Abstract
Background
Melanoma is the most aggressive type of skin cancer and is associated with environmental and genetic risk factors. It originates in melanocytes, the pigment-producing cells. Single nucleotide polymorphisms (SNPs) in pigmentation genes have been described in melanoma risk modulation, but knowledge in the field is still limited.
Methods
In a case-control approach (107 cases and 119 controls), we investigated the effect of four pigmentation gene SNPs (TYR rs1126809, HERC2 rs1129038, SLC24A5 rs1426654, and SLC45A2 rs16891982) on melanoma risk in individuals from southern Brazil using a multivariate logistic regression model and multifactor dimensionality reduction (MDR) analysis.
Results
Two SNPs were associated with an increased risk of melanoma in a dominant model: rs1129038AA and rs1426654AA [OR = 2.094 (95% CI: 1.106–3.966), P = 2.3 10− 2 and OR = 7.126 (95% CI: 1.873–27.110), P = 4.0 10− 3, respectively]. SNP rs16891982CC was associated with a lower risk to melanoma development in a log-additive model when the allele C was inherited [OR = 0.081 (95% CI: 0.008–0.782), P = 3 10− 2]. In addition, MDR analysis showed that the combination of the rs1426654AA and rs16891982GG genotypes was associated with a higher risk for melanoma (P = 3 10− 3), with a redundant effect.
Conclusions
These results contribute to the current knowledge and indicate that epistatic interaction of these SNPs, with an additive or correlational effect, may be involved in modulating the risk of melanoma in individuals from a geographic region with a high incidence of the disease.
Similar content being viewed by others
Background
Melanoma is the most aggressive skin tumor, and its incidence has been correlated with latitude of residence, occurring most frequently in fair-skinned individuals [1]. In fact, the risk of developing melanoma diverges markedly according to skin pigmentation and geographical area, mainly due to the causative effect of ultraviolet radiation [2, 3]. Currently, Australia and New Zealand have the highest incidence and mortality rates of melanoma in the world, with incidence reaching 33.6/100,000 and 33.3/100,000, respectively [4]. In those two countries, the risk of developing melanoma before age 75 years is 1/24 and 1/34 for males and females, respectively [5]. In Brazil, melanoma represents 4% of all skin cancers, and 6260 new diagnoses are estimated in 2018. The highest incidence rates per region are expected for southern Brazil, reaching 8.4/100.000 [6]. These high rates are attributed to geographical location– the southernmost state of Brazil, Rio Grande do Sul, is within the same latitude as Australia (30.0346° South) [7]. Social practices with intense and often unprotected sun exposure and a majority-European ancestry are associated with lighter pigmentation of the skin in these individuals [7, 8].
Approximately 10% of melanomas are caused by germline mutations in cancer predisposition genes [9]. These include genes predominantly associated with melanoma (such as CDKN2A and CDK4) but also genes related to multiple solid tumors including melanoma. Examples are, among others, the BAP1 gene, the PTEN gene related to Cowden’s syndrome and XPD, XPC and XPA genes related to Xeroderma pigmentosum. Most identifiable heritable mutations associated with hereditary melanoma have variable penetrance [10]. In addition to germline mutations, our understanding of the contribution of single nucleotide polymorphisms (SNP) proposed as risk modulators for melanoma is increasing [11]. Several common SNPs, usually of low penetrance, are commonly investigated in polygenic risk models. These models can assess the joint effect of independent SNPs in genes with lower and intermediate penetrance such as MC1R, ARNT, CDK10, identified by the GWAS, and can assist in the identification of individuals with a higher risk of susceptibility to melanoma [12]. Some of these SNPs are located in genes of the melanogenic pathway, and some have been described in association with melanoma in different populations across the world [13]. A complicating aspect of such studies is that, in multifactorial disorders such as melanoma, genetic and nongenetic factors, such as admixture, population substructure, and evolution patterns, can severely confound the results and result in false-positive associations [14]. Thus, differences in allele frequency between cases and controls could be associated with differences in ancestry rather than reflect an association of genes with disease [15].
The Consortium for Analysis of Diversity and Evolution in Latin America (CANDELA) is a multidisciplinary international study that involves researchers focused on studying the biological diversity of Latin Americans, analyzing samples from Mexico, Colombia, Peru, Chile, and Brazil for a wide range of issues relevant to anthropological, biological and medical research in these populations. In 2014, an analysis to evaluate a possible association between 18 SNPs in genes involved in the pigmentation pathway and Melanin Index (MI) was performed within the Brazilian cohort of Consortium for the Analysis of the Diversity and Evolution of Latin America (CANDELA) with participants born in Rio Grande do Sul (RS) and Bahia (BA), in the South and Northeast of Brazil, respectively. As a result of this analysis, four SNPs were associated with differences in MI in these populations: rs1126809 (p.Arg402Gln) on tyrosinase (TYR), rs1129038 (3’UTR) in the hect domain and rcc1-like domain (HERC2), rs1426654 (p.Thr111Ala) in solute support family 24, member 4 (SLC24A5) and rs16891982 (p.Phe374Leu) in the family of solute, member 2 (SLC45A2) carriers. Among these four SNPs, allele A of rs1426654 and allele G of rs16891982 were associated with less melanin content in the 352 participants of RS cohort (P < .001) [16].
