Abstract
Background
Borderline resectable pancreatic cancer (BRPC) is frequently associated with positive surgical margins and a poor prognosis because the tumor is in contact with major vessels. This study evaluated the relationship between the margin-negative (R0) resection rate and findings indicating peripancreatic vascular invasion on multidetector computed tomography (MDCT) imaging after neoadjuvant chemoradiotherapy (NACRT) in patients with BRPC.
Methods
Twenty-nine BRPC patients who underwent laparotomy after neoadjuvant S-1 with concurrent radiotherapy were studied retrospectively. Peripancreatic major vessel invasion was evaluated based on the length of tumor-vessel contact on MDCT. The R0 resection rates were compared between the progression of vascular invasion (PVI) group and the non-progression of vascular invasion (NVI) group.
Results
There were 3 patients with partial responses (10%), 25 with stable disease (86%), and 1 with progressive disease (3%) according to the RECISTv1.1 criteria. Regarding vascular invasion, 9 patients (31%) were classified as having PVI, and 20 patients (69%) were classified as having NVI. Of the 29 patients, 27 (93%) received an R0 resection, and all the PVI patients received an R0 resection (9/9; R0 resection rate = 100%) while 90% (18/20) of the NVI patients underwent an R0 resection. The exact 95% confidence interval of risk difference between those R0 resection rates was − 10.0% [− 31.7–20.4%].
Conclusions
Patients with BRPC after NACRT achieved high R0 resection rates regardless of the vascular invasion status. BRPC patients can undergo R0 resections unless progressive disease is observed after NACRT.
Trial registration
UMIN-CTR, UMIN000009172. Registered 23 October 2012
Similar content being viewed by others
Background
Pancreatic cancer is highly lethal and has an extremely poor prognosis [1, 2]. Previous reports have shown a median survival time of 15.2 months after curative-intent resection and 6–8 months without surgery [1, 3]. Surgical resection is the only possible cure, but most patients are diagnosed at an advanced stage, and only 15–20% of patients are considered candidates for curative-intent resection [4]. For patients undergoing curative-intent resection, margin-positive (R1) residual tumor resection is significantly correlated with a poorer survival outcome [5].
Borderline resectable pancreatic cancer (BRPC) is frequently associated with positive surgical margins and a poor prognosis because the tumor is in contact with major vessels. According to the National Comprehensive Cancer Network (NCCN) guidelines, neoadjuvant chemotherapy (NAC) or neoadjuvant chemoradiotherapy (NACRT) is recommended for patients with BRPC [6, 7]. Neoadjuvant therapy may assist in shrinking the primary tumor, controlling micrometastases, and identifying patients at risk of early disease progression before surgery [8, 9]. Moreover, neoadjuvant therapy may improve the rate of R0 resection, which is a strong prognostic factor in BRPC patients undergoing pancreatic resection. Although several clinical trials using either NAC or NACRT have been conducted for BRPC patients, a standard treatment for BRPC has not been established. Recently, a phase II multicenter prospective trial (JASPAC 05) elucidated the efficacy and feasibility of neoadjuvant S-1 and concurrent radiotherapy at increasing the R0 resection rate and improving survival in BRPC [10].
Whether surgery is indicated for patients with localized pancreatic cancer is usually assessed according to the degree of tumor-vessel contact measured using multidetector computed tomography (MDCT). However, in BRPC patients who have received NACRT, the decision to proceed with surgery can be difficult because the MDCT findings do not always correspond with the actual resectability, partly because of inflammatory change caused by NACRT. Some of our patients in whom the primary tumor had shrunk but the perivascular component of the tumor had paradoxically progressed after NACRT were able to receive an R0 resection [11]. Based on the above-mentioned experiences, we began to presume that the radiological progression of the perivascular component of the tumor after NACRT might not correspond with the actual progression if the primary tumor does not show signs of progression. If so, R0 resectability after NACRT should be evaluated according to not only the degree of tumor-vessel contact, but the radiological response of the primary tumor.
We tested this hypothesis by evaluating the R0 resection rates according to the combinations of radiological changes in the perivascular tumor and the tumor response in patients who had undergone a laparotomy after receiving neoadjuvant S-1 and concurrent radiotherapy, including the patients enrolled in the JASPAC 05 trial. The relationship between the perivascular tumor response and clinicopathological variables and the factors associated with the pathological tumor response were also examined.
