per 100,000 population) and Japan (285.9 per 100,000 population) [29]. In addition to variations in prior cancer prevalence, this may also be indicative of differences in genetic, lifestyle-related, and/or environmental risk factors. Differences in the prevalence of prior cancers among previous studies may also be explained in part by our non-inclusion of patients with carcinoma in situ, as this condition is generally curable and would not unduly affect survival. In addition, we did not use the recorded sequence numbers that indicate the order of cancer in individual cases, which may also have resulted in a lower apparent prevalence of prior cancer. We had decided to exclude prior cancers at the same site as the index cancer to avoid potential double counting because we would be unable to determine if the index cancer was metastatic or primary.
The prevalence of prior cancer was found to be higher in index cancer patients with localized tumors, and prior cancers were generally diagnosed in the early localized stage across all index cancers. A possible explanation for the former observation is that patients with prior cancer would undergo regular follow-up examinations, which would support the prompt incidental detection of new tumors in the early stages. This may also contribute to the high prevalence of prior cancer for patients whose index cancers were incidentally detected during examinations for another disease. For the latter observation, we posit that patients with cancers diagnosed in the earlier stages would receive prompt treatment and have longer survival, thereby increasing the opportunities for other cancers to develop. Our results also showed that surgical treatment was often selected for prior cancer, which may be due to the high proportion of early-stage cancers.
In the present study, the prevalence of prior cancers in male patients was higher than in female patients. In particular, the proportion of male patients with two or more prior cancers was higher than female patients for new cases of lung cancer, stomach cancer, and colorectal cancer. The cumulative proportions of the most recent prior cancers diagnosed within 5 years before the index cancer diagnosis were higher in male patients (Tables 2 and 3). These observations may be influenced by the higher incidence of cancers in men, lifestyle differences, and other sex-based differences [30].
While our data did not include information on tobacco consumption, smoking-related prior cancers were found to be less common in female patients newly diagnosed with lung, stomach, and colorectal cancer than their male counterparts. The proportions of smoking-related prior cancers were 73.1% in male patients and 53.0% in female patients among the general population of cancer patients (P < 0.001). In recent years, smoking rates have declined in Japan (45.7% in men and 15.2% in women in 2004 to 30.4% in men and 10.7% in women in 2016) [31]. At the same time, smoking-related cancers have also decreased, suggesting that the reduction in smokers is related to the reduction in smoking-related cancers (Supplementary Table S3). However, the proportion of index cancer patients with smoking-related prior cancers have decreased in men, but increased in women (Supplementary Table S4). Smoking prevalence may be associated with smoking-related prior cancers among male patients, but this relationship is less clear in female patients. This may indicate a biological difference in susceptibility to smoking-related cancer between the sexes, but more work is needed to explore this possibility [32, 33].
Furthermore, female patients with index breast cancer had a higher proportion of smoking-related prior cancers than those with other index cancers. Although several studies have reported that smoking is associated with an increased risk of breast cancer [34,35,36], the evidence remains inconclusive and further research is needed to understand these findings. Patients with cervical cancer had a significantly higher rate of smoking-related cancers than those with corpus uterine cancer, likely because cervical cancer is also a smoking-related cancer.
Our results also showed that patients with index breast cancer had a higher prevalence of prior corpus uterine cancer (8.3%) relative to the general cancer population (3.7%) in the OCR [15]. Similarly, patients with index corpus uterine cancer had a higher prevalence of prior breast cancer (46.6%) relative to the general cancer population (20.7%) in the OCR [15]. In addition, there was a slightly higher prevalence of prior ovary cancer in patients with index breast cancer (3.8%) than the general cancer population (2.4%) [15]. This may be indicative of hereditary cancers (such as hereditary breast and ovarian cancer syndrome, Lynch syndrome, and Li-Fraumeni syndrome) or the effects of previous treatments for prior cancers [37, 38].