In this study, we aimed to assess the association of these four SNPs with melanoma risk in southern Brazil, a region with important contribution of European ancestry and with the highest indices of melanoma in the country.
Methods
Samples and genotyping
This case-control study was conducted at a public University Hospital, Hospital de Clínicas de Porto Alegre (HCPA) in southern Brazil, was approved by the Institutional Review Board under number 07–139, and all participants provided informed consent. Overall, 255 unrelated individuals were recruited for the study between September 2007 and November 2008. All participants were born in the State Rio Grande do Sul, and of these, 120 had been diagnosed with melanoma. Among the melanoma patients, 19 had a family history of melanoma (melanoma in first-, second-, and/or third-degree relatives) and/or multiple primary melanomas and 101 had features of sporadic melanoma. All diagnoses were confirmed by pathology reports. The 135 individuals of the control group were recruited consecutively among patients who presented to the outpatient clinic of the same dermatology department for an initial consultation or regular follow-up for diseases other than skin cancer. None of the individuals included in the control group reported a family history of the disease, and within the patients and control groups, there were no familial relations. All patients willing to participate were clinically examined and demographic variables, pigmentation traits (eye and hair color), skin type, tanning ability, quantitative/qualitative presence of nevi, and data from primary lesions of patients with cutaneous melanoma were documented. Genomic DNA was obtained from peripheral blood, and genotyping of SNPs rs1126809 (TYR), rs1129038 (HERC2), rs1426654 (SLC24A5) and rs16891982 (SLC45A2) based on the results of the Consortium for the Analysis of the Diversity and Evolution of Latin America (CANDELA, http://www.ucl.ac.uk/silva/candela) was performed using Human Custom TaqMan® SNP Genotyping Assays 40X (Applied Biosystems, USA; Assay IDs: AHBKFKH; C_48033–10; C_2908190_10; C_2842665_10, respectively). Genotyping were conducted using 20 ng of genomic DNA in a StepOneTM Real-Time PCR System (Applied Biosystems, USA). Allelic discrimination and analysis was performed using the Real-Time PCR software v.2.2. The study was conducted according to the Declaration of Helsinki Principles.
Ancestry analysis
Because the population structure due to admixture is a known confounding factor in association studies, the proportion of African, European, and Amerindian ancestry of all individuals recruited was evaluated using a previously published panel containing 61 biallelic short insertion/deletion polymorphisms (INDELs) [17].
Statistical analysis
Genotype and allele frequencies were obtained by simple counting. Differences between groups were compared using Pearson’s chi square or Fisher’s exact tests. All tests were two-tailed, and significance was set at 0.05. Wilcoxon test was performed to compare ancestry profiles between cases and controls, skin types and carriers and noncarriers of fixed alleles in European populations. The study was conducted considering two scenarios: with and without population structure control.
After obtaining the proportion of African, European, and Amerindian ancestry of the individuals recruited for this study we observed that some individuals had strikingly different ancestry profiles when compared to the majority of the sample, indicating populational substructure (Additional file 1). To control for this substructure, we performed 10,000 bootstrap simulations and calculated the average 95% confidence interval of these simulations to obtain the ancestry distribution profile. Using this confidence interval, we were able to identify samples exceeding the interval. This approach identified 29 individuals, which were removed to reduce sample substructure, which could skew the analysis. The remaining samples were used in all subsequent analyses, such as in the Hardy-Weinberg equilibrium test, logistic regression and MDR. To estimate the risk of melanoma associated with selected variants, we calculated odds ratios and their 95% confidence intervals using multivariate logistic regression analysis and controlled for the following confounders: age (discrete variable); sex; skin type according to the Fitzpatrick scale in 6 subtypes, hair color, number of nevi (more or less than 50 nevi); European and African ancestry. Eye color was not used as a confounding variable because it is a covariable of the color of skin and hair. We chose these variants for adjustment since they are established risk factors for melanoma [18]. All statistical analyses were performed using SPSS®, version 18 (IBM, USA) and R.
Multifactor dimensionality reduction
Higher-order gene-gene interactions among the SNPs associated in the logistic regression analysis were used in a nonparametric and genetic model-free multifactor dimensionality reduction (MDR) approach (version 3.0.4.). Bivariate MDR analysis was performed to verify the contribution of each SNP in the interaction and included HERC2 rs1129038, SLC24A5 rs1426654, and SLC45A2 rs16891982. The model with the highest testing balance accuracy and with major cross-validation consistency was selected as the best model. Statistical significance was determined using a 1000-fold permutation test.
