Abstract
Background
In patients undergoing major liver resection, portal vein embolization (PVE) has been widely used to induce hypertrophy of the non-embolized liver in order to prevent post-hepatectomy liver failure. PVE is a safe and effective procedure, but does not always lead to sufficient hypertrophy of the future liver remnant (FLR). Hepatic vein(s) embolization has been proposed to improve FLR regeneration when insufficient after PVE. The sequential right hepatic vein embolization (HVE) after right PVE demonstrated an incremental effect on the FLR but it implies two different procedures with no time gain as compared to PVE alone.
We have developed the so-called liver venous deprivation (LVD), a combination of PVE and HVE during the same intervention, to optimize the phase of liver preparation before surgery. The main objective of this randomized phase II trial is to compare the percentage of change in FLR volume at 3 weeks after LVD or PVE.
Methods
Patients eligible to this multicenter prospective randomized phase II study are subjects aged from 18 years old suffering from colo-rectal liver metastases considered as resectable and with non-cirrhotic liver parenchyma. The primary objective is the percentage of change in FLR volume at 3 weeks after LVD or PVE using MRI or CT-Scan. Secondary objectives are assessment of tolerance, post-operative morbidity and mortality, post-hepatectomy liver failure, rate of non-respectability due to insufficient FLR or tumor progression, per-operative difficulties, blood loss, R0 resection rate, post-operative liver volume and overall survival. Objectives of translational research studies are evaluation of pre- and post-operative liver function and determination of biomarkers predictive of liver hypertrophy. Sixty-four patients will be included (randomization ratio 1:1) to detect a difference of 12% at 21 days in FLR volumes between PVE and LVD.
Discussion
Adding HVE to PVE during the same procedure is an innovative and promising approach that may lead to a rapid and major increase in volume and function of the FLR, thereby increasing the rate of resectable patients and limiting the risk of patient’s drop-out.
Trial registration
This study was registered on clinicaltrials.gov on 15th February 2019 (NCT03841305).
Similar content being viewed by others
Background
In patients undergoing major liver resection, portal vein embolization (PVE) has been widely used to induce hypertrophy of the non-embolized liver in order to prevent small-for-size and post-hepatectomy liver failure. PVE is a safe and effective procedure, but does not always lead to sufficient hypertrophy of the future liver remnant (FLR) [1]. Therefore, several approaches have been proposed to improve PVE:
- i.
combined technique with subsequent embolization of ipsilateral hepatic artery, was efficient for FLR hypertrophy, but has been abandoned regarding the increased risk of liver abscess
- ii.
intrahepatic biliary ablation using ethanol but seemed to increase the risk of damage to the bile ducts of the FLR;
- iii.
the adjunct of hematopoietic stem cells to PVE, which is still under study.
Recently, the ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) procedure has been developed by surgeons. Although a very high rate of liver hypertrophy has been reported [2], ALPPS was demonstrated to tremendously increase perioperative mortality and morbidity [3]. Another approach to improve FLR regeneration when insufficient after PVE consists in embolizing hepatic vein(s) [4]. Indeed, the sequential right hepatic vein embolization (HVE) after right PVE demonstrated an incremental effect on the FLR, but implies two different procedures with no time gain as compared to PVE alone. To optimize the phase of liver preparation before surgery, we developed the so-called liver venous deprivation (LVD) technique, a combination of PVE and HVE during the same intervention. We reported that LVD was safe and provided fast and important hypertrophy of the FLR at 3 weeks [5]. More recently, we showed that LVD could provide marked and very rapid increase not only in FLR volume but also in FLR function [6, 7] assessed with 99mTc mebrofenin hepatobiliary scintigraphy with SPECT which has been validated as a quantitative method for evaluating liver function [8].
Methods/design
Aim of the study
The main objective of this randomized multicenter phase II trial is to compare the percentage of change in FLR volume at 3 weeks after LVD or PVE using MRI or CT-scan. Secondary objectives are listed in Table 1. Translational research objectives are i) evaluation of pre- and post-operative liver function and ii) determination of biomarkers predictive of liver hypertrophy.