Methods
Patients and clinicopathological data collection
This was a retrospective observational study. Patients who were either enrolled in the JASPAC 05 trial or received NACRT with S-1 for BRPC at the National Cancer Center Hospital East (NCCHE) between 2008 and 2017 were examined. The patients were included in the present study if a laparotomy was conducted for curative-intent after NACRT. Patients who were found to have distant metastasis at the time of laparotomy were excluded. Among the 14 institutions that enrolled patients in JASPAC 05, 8 institutions agreed to collaborate with the present study. As a result, 24 patients with BRPC who had participated in the JASPAC 05 trial and 5 patients with BRPC who had received NACRT at the NCCHE were included. All 29 patients had a cytologically or histologically confirmed diagnosis of pancreatic ductal adenocarcinoma prior to undergoing NACRT.
Clinicopathological data were retrieved from the JASPAC 05 data and the medical records of the patients who were treated at the NCCHE. Patient characteristics including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor location, clinical staging as per the Union for International Cancer Control (UICC) classification (7th edition) [12], and type of tumor-vessel contact before NACRT were noted. Tumor size on CT imaging, the carcinoembryonic antigen (CEA) level, and the carbohydrate antigen 19–9 (CA 19–9) level were analyzed before and after NACRT. The tumor response was evaluated according to the response evaluation criteria in solid tumors classification (RECIST) v1.1 [13]. Intraoperative variables including the type of surgical procedure, portal vein resection, partial resection of the superior mesenteric artery (SMA) plexus, the number of retrieved lymph nodes, and blood loss were also examined.
Pathological findings including the tumor size, lymph node metastasis, portal vein invasion, plexus invasion, pathological staging as per the UICC classification (7th edition), resection margin, and pathological response were noted. R0 resection was defined as the absence of tumor cells present at the resection margin. The pathological response for NACRT was evaluated using the Evans classification [14]. The pathological response was classified as follows: grade I < 10% or no tumor cell destruction; grade IIa, destruction of 10–50% of tumor cells; grade IIb, destruction of 51–90% of tumor cells; grade III, < 10% viable-appearing tumor cells; and grade IV, no viable cells. A pathologist at each institution evaluated the histological examination results.
Neoadjuvant chemoradiotherapy
The NACRT regimen consisted of S-1 with concurrent radiotherapy, as reported previously [15]. Briefly, a total dose of 50.4 Gy was delivered in 28 fractions over 5.5 weeks. S-1 was administered orally at 40 mg/m2 twice daily for 28 consecutive days, followed by a 14-day rest period per course on the day of irradiation.
Surgical indication after NACRT
Patients who met the surgery-entry criteria, consisting of the absence of distant metastasis, no local progression precluding an R0 or R1 resection, a good performance status (PS 0/1), no massive ascites, no massive pleural effusion, no serious infection, no serious adverse NACRT events, and adequate organ system function, underwent surgery 15 to 56 days after the end of NACRT.
When diagnosing local progression precluding R0 or R1 resection, the occurrence of progressive disease (PD) according to the RECIST was basically used as our consensus-based standards for the criteria, while 1 patient with PD who conducted laparotomy at the NCCHE because of patient’s strong preference was included in the study. The perivascular findings obtained using MDCT after NACRT were not taken into consideration when determining the surgical indications unless the tumor response was PD.
Diagnosis and classification of CT imaging findings
BRPC was diagnosed via a 16- or 64-slice MDCT with a pancreas protocol including triphasic cross-sectional imaging with a 2-mm slice thickness. BRPC was defined as bilateral impingement on the superior mesenteric vein or portal vein (BR-PV) or tumor contact ≤180° with the superior mesenteric artery (BR-SMA), the common hepatic artery (BR-CHA), or the celiac artery (BR-CeA) based on the NCCN 2009 guidelines [6].
Two radiologists (T.K. and H.K.) who were blinded to the patients’ clinical histories retrospectively analyzed the CT images before and after NACRT. The radiological response of the perivascular component of the tumor was evaluated and graded as peripancreatic major vessel invasion. The response of the peripancreatic major vessel invasion was graded according to the change in the longitudinal length of tumor-vessel contact. The progression or the shrinkage of peripancreatic major vessel invasion was defined as an increase or decrease in the length of tumor-vessel contact ≥2 mm. Otherwise, the peripancreatic major vessel invasion was graded as stable. Patients were classified into 2 groups: progression of vascular invasion (PVI, progression) or non-progression of vascular invasion (NVI, shrinkage or stable).
The R0 resection rates according to the various combinations of peripancreatic major vessel invasion response and tumor response were analyzed. The relationships between the peripancreatic major vessel invasion response and other evaluation items such as the rates of reduction in CEA and CA 19–9 after NACRT, the tumor response according to the RECIST, and the pathological response according to the Evans classification were evaluated. Factors associated with a pathological response of Evans grade ≥ IIb were also analyzed.