Results
Sample
Clinical features of the 254 individuals recruited are summarized in Table 1. Most individuals were female, older than 50 years at recruitment and fair-skinned (56.8% skin types I and II). The mean age was higher in the control group and the number of nevi was higher in the case group. A trihybrid ancestry profile with predominant European contribution was observed. Although admixture in the Brazilian population is expected and significant, in our sample, a homogeneity of European ancestry and a difference of European and African ancestry profiles between cases and controls were observed (P = .004 and P = .008, respectively). Additionally, mean European ancestry in individuals with skin types I and II was different than that observed in those with skin types III, IV and V (0.946, CI 95% [0.934–0.959] versus 0.902, CI 95% [0.875–0.928], P = .002). Moreover, carriers of almost all fixed alleles in European populations had a different ancestry profile when compared with noncarriers (the European ancestry profile A in rs1426654 was 0.928, CI 95% [0.915–0.942] and 0.746, CI 95% [0.021–1.470] in carriers and noncarriers, respectively, P = .033; the European ancestry profile G in rs16891982 was 0.936, CI 95% [0.924–0.947] and 0.740, CI 95% [0.561–0.918] in carriers and noncarriers, respectively, P < 0.001). Among melanoma patients, the average age at diagnosis was 53.72 years (SD15.5), and 23.5% had intraepithelial tumors, with the most common histological subtype being superficial spreading melanoma (Table 2).
Population substructure control
Using the 95% confidence interval calculated for each ancestry, we detected a population substructure (mean European ancestry was 0.971, CI 95% [0.583–0.991], mean African ancestry was 0.010, CI 95% [0.002–0.206], and mean Native-American ancestry was 0.013, CI 95% [0.003–0.280], see Additional file 1A. A total of 29 samples (13 cases and 16 controls) were outside the ancestry confidence interval and were excluded from the analysis of Hardy-Weinberg equilibrium and logistic regression (see Additional file 1B) in order to control for the population substructure (the entire list of excluded samples can be found in Additional Table 1).
Genotyping
Genotyping results are summarized in Table 3. Initially, we undertook a separate analysis of the SNP frequencies in individuals with and without a family history of melanoma in the case group and did not identify a significant difference between groups (data not shown). Therefore, we have opted to continue the additional analyses including all individuals in the case group (those with and without a family history of melanoma). Linkage disequilibrium was not observed, and only rs16891982 in SLC45A2 did not follow Hardy-Weinberg equilibrium when considering the entire sample. After controlling for substructure, all SNPs were in Hardy-Weinberg equilibrium (α = 0.05; rs1126809 TYR χ2 = 0.089, P = 1; rs1129038 HERC2 χ2 = 1.361, P = 1; rs1426654 SLC24A5 χ2 = 0.796, P = 1; rs16891982 SLC45A2 χ2 = 3.182, P = 0.498). Further analysis showed a statistically significant difference in genotypic and allelic frequencies between cases and controls for rs1426654, rs16891982, and rs1129038. Comparisons of allelic frequencies between the main population databases and other population data of southern Brazilians are shown in Additional Table 2. Allelic frequency data reinforce similarities between South Brazilians and Europeans.
Genetic variants and skin pigmentation
Details from the comparative data on the associations between genotypic frequencies and skin pigmentation parameters in cases and controls are shown in Additional Table 2. With the exception of SNP TYR rs1126809, all the SNPs were associated with certain phenotypes. The SNP HERC2 rs1129038 AA genotype was more frequent in individuals with light skin and eyes and blond hair in both cases and controls (P < .001 for all analysis). The SNP SLC24A5 rs1426654 was also associated with lighter skin and eye color, but only in the control group (P = .0017). The SNP SLC45A2 rs16891982 GG genotype was more frequent in individuals with fair skin and hair both in cases and controls (P > .001 and P = .008; P < .001 and P = .004, for cases and controls, respectively). The same genotype was associated with light eye color only among cases (P < .001).
Genetic variants as risk factors for melanoma
Clinical features of the melanoma patients are summarized in Table 2. Three of the four SNPs were associated with melanoma outcome. The HERC2 rs1129038AA and SLC45A2 rs16891982GG genotypes and the rs1426654A allele were more frequently observed in cases than controls (P = .0016, P = .002, and P < 0.001, respectively). In a regression logistic model, including the following risk factors: sex, age, hair color, skin type, number of nevi, and African ancestry, these associations remained strong, suggesting that they may be independent risk factors. Odds ratios (OR) for melanoma associated with genetic effect models that were obtained before and after genetic substructure control and are shown in Table 4. The dominant model for HERC2 rs1129038 and SLC24A5 rs1426654 was considered the best model, and in both, an increase in OR was observed after substructure control: [OR = 2.212 (95% CI: 1.106–4.426), P = .025] and [OR = 13.996 (95% CI: 1.711–113.995), P = .014], respectively. For SLC45A2 rs16891982, the most suitable genetic model was log-additive, showing a slight reduction in the effect modification after substructure reduction when compared to the entire sample [OR = 0.068 (95% CI: 0.007–0.692), P = .023] and [OR = 0.081 (95% CI: 0.008–0.782), P = .030].