Sample size and follow-up period
Our hypotheses for sample size calculation are based on a systematic review on PVE before liver resection, involving 1791 patients [9]: the mean increase of the FLR volume was 37.9% at 26 days. In our preliminary study [5] and in a more recent paper by Le Roy et al. [10], a mean increase of 53% of the FLR volume was observed after 3 weeks. Therefore, it is realistic to expect a difference of 12% (or more) between the 2 procedures at 21 days. With a standard deviation of 14% in each arm, a two-sided α = 5% and a power of 90%, according to a Student Test, 30 patients have to be randomized by arm. Taking into account that 5% of the patients could not be evaluable, 32 patients have to be randomized per arm. Finally, planned enrollment will be 64 subjects. The expected duration of the recruitment of all patients is 24 months with a minimal duration of the subject participation of 5 months.
Selection of study population
Study population
Subjects aged from 18 years old suffering from liver metastases considered as resectable could be enrolled in this study if inclusion and exclusion criteria are satisfied.
Inclusion criteria
Patients eligible for inclusion in this study have to meet all the following criteria:
Liver metastases considered as resectable from colo-rectal origin (as validated by a multidisciplinary committee with at least one senior hepatic surgeon)
Percentage of FLR volume < 30%
Age ≥ 18 years
General health status WHO 0 or 1
Estimated life expectancy > 3 months
Patients whose biological parameters are:
Platelets ≥100,000/mm3,
Polynuclear neutrophils ≥1000/mm3,
Hemoglobin ≥9 g/dL (even transfused patients can be included)
Creatininemia < 1.5 N
Bilirubinemia ≤2 N
AST and ALT ≤5 N
PT > 70%
Reference liver CT-scan or MRI done during the 30 days preceding PVE or LVD
Written informed consent
National health insurance cover
Exclusion criteria
Patients eligible for this study must not meet any of the following criteria:
Cirrhosis
Presence of clinical ascites
Ongoing participation or participation within the 21 days prior to inclusion in the study in another therapeutic trial with an experimental drug
Serious non-stabilized disease, active uncontrolled infection or other serious underlying disorder likely to prevent the patient from receiving the treatment
Pregnancy (βHCG positive), breast-feeding or the absence of effective contraception for women of child-bearing age
Contraindication to MRI (in the following cases, a CT-scan must be used instead): Pacemaker or neurosensorial stimulator or implantable defibrillator, cochlear implant, ferromagnetic foreign body
Allergy or contra-indication to iodine contrast agents
Treatment with anticoagulants (heparin or AVK) that cannot be interrupted for 48 h
Treatment with anti-platelets that cannot be interrupted for 5 days for aspirin or clopidogrel
Legal incapacity (persons in custody or under guardianship)
Deprived of liberty Subject (by judicial or administrative decision)
Impossibility to sign the informed consent document or to adhere to the medical follow-up of the trial for geographical, social or psychological reasons
Randomization
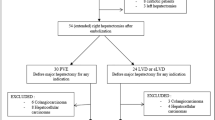
The randomization will be done according to the minimization method (ratio 1:1) and stratified on center and on type of resection scheduled (≤ 4 segments, > 4 segments). The standard arm is Portal Vein Embolization (PVE), the experimental arm is Liver Venous Deprivation (LVD). Figure 1 summarizes the design of the study.
Study endpoints
The primary endpoint for the current trial is the percentage of change in future liver remnant (FLR) volume at 3 weeks after liver venous deprivation (LVD) or portal vein embolization (PVE) using MRI/CT-Scan. Secondary endpoints are listed in Table 2. Endpoints for the translational research are:
Evaluation of pre- and post-operative liver function. This will be evaluated using 99mTc-mebrofenin scintigraphy through SPECT/CT acquisitions by calculating mebrofenin clearance in %/min/m2 of whole liver and FLR (described in [8]) at the same time points as CT/MRI (central review).
To search for biomarkers predictive of liver hypertrophy (EGF, HGF, VEGF, H-EGF, TGF-beta, TNF-alpha, IL-10, IL-6, surviving, FGL-1). Blood samples will be stored by sponsor’s biological resource center (CRB MONTPELLIER). The biological studies on the samples will be managed by a biological committee and funded separately.