Cases with prior cancer are frequently excluded from clinical research. However, the relatively high prevalence of prior cancer in newly diagnosed cancer patients suggests that their exclusion would have a substantial effect on research outcomes. Approximately 80% of previous clinical trials for lung cancer patients excluded those with prior cancer, and most trials employ a 5-year exclusion window [13, 18]. Patients with prior cancer are also sometimes excluded from observational studies due to concerns that they may affect outcome measurements [39, 40]. Our present study found that approximately 70% of lung, stomach, and colorectal cancer patients with prior cancer had a diagnostic interval of 5 years or less between the prior and index cancers. In addition, the most frequent diagnostic interval was 1–5 years for all index cancer sites. Even after excluding cancer patients with a diagnostic interval of 3 months or less, individuals who had a diagnostic interval of 5 years or less still accounted for more than 60% of patients with prior cancer. Accordingly, a considerable proportion of patients with prior cancer would not be eligible to participate in trials with a 5-year exclusion window. The impact of prior cancer on survival should be examined with scientific evidence, and cancer survivors should not be excluded from studies as a matter of course [41]. Quantifying the prevalence of prior cancer in newly diagnosed cancer patients can aid our understanding of these patients and support the formulation of comprehensive treatment strategies. For example, medical institutions, government agencies, and insurers may be able to design more efficient strategies to allocate health resources and develop treatment plans that account for cancer survivors [42,43,44,45].
This study has the following limitations. First, we created sequence numbers for multiple cancers in the OCR with the assumption that patients did not move outside of the cancer registry catchment area (i.e., Osaka prefecture). If the population had grown during the study period, the index cancer prevalences may increase, but the prior cancer prevalences could be underestimated due to a reduced coverage of medical history in new residents. If the population had declined during the study period, the index cancer prevalences may fall, and the accuracy of prior cancer prevalences may also be affected. In Osaka prefecture, out-migration exceeded in-migration until 2010, and this pattern was reversed from 2011 onward [46]. Overall, the prefectural population had increased from 8.81 million in 2004 to 8.83 million in 2015 [46]. Among the age groups, there were many in-migrants aged 15–24 years, whereas the other age groups tended to be out-migrants [46]. Accordingly, it is possible that the prevalences of index cancers and prior cancers were underestimated. Second, the registry lacked several types of patient information (e.g., tobacco use, alcohol use, and obesity), which prevented a more detailed analysis of patient background factors. Third, our study did not examine the competing risk of dying from the first cancer (which would preclude the occurrence of a second cancer) when considering second cancers in patients. Furthermore, cancers diagnosed in the earlier stages may involve lead time bias or length time bias because of earlier detection and slowly progressing diseases. Future studies are therefore needed to explore these aspects. Fourth, it is possible that cancers that occurred at a younger age had been overlooked due to the use of a 15-year retroactive observation period for identifying prior cancers.
Despite these limitations, our study has several strengths. Using a historic, large-scale cancer registry database, we were able to identify and characterize prior cancers in newly diagnosed cancer patients residing in a major metropolitan area in Japan. To the best of our knowledge, this is the first study to reveal recent trends in the age-adjusted prevalence of prior cancers stratified by sex.
Conclusions
This study provided globally comparable estimates of the prevalence and characteristics of prior cancer in newly diagnosed cancer patients using a long-term population-based cancer registry. The prevalence of prior cancer increased in recent years, and approximately 70% of prior cancers in male patients were potentially smoking-related. In light of the increasing number of cancer survivors worldwide, our findings suggest that a deeper understanding of the prevalence and characteristics of prior cancer in cancer patients is needed to promote more inclusive clinical research and support the expansion of treatment options.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OCR:
-
Osaka Cancer Registry
- ICD-10:
-
International Classification of Diseases, Tenth Revision
References
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin. 2019;69: 363–85. https://doi.org/10.3322/caac.21565.
Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103(6):1111–20.
Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior Cancer among persons newly diagnosed with Cancer: an initial report from the surveillance, epidemiology, and end results program. JAMA Oncol. 2018;4(6):832–6.
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734–45.
Andrew L. Laccetti, Sandi L. Pruitt, Lei Xuan, Ethan A. Halm, David E. Gerber, Effect of Prior Cancer on Outcomes in Advanced Lung Cancer: Implications for Clinical Trial Eligibility and Accrual. J Natl Cancer Inst. 2015;107(4):djv002. https://doi.org/10.1093/jnci/djv002.
Pruitt SL, Laccetti AL, Xuan L, Halm EA, Gerber DE. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer. 2017;116(6):717–25.
Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Prior cancer does not adversely affect survival in locally advanced lung cancer: a national SEER-medicare analysis. Lung Cancer. 2016;98:106–13.
He C, Zhang Y, Cai Z, Lin X. Effect of prior cancer on survival outcomes for patients with pancreatic adenocarcinoma: a propensity score analysis. BMC Cancer. 2019;19(1):509.