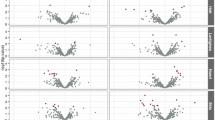
Additionally, we performed nonparametric Multifactor dimensionality reduction (MDR, v. 3.0.4.) to detect and characterize gene-gene interactions among HERC2 (rs1129038), SLC24A5 (rs1426654), and SLC45A2 (rs16891982) in risk of developing melanoma (Moore et al., 2006). Significant two- (P < .001) and three-locus interactions (P = .031) were identified in our analysis (Table 5). According to these results, the best model for predicting melanoma development was the combination of the three factors (HERC2 rs1129038, SLC24A5 rs1426654, and SLC45A2 rs16891982). More details about the criteria for selecting the best model can be found in [19]. The largest main effect with the higher information gain (IG) was observed for SLC24A5 rs1426654 (5.63%), with 100% accuracy for this model. The contribution of the other two markers, SLC45A2 rs16891982 (3.80%) and HERC2 rs1129038 (2.34%), indicated that they also have an important role in predicting melanoma risk. This interaction represents a high redundancy effect, which can be interpreted as an additive or correlation effect (Fig. 1). These finding are confirmed when analyzing the interaction graph with genotypic associations (Fig. 2). The combination of SLC24A5 rs1426654AA and SLC45A2 rs16891982GG was significantly associated with melanoma risk regardless of the genotype of HERC2 rs1129038, confirming the lower weight of HERC2 (2.34%) in the analysis of gene interaction. However, even isolated HERC2 rs1129038 analysis shows that the AA genotype confers an increased risk for melanoma. Logistic regression models were created with the risk genotypes pointed out by MDR for rs1426654 and rs16891982 (AA and GG genotypes, respectively) and compared to a model without these risk alleles to identify the best model. The comparison, using ROC curve analysis, demonstrated that the model with the risk alleles is more appropriate than the model without these alleles (AUC 0.702, 95% CI 0.637–0.766 versus AUC 0.669, 95% CI 0.602–0.736).
Multifactor dimensionality reduction (MDR) interaction models. Interaction circle graph comprised of nodes with pairwise connections. Values in nodes represent information gain (IG) of individual genes. While values between nodes are the IG of each pairwise combination. The type of interaction is showed by color of the line. The blue line represents negative entropy. Redundancy or linkage disequilibrium
The SNPs interaction to risk with melanoma development. A high-frequency genotype combination is displayed in Dark Square. while low-frequency combinations are in lightly shaded. For each cell. The left bar indicates the absolute number of cases and the right bar the absolute number of controls. a The effect of HERC2 rs1129038AA genotype. b The combination effect of SLC24A5 rs1426654AA and SLC45A2 rs16891982GG genotypes. c The combination effect of SLC24A5 rs1426654AA. SLC45A2 rs16891982GG. and HERC2 rs1129038AA genotypes
Discussion
Based on a study developed by CANDELA, we investigated 4 variants previously associated with skin pigmentation in southern Brazil in melanoma patients and unaffected controls from the same geographic region [16]. A model of logistic regression including ancestry, melanoma risk factors and SNPs HERC2 rs1129038 and SLC24A5 rs1426654 in dominant models of inheritance showed significant associations with melanoma. The SLC45A2 rs16891982CC SNP, in a log-additive model, was associated with a lower risk for developing the disease.
Pigmentation is a polygenic trait. and different variants have been associated with melanin levels in populations over the world [20]. One of the four SNPs investigated rs1129038, occurs in the untranslated region of HERC2, and three. TYR rs1126809, SLC24A5 rs1426654, and SLC45A2 rs16891982, are present in genes involved in the synthesis of melanosomes, the vesicles where melanin production and deposition occurs. In fact, rs1426654 and rs16891982 polymorphisms are determinants of pigmentation in Europeans [21], as well as in other populations [20, 22]. Our findings corroborate the association of some genotypes with lighter pigmentation and predominant European ancestry. The association of European ancestry and fair skin, eyes and hair was previously demonstrated in a sample of 1594 individuals from the same geographic region of the present study [23]. and the different ancestry profiles between darker and lighter individuals has also been previously reported [24]. Although our sample is not representative of the tri-hybrid pattern seen in most Brazilians [25], it reflects the massive colonization by Europeans in the specific region of the study [16, 23]. Just as there are regional differences in the proportions of ancestral populations in Brazil, we also expect heterogeneity in the frequency of genetic variants of specific genes, especially those related to skin, eye and hair pigmentation. Although research with admixed populations can be useful for allele detection involved in susceptibility to common diseases, the population substructure is a potential bias and should be controlled [26, 27]. In addition to a limitation in sampling (the sample was partially paired), the Hardy-Weinberg deviation found in our allelic frequencies for SLC45A2 rs16891982 can also be explained by the occurrence of interethnic mix and population substructure. Previously identified in Europe and in other Brazilian populations [28].