Statistical analysis
Categorical variables were analyzed using the Fisher exact test and are shown as numbers with percentages. Continuous variables were analyzed using the Mann-Whitney U test and are shown as the median and range. All P values were based on two-sided statistical tests, and the significance level was set at 0.05. The exact 95% confidence interval (CI) of risk difference was constructed. All the statistical analyzes were performed using JMP12.0.1 (SAS Institute, Cary, NC, USA).
This study was approved by the National Cancer Center Institutional Review Board (IRB) and by the IRBs of the other 7 participating institutions.
Results
Clinicopathological characteristics
Table 1 shows the clinical variables of the 29 patients who were studied. Nine patients (31%) had BR-PV, and 19 patients (66%) had BR-SMA (overlap allowed). The overall tumor size decreased from 29 mm to 26 mm after NACRT. The overall CA 19–9 level also exhibited a sharp decrease from 202.2 U/mL to 68.7 U/mL. A radiologic tumor response to NACRT with S-1 was observed in 3 patients (10%). There were 3 patients with partial responses (PRs; 10%), 25 with stable diseases (SDs; 86%), and 1 with PD (3%) according to the RECISTv1.1 criteria.
Table 2 shows the pathological variables among the patients. In terms of the residual tumor status, 27 (93%) of the 29 patients underwent R0 resection, and one each underwent R1 or RX resections, respectively. RX resection means that the resection margin could not be evaluated. R2 resection, probe laparotomy, and by-pass operations were not performed. The destruction of more than 50% of the tumor cells (grade ≥ IIb according to the Evans classification) was observed in 8 patients (28%).
R0 resection rates based on the imaging findings of peripancreatic vascular invasion and tumor response
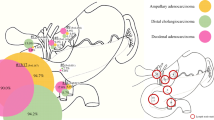
Nine patients (31%) were classified as PVI, and 20 (69%) were classified as NVI. All the patients in the PVI group underwent an R0 resection (9/9; R0 resection rate = 100%), while 90% (18/20) of the patients in the NVI group underwent an R0 resection. The exact 95% CI of risk difference between the PVI and NVI groups was − 10.0% [− 31.7–20.4%]. Furthermore, patients were classified into 6 groups according to the CT imaging findings for peripancreatic vascular invasion and tumor response according to the RECIST (Table 3). The R0 resection rates were not different among these 6 groups either. Figure 1 presents a representative patient from the PVI group in whom an R0 resection was achieved.
A patient with borderline resectable pancreatic cancer (a) before and (b) after neoadjuvant chemoradiotherapy. Legend; The pancreatic cancer in the uncinate process involved the superior mesenteric artery (SMA) and the superior mesenteric vein. The primary tumor (T) showed shrinkage (arrows), but the vascular invasion of the SMA (arrowheads) showed continued progression after neoadjuvant S-1 and concurrent radiotherapy
Relationships between grade of peripancreatic major vessel invasion and other evaluation items
Table 4 shows the rates of reduction in CEA and CA 19–9 after NACRT, the tumor responses according to RECIST, and the pathological responses in both the NVI and PVI groups. None of the aforementioned factors differed significantly between the PVI and NVI groups.
Factors associated with pathological response of Evans grade ≥ IIb
Twenty-one patients were classified as having a pathological response of Evans grade ≤ IIa; 8 were classified as having Evans grade ≥ IIb. When these patients were compared, neither the rate of decrease in tumor markers (CEA or CA 19–9) after NACRT, the grade of peripancreatic major vessel invasion, nor the tumor response according to the RECIST differed significantly (Table 5). As a result, no evaluation factor was determined to be associated with the pathological response.
Discussion
The present study showed that the response of peripancreatic vascular invasion after NACRT did not reflect the tumor response according to the RECIST. In the 29 BRPC patients, 9 (31%) experienced the progression of peripancreatic vascular invasion after NACRT, whereas local progression corresponding to PD according to the RECIST was only observed in 1 patient (3%). Moreover, the response of peripancreatic vascular invasion was likely to be uncorrelated with the actual resectability, since a high rate of R0 resection was achieved not only in the NVI group (90%), but also in the PVI group (100%) with the risk difference of − 10% [− 31.7–20.4%]. The progression of vascular invasion also seemed unrelated to the pathological response. These results would support our hypothesis that the radiological progression of the perivascular component of the tumor after NACRT might not reflect actual progression if the primary tumor does not exhibit progressive disease.