Zhou H, Huang Y, Qiu Z, Zhao H, Fang W, Yang Y, Zhao Y, Hou X, Ma Y, Hong S, et al. Impact of prior cancer history on the overall survival of patients newly diagnosed with cancer: a pan-cancer analysis of the SEER database. Int J Cancer. 2018.
Gerber DE, Pruitt SL, Halm EA. Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res. 2015;4(4):289–91.
Tsukuma H, Fujimoto I, Hanai A, Hiyama T, Kitagawa T, Kinoshita N. Incidence of second primary cancers in Osaka residents, Japan, with special reference to cumulative and relative risks. Japan J Cancer Res. 1994;85(4):339–45.
Osaka Prefectural Department of Public Health and Welfare, Osaka Medical Association, Osaka International Cancer Institute: Annual Report of Osaka Cancer Registry No.82 - Cancer Incidence and Medical Care in Osaka in 2015・2014 and the Survival in 2010 -. OPDPHW 2019, 82.
Parkin DM, Chen VW, Ferlay J, Galceran J, Storm HH, SL W. Comparability and Quality Control in Cancer Registration, IARC Technical Report No. 19. Lyon: IARC; 1994.
Group RW. International rules for multiple primary cancers (ICD-O third edition). Eur J Cancer Prev. 2005;14(4):307–8.
David E. Gerber, Andrew L. Laccetti, Lei Xuan, Ethan A. Halm, Sandi L. Pruitt, Impact of Prior Cancer on Eligibility for Lung Cancer Clinical Trials. J Natl Cancer Inst. 2014;106(11):dju302, https://doi.org/10.1093/jnci/dju302.
World Health Organization: International Statistical Classification of Diseases and Health Related Problems (The) ICD-10 Second Edition. World Health Organization 2004.
Department of Public Health and Medical Affairs Osaka Prefectural Government, Cancer Control Center Osaka International Cancer Institute: Annual Report of Osaka Cancer Registry vol. 84: Cancer Incidence and Treatment in 2016 and Cancer Survival in 2011 in Osaka. Osaka Prefectural Government 2020, 84.
Aguilo R, Macia F, Porta M, Casamitjana M, Minguella J, Novoa AM. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg. 2008;34(5):1075–80.
Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 surgeon General's report: "the health consequences of smoking--50 years of progress": a paradigm shift in cancer care. Cancer. 2014;120(13):1914–6.
Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Beane Freeman L, Berrington de Gonzalez A, Caporaso N, Curtis RE, Elena J, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989–95.
Fircanis S, Merriam P, Khan N, Castillo JJ. The relation between cigarette smoking and risk of acute myeloid leukemia: an updated meta-analysis of epidemiological studies. Am J Hematol. 2014;89(8):E125–32.
Ugai T, Matsuo K, Oze I, Ito H, Wakai K, Wada K, Nagata C, Nakayama T, Liu R, Kitamura Y, et al. Smoking and subsequent risk of acute myeloid leukaemia: a pooled analysis of 9 cohort studies in Japan. Hematol Oncol. 2018;36(1):262–8.
Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11(3):375–86.
Osaka Prefectural Department of Public Health and Welfare, Osaka Medical Association, Osaka Medical Center for Cancer and Cardiovascular Disease: Annual Report of Osaka Cancer Registry No.80 - Cancer Incidence and Medical Care in Osaka in 2012・2011 and the Survival in 2009 -. OPDPHW 2016, 80.
Osaka Prefectural Department of Public Health and Welfare, Osaka Medical Association, Osaka International Cancer Institute: Annual Report of Osaka Cancer Registry No.81 - Cancer Incidence and Medical Care in Osaka in 2013 -. OPDPHW 2017, 82.
Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer Incidence in Five Continents, Vol. XI (electronic version). IntAgency Res Cancer. 2017:166.
Shin JY, Jung HJ, Moon A. Molecular markers in sex differences in Cancer. Toxicol Res. 2019;35(4):331–41.
Cancer Information Service, National Cancer Center, Japan: Cancer Registry and Statistics. https://ganjoho.jp/reg_stat/statistics/dl/index.html.
Yu Y, Liu H, Zheng S, Ding Z, Chen Z, Jin W, Wang L, Wang Z, Fei Y, Zhang S, et al. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer. 2014;85(3):351–60.
Li WY, Han Y, Xu HM, Wang ZN, Xu YY, Song YX, Xu H, Yin SC, Liu XY, Miao ZF. Smoking status and subsequent gastric cancer risk in men compared with women: a meta-analysis of prospective observational studies. BMC Cancer. 2019;19(1):377.