The nonsynonymous SNP TYR rs1126809 (p.Arg402Gln) has been previously associated with light pigmentation of skin and is frequent in Caucasians. Its presence results in the reduction of activity of tyrosinase, a key enzyme in of the melanin production pathway, and some authors reported an increased risk of melanoma in carriers. Both in Europe and Australia [29]. However, we did not observe a consistent association of this SNP with pigmentation nor with risk for melanoma in our series.
On other hand, HERC2 rs1129038, which was previously associated with lighter eye pigmentation in European populations [30, 31], showed a significantly association with fair skin. Eyes and hair in our sample. Forensic associations have described this SNP as a good predictor of blue eyes in Europeans [30, 32] and Brazilians [33]. and our findings reinforce these predictions.
Finally, SNPs SLC24A5 rs1426654 and SLC45A2 rs16891982 were associated with fair skin, eyes, and hair and with melanoma. SLC24A5 rs1426654 (p.Thr111Ala) was first described in zebrafish as responsible for the golden phenotype due to a delay in melanin production during embryonic development [34]. In melanocyte cultures, homozygous GG leads to an increase in SLC24A5 gene transcripts and a consequent increase in tyrosinase activity and melanin production [35]. The decrease of G allele frequency is gradual from Africa to Europe, indicating that a selection pressure in favor of the A allele acted on the determination of fair skin in places where the intensity of UV radiation is lower [36, 37]. Evidence of natural selection makes this SNP a frequent component of ancestral and forensic informative panels [38]. In our study, we confirmed the association of the AA genotype with fair skin and light eyes. and we identified allelic frequencies consistent with those observed in European populations [3] and in previous studies of Brazilians from other regions [39]. Likewise, SNP SLC45A2 rs16891982 is also widely studied regarding its relationship with pigmentation in different populations. SLC45A2 encodes the membrane-associated transporter protein carrier involved in melanin synthesis, and experimental studies in zebrafish. Mice and yeast have clearly demonstrated that the presence of the missense variant rs16891982 (p.Phe374Leu) results in decreased protein activity [40]. This SNP is also considered an ancestry informative marker (AIM), since it is able to differentiate European populations due to G allele frequency. Which is similar to the rs1426654 A allele [41]. These findings are aligned with the theory of vitamin D synthesis. Which proposes that light skin is a feature selected to compensate for the lower solar incidence in populations living far from the Equator [42] and with increased ability of the skin to respond to ultra violet (UV) radiation [43].
Alleles associated with lighter pigmentation were also associated with melanoma in our study, and this result remained significant after analysis with multivariate logistic regression adjusted for the risk factors ancestry, gender, age, eye, hair, and skin color, and number of Nevi. We considered this analysis essential to identify whether the variants studied could be considered independent risk factors for the occurrence of melanoma. Thus, SNPs HERC2 rs1129038 and SLC24A5 rs1426654 remained strongly associated with risk for the development of melanoma in a dominant model. The presence of homozygous genotypes of either SNP (AA for rs1426654 and AA for rs1129038) were associated with increased melanoma risk, while the SLC45A2 rs16891982 C allele was associated with protection for melanoma as shown previously in a GWAS study in Greece composed of 284 patients and 284 controls (OR = 0.51. 95% CI 0.34–0.76; P = 0.001) [44]. On the other hand, an Australian sample with individuals of 100% Northern European ancestry (1.062 cases and 1.262 controls) showed the same allele associated with the risk to melanoma in logistic regression models including pigmentation features and ancestry, similar to the one presented here (OR = 2.04. 95% CI 1.27–3.40) [45] The results remained unchanged after population substructure analysis. Furthermore, the independent effects of each of these SNP were also accessed by MDR analysis, and the analysis considering the entire sample showed a redundancy interaction between the same SNPs that displayed significance through logistic regression (P = .031). The interaction illustrated in Fig. 1 shows that these three genes act redundantly to increase the risk of melanoma. The genotypic combinations SLC24A5 rs1426654AA and SLC45A2 rs16891982GG present a greater contribution in determining the risk for the disease, presenting a possible epistatic effect similar to that found between SLC45A2 and VDR [46], SLC45A2 and OCA2. and MC1R and SLC24A5 [45].