Intervention description
The SPIRIT flow chart is presented in Table 3.
Standard arm (PVE group)
The portal system will be accessed using a micropuncture set either through the left or through the right portal vessels. 2D and/or 3D portography will be performed by inserting a 4F or 5F catheter in the main portal trunk. Portal pressure will be measured. Then portal vessels supplying the future resected liver will be embolized using a mixture of cyanoacrylate and lipiodol (ratio 3–6/1 depending on operator’s preference). If segment IV is scheduled to be resected, PVE of portal vein branches of segment IV is allowed.
Experimental arm (LVD group)
If right hemihepatectomy is scheduled: Right hepatic vein as well as accessory right hepatic vein(s) (when present) are accessed using a micropuncture set. After opacification, a 0.018″ microguidewire is left in place in each hepatic vein.
If right hemihepatectomy and resection of segment IV (+/− other segments) is scheduled: Middle & right hepatic veins as well as accessory right hepatic vein(s) (when present) are accessed using a micropuncture set. After opacification, a 0.018″ microguidewire is left in place in each hepatic vein.
Then, the portal system will be accessed using a micropuncture set either through the left or through the right portal vessels. 2D and/or 3D portography will performed by inserting a 4F or 5F catheter in the main portal trunk. Portal pressure will be measured. Then portal vessels supplying the future resected liver will be embolized using a mixture of cyanoacrylate and lipiodol (ratio 3–6/1 depending on operator’s preference). If segment IV is scheduled to be resected, PVE of portal vein branches of segment IV is allowed.
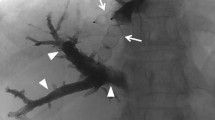
After PVE is completed, microguidewire(s) left in hepatic veins are used to introduce a Neff set. Through the Neff set, a 0.035″ guidewire is inserted to introduce a 7F Destination (Terumo, Japan) sheath in order to deploy an Amplatzer Vascular Plug II (100% oversizing: 14-22 mm) 10-15 mm before the origin of the hepatic vein to keep place for further surgical ligature. After plug deployment, opacification is performed through the sheaths to check for plug occlusion and potential veno-venous collaterals. As soon as the plug is occluded, embolization of distal venous branches is conducted using a mixture of cyanoacrylate (Purefill, Peters Surgical) and lipiodol (ratio 2–3/1). At last, tract embolization is performed using the same mixture. Tract embolization of portal vein access is performed using the mixture used for PVE.
Post-procedural prescriptions (both arms)
Pain medication is administered following the recommendations of each center. Morphine administration is allowed.
Day 0: IV multivitamin supplementation.
Day 1: Hydrosol® multivitamin drinkable solution (25 drops morning and evening). Per-os phosphorus supplementation (except if phosphoremia or calcemia > ULN) to maintain phospheremia within the limits of normal.
Day 2: Hydrosol® multivitamin drinkable solution (25 drops in the morning).
Discussion
We developed an innovative trans-hepatic technique (called LVD) for both PVE and HVE, easy to practice by interventional radiologists. Hepatic vein(s) are accessed under US guidance using micropuncture sets and embolized using Amplatzer vascular plug(s) and cyanoacrylate for distal branches and veno-venous collaterals. In two preliminary studies [6, 7] we showed that LVD is safe (no migration of embolic material was observed) and provided a strong increase in both FLR volume and function at 3 weeks (respectively 52.6% (range, 1–175.6%) and 68.2% (range, 25.4–121.4%)). In a retrospective analysis, we also showed similar mortality/morbidity rates during and after surgery compared to PVE [11]. A paper from another team [12] studying LVD in association with biliary drainage in 6 patients with Klatskin tumors also showed no adverse events and a FLR hypertrophy of 67% (range 29–123) 3 weeks after the procedure. Given the high morbidity and mortality rate following ALPPS [3], LVD could be an attractive alternative technique to increase FLR volume in a short period of time and has the potential to replace PVE as a standard of care. This project also includes functional evaluations of both the whole liver and FLR using 99 m-Tc mebrofenin scintigraphy. This will bring additional useful data given the great potential of liver function to become a more accurate predictor of post-operative liver dysfunction than liver volume.