Jones ME, Schoemaker MJ, Wright LB, Ashworth A, Swerdlow AJ. Smoking and risk of breast cancer in the generations study cohort. Breast Cancer Res. 2017;19(1):118.
Kispert S, McHowat J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer (Dove Med Press). 2017;9:127–32.
Goldvaser H, Gal O, Rizel S, Hendler D, Neiman V, Shochat T, Sulkes A, Brenner B, Yerushalmi R. The association between smoking and breast cancer characteristics and outcome. BMC Cancer. 2017;17(1):624.
Therkildsen C, Ladelund S, Smith-Hansen L, Lindberg LJ, Nilbert M. Towards gene- and gender-based risk estimates in lynch syndrome; age-specific incidences for 13 extra-colorectal cancer types. Br J Cancer. 2017;117(11):1702–10.
Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. 2019.
Bradley CJ, Yabroff KR, Mariotto AB, Zeruto C, Tran Q, Warren JL. Antineoplastic treatment of advanced-stage non-small-cell lung Cancer: treatment, survival, and spending (2000 to 2011). J Clin Oncol. 2017;35(5):529–35.
Brooks GA, Austin AM, Uno H, Dragnev KH, Tosteson ANA, Schrag D. Hospitalization and survival of Medicare patients treated with carboplatin plus paclitaxel or Pemetrexed for metastatic, nonsquamous, non-small cell lung Cancer. JAMA Netw Open. 2018;1(6):e183023.
Kim ES, Bruinooge SS, Roberts S, Ison G, Lin NU, Gore L, Uldrick TS, Lichtman SM, Roach N, Beaver JA, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and friends of Cancer research joint research statement. J Clin Oncol. 2017;35(33):3737–44.
Rothman KJ, Greenland S, Lash TL: Modern Epidemiology, Third Edition: Lippincott Williams & Wilkins; 2008.
Rothman KJ: Epidemiology: an introduction, 2nd edition: Oxford university press; 2012.
Pearce N. Effect measures in prevalence studies. Environ Health Perspect. 2004;112(10):1047–50.
Pearce N. Classification of epidemiological study designs. Int J Epidemiol. 2012;41(2):393–7.
Statistics Bureau of Japan, Ministry of Internal Affairs and Communications.: Annual report on internal migration in Japan derived from the basic resident registration. https://www.stat.go.jp/english/data/idou/1.html.
Acknowledgements
We are grateful to Dr. Takahiro Tabuchi and Dr. Akira Oshima of the Cancer Control Center, Osaka International Cancer Institute, for their technical assistance.
Funding
This study was supported by grants from the Osaka Health and Medical Foundation, the Osaka Foundation for the Prevention of Cancer and Lifestyle-related Diseases, and the Ministry of Health, Labour and Welfare, Japan (H30-Gantaisaku-Ippan-009). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation and methodology: AS. Investigation and writing, review, and/or revision of the manuscript: AS, KM, TM, KN, KK, IM. Supervision: KK, IM. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Osaka International Cancer Institute (Approval No. 18–0020; December 6, 2018) and the Kyoto University Graduate School of Medicine Ethics Committee (Approval No. R1808; December 18, 2018). The ethics committees waived the need for informed consent from the OCR-registered patients as the data were anonymized before being received by the authors. Osaka Cancer Registry data were accessed with permission from the Osaka Prefectural Government. The study was performed in accordance with the ethical standards outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
KK has received research funds from Stella Pharma Corporation. The sponsor had no control over the interpretation, writing, or publication of this work. No potential conflicts of interest were disclosed by the other authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Temporal trends in age-adjusted index cancer prevalence and the expected number of index cancer patients with prior cancer
Additional file 2: Supplementary Table S2.
Temporal trends in the method of index cancer detection
Additional file 3: Supplementary Table S3.
Temporal trends in the proportion of smoking-related index cancers
Additional file 4: Supplementary Table S4.
Temporal trends in the proportion of smoking-related prior cancers among index cancer patients
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sato, A., Matsubayashi, K., Morishima, T. et al. Increasing trends in the prevalence of prior cancer in newly diagnosed lung, stomach, colorectal, breast, cervical, and corpus uterine cancer patients: a population-based study. BMC Cancer 21, 264 (2021). https://doi.org/10.1186/s12885-021-08011-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08011-3