The two most important limitations of our study are sampling process (individuals showing mostly Euro descendant ancestry in the entire sample) and relatively limited sample size. However, despite these limitations, our results are in line with previous studies and demonstrate that SNPs in genes related to pigmentation confer an independent increase in the risk for developing melanoma. In determining complex human traits in general, common genetic variants tend to have small effect sizes individually, but together. They may reveal important information and contribute to the assessment of individual risk for complex diseases such as cancer [47]. The development and evaluation of predictive models that combine environmental and genomic risk factors can help improve melanoma prevention and population screening by motivating risk reduction behaviors, especially in regions with high incidence rates. High UV radiation exposure and predominantly European ancestry [48].
Additional studies should be performed to verify whether the same scenario occurs in other regions of Brazil and Latin America. Although an association between SLC24A5 rs1426654 and SLC45A2 rs16891982 and melanoma has been previously described in Europeans, to our knowledge, this is the first study that confirms this association in a South American high-risk population.
Conclusions
In this case-control study conducted in Southern Brazil, SNPs SLC24A5 rs1426654 and SLC45A2 rs16891982 were associated with an increased risk for melanoma, which was found to be additive and independent of pigmentation profile. These results contribute to the current knowledge about melanoma risk factors in individuals from a geographic region with a high incidence of the disease.
Availability of data and materials
Not applicable.
Abbreviations
- SNP:
-
Single nucleotide polymorphisms
- MDR:
-
Multifactor dimensionality reduction
- CANDELA:
-
Consortium for Analysis of Diversity and Evolution in Latin America
- MI:
-
Melanin Index
- RS:
-
Rio Grande do Sul
- BA:
-
Bahia
- HCPA:
-
Hospital de Clínicas de Porto Alegre
- CI:
-
Interval confidence
- OR:
-
Odds ratio
- IG:
-
Information gain
- AIM:
-
Ancestry informative marker
- UV:
-
Ultravioleta radiation
References
Yamaguchi Y, Hearing VJ. Melanocytes and their diseases. Cold Spring Harb Perspect Med. 2014;4(5):1–18.
Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, Berwick M, Bracci PM, Elwood JM, Ernstoff MS, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38(3):814–30.
Lian CG. Jr Mihm MC: Skin Cancer. In: BWSaCP W, editor. World Cancer Report 2014, vol. 2014. Lyon Cedex: International Agency for Research on Cancer. p. 495–502.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer observatory: Cancer today. Lyon: International Agency for Research on Cancer; 2018.
Erdmann F, Lortet-Tieulent J, Schüz J, Zeeb H, Greinert R, Breitbart EW, Bray F. International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400.
da Silva INdCJAG. Estimativa 2018 Incidência de Câncer no Brasil. In: Diegez C, editor. , vol. 1. Rio de Janeiro: Coordenação de Ensino do Instituto Nacional do Câncer-INCA; 2017. p. 128.
Pesavento SJ. O Rio Grande de São Pedro. In: Martins M, editor. A História do Rio Grande do Sul, vol. 1. Porto Alegre: Martins Livreiro Editora LTDA; 2014. p. 9–20.
Bakos RM, Besch R, Zoratto GG, Godinho JM, Mazzotti NG, Ruzicka T, Bakos L, Santos SE, Ashton-Prolla P, Berking C, et al. The CDKN2A p.A148T variant is associated with cutaneous melanoma in southern Brazil. Exp Dermatol. 2011;20(11):890–3.
Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H. Hereditary melanoma: update on syndromes and management: emerging melanoma cancer complexes and genetic counseling. J Am Acad Dermatol. 2016;74(3):411–20 quiz 421-412.
Abdo JF, Sharma A, Sharma R. Role of hereditary in melanoma susceptibility. A primer for the practicing surgeon. Surg Clin North Am. 2020;100(1):13–28.
Gibbs DC, Orlow I, Kanetsky PA, Luo L, Kricker A, Armstrong BK, Anton-Culver H, Gruber SB, Marrett LD, Gallagher RP, et al. Inherited genetic variants associated with occurrence of multiple primary melanoma. Cancer Epidemiol Biomark Prev. 2015;24(6):992–7.
Fang S, Han J, Zhang M, Wang L, Wei Q, Amos CI, Lee JE. Joint effect of multiple common SNPs predicts melanoma susceptibility. PLoS One. 2013;8(12):1–9.
Helsing P, Nymoen DA, Rootwelt H, Vårdal M, Akslen LA, Molven A, Andresen PA. MC1R. ASIP. TYR. And TYRP1 gene variants in a population-based series of multiple primary melanomas. Genes Chromosom Cancer. 2012;51(7):654–61.
Berger MF, Garraway LA. Applications of genomics in melanoma oncogene discovery. Hematol Oncol Clin North Am. 2009;23(3):397–414 vii.
Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, Gabriel SB, Topol EJ, Smoller JW, Pato CN, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36(4):388–93.