In conclusion, we believe that LVD is a promising method to improve liver preparation before major hepatectomy, thereby increasing the number of patients undergoing curative surgery and preventing drop-out due to tumor progression. This prospective, multicenter and randomized phase II trial is mandatory to confirm our preliminary results. Serial evaluations of liver function, based on 99 m-Tc mebrofenin scintigraphies, will be helpful to define the optimal time for resection.
Availability of data and materials
Not applicable.
Abbreviations
- ALPPS:
-
Associating Liver Partition and Portal vein ligation for Staged hepatectomy
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- FLR:
-
Future Liver Remnant
- HVE:
-
Hepatic Vein Embolization
- LVD:
-
Liver Venous Deprivation
- PHLF:
-
Post-Hepatectomy Liver Failure
- PT:
-
Prothrombin time
- PVE:
-
Portal Vein Embolization
- SPECT/CT:
-
Single Photon Emission Computed Tomography with Computed Tomography
References
Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31(2):367–74.
Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255(3):405–14.
Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, et al. Early survival and safety of ALPPS: first report of the international ALPPS registry. Ann Surg. 2014;260(5):829–36 discussion 36-8.
Hwang S, Lee SG, Ko GY, Kim BS, Sung KB, Kim MH, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249(4):608–16.
Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26(12):4259–67.
Guiu B, Quenet F, Escal L, Bibeau F, Piron L, Rouanet P, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol. 2017;27(8):3343–52.
Guiu B, Quenet F, Panaro F, Piron L, Cassinotto C, Herrerro A, et al. Liver venous deprivation versus portal vein embolization before major hepatectomy: future liver remnant volumetric and functional changes. Hepatobiliary Surg Nutr. 2020. https://doi.org/10.21037/hbsn.2020.02.06.
de Graaf W, van Lienden KP, van Gulik TM, Bennink RJ. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51(2):229–36.
van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36(1):25–34.
Le Roy B, Perrey A, Fontarensky M, Gagniere J, Abergel A, Pereira B, et al. Combined preoperative portal and hepatic vein embolization (Biembolization) to improve liver regeneration before major liver resection: a preliminary report. World J Surg. 2017;41(7):1848–56.
Panaro F, Giannone F, Riviere B, Sgarbura O, Cusumano C, Deshayes E, et al. Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. Hepatobiliary Surg Nutr. 2019;8(4):329–37.
Hocquelet A, Sotiriadis C, Duran R, Guiu B, Yamaguchi T, Halkic N, et al. Preoperative portal vein embolization alone with biliary drainage compared to a combination of simultaneous portal vein, right hepatic vein embolization and biliary drainage in Klatskin tumor. Cardiovasc Intervent Radiol. 2018;41(12):1885–91.
Acknowledgements
Not applicable.
Funding
This trial (NCT03841305) is financially supported by a governmental grant from the French National Institute of Cancer, reference K17–019. The authors declare that they have no competing interests relative to this study. The National Institute of Cancer was not involved in the design of the study and collection, analysis, interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
ED, FQ and BG developed the study concept and protocol. LP, AB, BL, EL, LV, CL, JBP, PC, AD, AR, CS and CG were major contributors in further development of the protocol. All authors will be involved in the conduct of the clinical trial. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has received ethical approval from the “Comité de Protection des Personnes Sud Est I” in January 2019 (project reference 2019–02) and from National Agency for Medical and Health products Safety (project reference 2019012200143) in January 2019. All patients will give their written informed consent before any study-related assessment start.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deshayes, E., Piron, L., Bouvier, A. et al. Study protocol of the HYPER-LIV01 trial: a multicenter phase II, prospective and randomized study comparing simultaneous portal and hepatic vein embolization to portal vein embolization for hypertrophy of the future liver remnant before major hepatectomy for colo-rectal liver metastases. BMC Cancer 20, 574 (2020). https://doi.org/10.1186/s12885-020-07065-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07065-z