Patients with BRPC have a greater risk of being unable to receive an R0 resection than those with resectable pancreatic cancer, even when vascular resection and reconstruction are utilized [16]. Currently, BRPC is treated using a multimodality approach, usually consisting of neoadjuvant systemic therapy with or without radiotherapy to sterilize the tumor boundaries that are in contact with the peripancreatic vessels to enable a successful R0 resection [17,18,19].
Several studies have highlighted the difficulty in assessing local tumor extension using CT after NACRT for pancreatic cancer [20,21,22]. Cassinotto et al. found that 39% of patients who had a high risk of an R1 resection based on their CT findings after neoadjuvant therapy were able to receive an R0 resection. They surmised that the significantly lower accuracy and specificity in determining R0 resectability after neoadjuvant therapy was probably due to the inability of CT examinations to differentiate tumor tissue from necrosis, fibrosis, and inflammatory changes caused by neoadjuvant treatment.
Katz et al. demonstrated that 81 of the 85 patients (95%) received an R0 resection after being treated with neoadjuvant therapy for BRPC. In that study, only 1 patient was downstaged to resectable pancreatic cancer [23]. Ferrone et al. also demonstrated that 92% of patients with borderline resectable or locally advanced pancreatic cancer after receiving the neoadjuvant FOLFIRINOX regimen received an R0 resection regardless of having been judged as being unresectable based on the post-FOLFIRINOX CT imaging findings [24]. Both studies showed that downstaging to resectable pancreatic cancer from the CT imaging findings was rare after neoadjuvant therapy; however, R0 resection could be performed regardless of the radiological response when no distant metastatic lesions were observed.
In most of the resectability criteria, the extent of tumor-vessel contact was evaluated according to the degree of circumferential tumor–vessel interface. In the present study, however, we tried to assess tumor-vessel contact by the longitudinal distance of tumor–vessel interface to observe more detailed morphological changes. Specifically, we defined the progression of peripancreatic major vessel invasion as an increase in the length of tumor-vessel contact ≥2 mm because this definition enabled a good agreement with the interpreting physicians’ impressions of progression. Using this definition, 31% of the BRPC patients showed the progression of peripancreatic major vessel invasion after NACRT. However, 93% of the BRPC patients were able to receive an R0 resection irrespective of their peripancreatic major vessel invasion status. Furthermore, the peripancreatic major vessel invasion status did not seem correlated with any of the other items that were used to evaluate the efficacy of neoadjuvant treatment, such as the rate of reduction in CA 19–9, the tumor response, or the pathological response. These results would agree with the results of the aforementioned studies and lead us to conjecture that BRPC can be resected locally whether or not radiological perivascular progression is observed whenever the tumor response is not PD according to the RECIST. However, the surgical indications for BRPC with a PD tumor response remain uncertain because only one patient who met the above condition was included in the present study.
The present study had several limitations. First, the retrospective design introduced a selection bias. Several patients who underwent a probe laparotomy or bypass operation because of local tumor progression were excluded because the institutions where the patients had undergone these treatments did not participate in this study. This selection bias might have affected the results of the study. Second, the number of patients included in this study was relatively small, even considering the rarity of this disease. The small sample size probably had an affect on the wide CI of the risk difference of R0 resection rates between PVI and NVI groups. However, those R0 resection rates themselves were acceptable. Then, the conclusions which were drawn from the results including the R0 resection rates might be considered of value. Validation of the study’s hypothesis in a larger cohort is warranted.
Conclusions
Patients with BRPC achieved a high R0 resection rate after NACRT regardless of the progression of peripancreatic vascular invasion. Patients with BRPC without PD according to the RECIST after NACRT can be considered as candidates for surgical resection.
Availability of data and materials
We promise to that the materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BRPC:
-
Borderline resectable pancreatic cancer
- MDCT:
-
Multidetector computed tomography
- NACRT:
-
Neoadjuvant chemoradiotherapy
- PVI:
-
Progression of vascular invasion
- NVI:
-
Non-progression of vascular invasion
- NCCN:
-
National Comprehensive Cancer Network
- NAC:
-
Neoadjuvant chemotherapy
- NCCHE:
-
National Cancer Center Hospital East
- ECOG:
-
Eastern Cooperative Oncology Group
- UICC:
-
Union for International Cancer Control
- CEA:
-
Carcinoembryonic antigen
- CA 19–9:
-
Carbohydrate antigen 19–9
- RECIST:
-
Response evaluation criteria in solid tumors classification
- SMA:
-
Superior mesenteric artery
- PD:
-
Progressive disease
- PV:
-
Portal vein
- CHA:
-
Hepatic artery
- CeA:
-
Celiac artery
References
Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012;18:4944–58.
Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. https://doi.org/10.1371/journal.pmed.1000267.
Bjerregaard JK, Mortensen MB, Schønnemann KR, Pfeiffer P. Characteristics, therapy and outcome in an unselected and prospectively registered cohort of pancreatic cancer patients. Eur J Cancer. 2013;49:98–105.
Russo S, Ammori J, Eads J, Dorth J. The role of neoadjuvant therapy in pancreatic cancer: a review. Future Oncol. 2016;12:669–85.
Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–9.
NCCN. Clinical practice guidelines in oncology (NCCN guidelines) pancreatic adenocarcinoma version 3; 2019. www.nccn.org/patients.
Palmer DH, Stocken DD, Hewitt H, Markham CE, Bassim Hassan A, Johnson PJ, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–96.
Von Hoff DD, Ervin T, Arena FP, Gabriela Chiorean E, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502.
Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, et al. Neoadjuvant S-1 with concurrent radiotherapy followed by surgery for borderline resectable pancreatic cancer: a phase II open-label multicenter prospective trial (JASPAC 05). Ann Surg. 2020;7(10):e018445. https://doi.org/10.1097/SLA.0000000000004535.
Suenaga M, Fujii T, Yamada S, Okumura N, Takeda S, Nakao A, et al. Three cases showing discrepancy between preoperative imaging and postoperative histopathological finding after neoadjuvant chemoradiation therapy for pancreatic cancer. Jpn J Gastroenterol Surg. 2013;46:106–13.
Sobin LH, Wittekind C, Gospodorowicz MK. UICC TNM classification of malignant Tumours. 7th ed. Hoboken: Wiley-Backwell; 2010.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, Yamao K, et al. A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:163–9.
Evans DB, George B, Tsai S. Non-metastatic pancreatic cancer: Resectable, borderline resectable, and locally advanced-definitions of increasing importance for the optimal delivery of multimodality therapy. Ann Surg Oncol. 2015;22:3409–13.
Faria SC, Tamm EP, Loyer EM, Szklaruk J, Choi H, Charnsangavej C. Diagnosis and staging of pancreatic tumors. Semin Roentgenol. 2004;39:397–411.
Kulig J, Popiela T, Zajac A, Kłek S, Kołodziejczyk P. The value of imaging techniques in the staging of pancreatic cancer. Surg Endosc. 2005;19:361–5.
Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming B, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–46.
Kim YE, Park MS, Hong HS, Kang CM, Choi JY, Lim JS, et al. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology. 2009;250:758–65.
Morgan DE, Waggoner CN, Canon CL, Lockhart ME, Fineberg NS, Posey JA 3rd, et al. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR Am J Roentgenol. 2010;194:615–22.
Cassinotto C, Cortade J, Belleannée G, Lapuyade B, Terrebonne E, Vendrely V, et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol. 2013;82:589–93.
Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–56.
Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–7.
Acknowledgements
Not applicable.
Funding
This work was supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (19ck0106441h0003).
Author information
Authors and Affiliations
Contributions
Study concept: TK, HA, ST, MI, MK1, and MK2. Study design: TK, HA and ST. Acquisition of data: HA, KU, SM, AM, HT, NT, KS, and WT. Interpretation of data: TK, HA and ST. Drafting the work: SY. Revising the draft critically: TK, ST, and HK. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the National Cancer Center Institutional Review Board (the reference number: 2016–455) and by the Institutional Review Boards of Shizuoka Cancer Center, Kanagawa Cancer Center, National Hospital Organization Osaka National Hospital, Kobe University Hospital, Fukuyama City Hospital, National Hospital Organization Kyusyu Cancer Center, and Chiba Cancer Center. Obtaining informed consent was not necessarily required by Ethical Guidelines for Medical and Health Research Involving Human Subjects because the present study was utilizing existing information retained by the research implementing entity and did not utilize human biological specimens. Access to the clinical/personal patient data used in the present study was implemented according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the permission of the National Cancer Center Institutional Review Board and the IRBs of the other 7 participating institutions. The research met the standards of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yasuta, S., Kobayashi, T., Aizawa, H. et al. Relationship between surgical R0 resectability and findings of peripancreatic vascular invasion on CT imaging after neoadjuvant S-1 and concurrent radiotherapy in patients with borderline resectable pancreatic cancer. BMC Cancer 20, 1184 (2020). https://doi.org/10.1186/s12885-020-07698-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07698-0