Cerqueira CC, Hünemeier T, Gomez-Valdés J, Ramallo V, Volasko-Krause CD, Barbosa AA, Vargas-Pinilla P, Dornelles RC, Longo D, Rothhammer F, et al. Implications of the admixture process in skin color molecular assessment. PLoS One. 2014;9(5):e96886.
Andrade RB, Amador MAT, Cavalcante GC, Leitão LPC, Fernandes MR, Modesto AAC, Moreira FC, Khayat AS, Assumpção PP, Ribeiro-Dos-Santos Â, et al. Estimating Asian Contribution to the Brazilian Population: A New Application of a Validated Set of 61 Ancestry Informative Markers. G3 (Bethesda). 2018;8(11):3577–82.
Swetter SM, Bichakjian CK. Risk Factors for Development of Single or Multiple Primary Melanomas. In: National Comprehensive Câncer Network Clinical Pactic Guidelines in Oncology (NCCN Guidelines). Fort Washington: National Comprehensive Cancer Network Foundation; 2018. p. MA1–2.
Valverde-Villegas JM, de Medeiros RM, de Andrade KP, Jacovas VC, Dos Santos BR, Simon D, de Matos Almeida SE, JAB C. Novel genetic associations and gene-gene interactions of chemokine receptor and chemokine genetic polymorphisms in HIV/AIDS. AIDS. 2017;31(9):1235–43.
Hernandez-Pacheco N, Flores C, Alonso S, Eng C, Mak AC, Hunstman S, Hu D, White MJ, Oh SS, Meade K, et al. Identification of a novel locus associated with skin colour in African-admixed populations. Sci Rep. 2017;7:44548.
Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, Cheng K, Canfield VA, Bradley DG, McEvoy B, Shriver MD. Genetic evidence for the convergent evolution of light skin in Europeans and east Asians. Mol Biol Evol. 2007;24(3):710–22.
Sarkar A, Nandineni MR. Association of common genetic variants with human skin color variation in Indian populations. Am J Hum Biol. 2018;30(1).
Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, Quinto-Sanchez M, Jaramillo C, Arias W, Fuentes M, Pizarro M, Everardo P, de Avila F, et al. Admixture in Latin America: geographic structure. phenotypic diversity and self-perception of ancestry based on 7.342 individuals. PLoS Genet. 2014;10(9):e1004572.
Leite TK, Fonseca RM, de França NM, Parra EJ, Pereira RW. Genomic ancestry. self-reported "color" and quantitative measures of skin pigmentation in Brazilian admixed siblings. PLoS One. 2011;6(11):e27162.
Santos HC, Horimoto AV, Tarazona-Santos E, Rodrigues-Soares F, Barreto ML, Horta BL, Lima-Costa MF, Gouveia MH, Machado M, Silva TM, et al. A minimum set of ancestry informative markers for determining admixture proportions in a mixed American population: the Brazilian set. Eur J Hum Genet. 2016;24(5):725–31.
Healy ME, Hill D, Berwick M, Edgar H, Gross J, Hunley K. Social-group identity and population substructure in admixed populations in New Mexico and Latin America. PLoS One. 2017;12(10):e0185503.
Berger M, Stassen HH, Köhler K, Krane V, Mönks D, Wanner C, Hoffmann K, Hoffmann MM, Zimmer M, Bickeböller H, et al. Hidden population substructures in an apparently homogeneous population bias association studies. Eur J Hum Genet. 2006;14(2):236–44.
NCA F, de Andrade ES, CEV W, CCF A, Zanão LR, da Silva MS, Marano LA, Donadi EA, Castelli EC, Simões AL, et al. Haplotypes from the SLC45A2 gene are associated with the presence of freckles and eye, hair and skin pigmentation in Brazil. Leg Med (Tokyo). 2017;25:43–51.
Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes. Pigmentary phenotypes. And risk of skin cancer in Caucasians. Int J Cancer. 2009;125(4):909–17.
Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Mengel-From J, Kjaer KW, Hansen L. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum Genet. 2008;123(2):177–87.
Mengel-From J, Børsting C, Sanchez JJ, Eiberg H, Morling N. Human eye colour and HERC2. OCA2 and MATP. Forensic Sci Int Genet. 2010;4(5):323–8.
Ruiz Y, Phillips C, Gomez-Tato A, Alvarez-Dios J, Casares de Cal M, Cruz R, Maroñas O, Söchtig J, Fondevila M, Rodriguez-Cid MJ, et al. Further development of forensic eye color predictive tests. Forensic Sci Int Genet. 2013;7(1):28–40.
Freire-Aradas A, Ruiz Y, Phillips C, Maroñas O, Söchtig J, Tato AG, Dios J, de Cal MC, Silbiger VN, Luchessi AD, et al. Exploring iris colour prediction and ancestry inference in admixed populations of South America. Forensic Sci Int Genet. 2014;13:3–9.
Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, et al. SLC24A5. A putative cation exchanger. Affects pigmentation in zebrafish and humans. Science. 2005;310(5755):1782–6.
Cook AL, Chen W, Thurber AE, Smit DJ, Smith AG, Bladen TG, Brown DL, Duffy DL, Pastorino L, Bianchi-Scarra G, et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP. SLC24A5/NCKX5. And OCA2/P loci. J Invest Dermatol. 2009;129(2):392–405.
Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, Xie X, Byrne EH, McCarroll SA, Gaudet R, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–8.
Canfield VA, Berg A, Peckins S, Wentzel SM, Ang KC, Oppenheimer S, Cheng KC. Molecular phylogeography of a human autosomal skin color locus under natural selection. G3 (Bethesda). 2013;3(11):2059–67.
Pietroni C, Andersen JD, Johansen P, Andersen MM, Harder S, Paulsen R, Børsting C, Morling N. The effect of gender on eye colour variation in European populations and an evaluation of the IrisPlex prediction model. Forensic Sci Int Genet. 2014;11:1–6.
Lima FA, de Araújo Lima F, Gonçalves FT, de Toledo Gonçalves F, Fridman C. SLC24A5 and ASIP as phenotypic predictors in Brazilian population for forensic purposes. Leg Med (Tokyo). 2015;17(4):261–6.
Reinders A, Ward JM. Investigating polymorphisms in membrane-associated transporter protein SLC45A2. Using sucrose transporters as a model. Mol Med Rep. 2015;12(1):1393–8.
Soejima M, Koda Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int J Legal Med. 2007;121(1):36–9.
Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962–8.
Hernando B, Sanz-Page E, Pitarch G, Mahiques L, Valcuende-Cavero F, Martinez-Cadenas C. Genetic variants associated with skin photosensitivity in a southern European population from Spain. Photodermatol Photoimmunol Photomed. 2018;34(6):415–22.
Stefanaki I, Panagiotou OA, Kodela E, Gogas H, Kypreou KP, Chatzinasiou F, Nikolaou V, Plaka M, Kalfa I, Antoniou C, et al. Replication and predictive value of SNPs associated with melanoma and pigmentation traits in a southern European case-control study. PLoS One. 2013;8(2):e55712.
Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130(2):520–8.
Kosiniak-Kamysz A, Marczakiewicz-Lustig A, Marcińska M, Skowron M, Wojas-Pelc A, Pośpiech E, Branicki W. Increased risk of developing cutaneous malignant melanoma is associated with variation in pigmentation genes and VDR. And may involve epistatic effects. Melanoma Res. 2014;24(4):388–96.
Fesenko DO, Chudinov AV, Surzhikov SA, Zasedatelev AS. Biochip-based genotyping assay for detection of polymorphisms in pigmentation genes associated with cutaneous melanoma. Genet Test Mol Biomarkers. 2016;20(4):208–12.
Cust AE, Drummond M, Kanetsky PA, Goldstein AM, Barrett JH, MacGregor S, Law MH, Iles MM, Bui M, Hopper JL, et al. Assessing the incremental contribution of common genomic variants to melanoma risk prediction in two population-based studies. J Invest Dermatol. 2018;138(12):2617–24.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and by Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and by Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA). The role of CAPES was to supply a master’s scholarship and the role of FIPE-HCPA was to supply the finance to realize the experiments. FSLV is recipient of a CNPq scholarship grant [grant number CNPq 312993/2017-0.
Author information
Authors and Affiliations
Contributions
LBR, GSM and PAP conceived the experiment(s); LBR, RMB and VCJ, conducted the experiment(s); LBR, FSLV, VCJ, SS and AMR-D-S analyzed the results. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki Principles. and the protocol approved by the Institutional Review Board of Hospital de Clínicas de Porto Alegre (HCPA) under number 07–139. and all participants provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Ancestry profile of samples (A) Individual European. African. and Native American ancestry inferred from 61 ancestry-informative markers in our all sample. Patients (green) and controls (orange) were compared with individuals from the putative parental populations used to infer admixture: Europeans. African. and Native Americans. (b) Individual European. African. and Native American ancestry inferred from 61 ancestry-informative markers in sample after substructure reduction. Patients (green) and controls (orange) were compared with individuals from the putative parental populations used to infer admixture: Europeans. African. and Native Americans. Admixture was estimated using STRUCTURE V.2.3.4 software.
Additional file 2: Additional Table 1.
Samples excluded in order to reduce Population Substructure. Additional Table 2. Allelic and genotipic frequencies of SNPs TYR rs1126809, HERC2 rs1129038, SLC24A5 rs1426654, and SLC45A2 rs16891982 in Southern Brazil samples and in main populacional databases
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reis, L.B., Bakos, R.M., Vianna, F.S.L. et al. Skin pigmentation polymorphisms associated with increased risk of melanoma in a case-control sample from southern Brazil. BMC Cancer 20, 1069 (2020). https://doi.org/10.1186/s12885-020-07485-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07485-x