Abstract
Background
To investigate the long-term efficacy of the minimally invasive Ivor Lewis esophagectomy (MIILE) in esophageal squamous cell carcinoma (ESCC) patients, a retrospective comparison of the quality of life (QOL) and survival between patients who underwent MIILE and left transthoracic esophagectomy (Sweet approach) was conducted.
Methods
A detailed database search identified 614 patients who underwent MIILE and 243 patients who underwent Sweet esophagectomy between January 2011 and December 2017. After propensity score matching, 216 paired cases were selected for statistical analysis. Survival was evaluated with Kaplan-Meier curves or Cox models.
Results
MIILE was associated with a longer duration, less blood loss and more lymph node dissected than Sweet esophagectomy. MIILE patients suffered from less pain, less frequently developed pneumonia, and had fewer postoperative complications. Additionally, MIILE patients began oral intake earlier and had a shorter postoperative hospital stay, and enhanced recovery of QOL. There was no significant difference between the approaches regarding the recurrence pattern, 2-year and 5-year overall survival (OS) or disease-free survival (DFS), except that patients with tumor-node-metastasis (TNM) stage I in the MIILE group demonstrated superior OS and DFS. Pathological TNM stage and postoperative complications were determined to be independent prognostic factors based on the multivariate analysis.
Conclusion
MIILE is a safe and feasible approach for treating ESCC patients. MIILE approach may provide more postoperative advantages, enhanced QOL improvement, and more favorable long-term survival in early stage patients than the Sweet procedure.
Similar content being viewed by others
Background
Surgical resection with lymphadenectomy remains the curative choice for esophageal cancer; however, the optimal surgical approach is uncertain. Unlike in the Western world, where the use of transthoracic and transhiatal esophagectomy is debated, transthoracic esophagectomy, especially the left transthoracic (Sweet) approach, has been widely adopted in China [1]. Minimally invasive Ivor Lewis esophagectomy (MIILE) is gaining popularity because of its short-term advantages over open approaches in treating esophageal adenocarcinoma [2, 3]. However, the long-term outcomes of esophageal squamous cell carcinoma (ESCC) patients undergoing MIILE need further investigation, as the biological and clinical patterns of ESCC significantly differ from those of esophageal adenocarcinoma [4, 5].
To investigate the long-term effects of MIILE on ESCC patients, a propensity score-matched study was conducted. We retrospectively compared clinical data from patients who underwent MIILE or the Sweet approach and evaluated postoperative outcomes, quality of life (QOL), and survival.
Methods
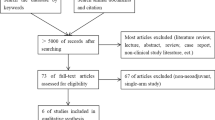
From January 2011 to December 2017, 1160 ESCC patients who did not receive neoadjuvant therapy were admitted for surgical assessment. The operability evaluation included a panel of oncological assessments (including contrast computed tomography of the chest and upper abdomen, esophageal gastroscopy, barium esophagography, endoscopic ultrasound, and positron emission tomography), and standard pulmonary and cardiac function tests. The treatment regimen was decided by a multidisciplinary team and the patient based on the clinical stage and the National Comprehensive Cancer Network (NCCN) guidelines [6]. For patients with unresectable tumors, conservative treatment was performed. For patients with a cTisN0M0 or cT1aN0M0 classification, endoscopic mucosal resection or endoscopic submucosal dissection was implemented. For other patients, the choice between MIILE and Sweet esophagectomy was made by the patient after informed consent was obtained (Fig. 1). Detailed data were gathered from a highly maintained in-house database. Techniques used in the two esophagectomy procedures have previously been described in detail [7, 8].
Surgical procedures
General anesthesia and double-lumen endotracheal intubation were routinely used, combined with epidural anesthesia or thoracic paravertebral nerve blocks.
For MIILE, the patient was initially placed in a supine position. Through laparoscopy, gastric tubulization and omental flap mobilization were performed, accompanied by abdominal lymph node dissection (paracardiac left and right, common hepatic artery, splenic artery, left gastric artery, and celiac). This procedure was followed by the insertion of a jejunal feeding tube. Before closure, the abdomen was drained. Subsequently, the patient was turned to the left lateral decubitus position. After esophageal mobilization and lymphadenectomy (peri-esophageal, lower posterior mediastinal, right and left recurrent laryngeal nerve (RLN), carina, and right paratracheal), intrathoracic anastomosis was completed using a circular stapler or the hand-sewn method. Thoracic duct ligation was routinely performed. Finally, a nasogastric tube was placed, and the thorax was drained.
For the Sweet approach, the patient was placed in the right lateral decubitus position. A left posterolateral thoracotomy was performed through the sixth or seventh intercostal space. After esophageal isolation and thoracic lymphadenectomy, the diaphragm was incised to mobilize the stomach and dissect the abdominal lymph nodes. A gastric tube, normally 4–5 cm in width, was formed along the arcus major ventriculi. Intrathoracic esophagogastric anastomosis was constructed using a circular stapler or the hand-sewn method. A nasoenteric feeding tube was inserted in the jejunum, and a nasogastric tube was placed. Subsequently, the thorax was drained.
Postoperative treatment
Patients were routinely monitored in the intensive care unit (ICU) until their vital signs were stabilized. Enteral nutrition was initiated on the first day after surgery. Oral intake was usually permitted after the presence of an intact anastomosis was verified by an esophagogram, the implementation of which was closely related to each patient’s recovery status. Chest drains were removed when drainage volumes were less than 100 ml/24 h. The jejunostomy feeding tube was retained for home enteric nutrition until three months after discharge. Adjuvant therapy (chemotherapy/radiotherapy/chemoradiation) was administered in patients with advanced stage (pathological stage more than T3 or N1) based on the tumor-node-metastasis (TNM) stage and the NCCN guidelines. The clinical and pathological stage was determined according to the 7th edition of the American Joint Committee on Cancer [9].
Adjuvant therapy
Chemotherapy included four cycles of cisplatin (75–100 mg/m2) on day 1 coupled with paclitaxel (120–175 mg/m2) or taxane (60–75 mg/m2) on day 2 every 21 days or four cycles of cisplatin or nedaplatin (75–100 mg/m2) on day 1 coupled with 5-fluorouracil (500–750 mg/m2) on days 1–5 every 21 days.
Radiotherapy was administered with a Trilogy® (Varian Medical Systems) linear accelerator. A dose of 45–50.4 Gy was administered in 1.8–2.0 Gy daily fractions for 5 weeks.
Chemoradiotherapy included cisplatin-based chemotherapy coupled with 45–50.4 Gy of radiation at a dose of 1.8–2.0 Gy per fraction for 5 weeks.
Health-related quality of life evaluation
QOL parameters were measured using the European Organization for Research and Treatment of Cancer (EORTC) QOL C30 questionnaire and the Supplemental QOL-Esophageal Module 18 questionnaire [10, 11]. The questionnaire was completed at admission and during postoperative follow-up dates (3, 6, 12, and 24 months) via direct or indirect communication (mail, email or telephone).
Follow-up
Patients had follow-up appointments every three months during the first year and every six months thereafter. Clinical examinations included a physical examination, evaluation of tumor biomarkers (carbohydrate antigen 19–9, carbohydrate antigen 242, carcinoembryonic antigen, and squamous cell carcinoma antigen levels), cervical region ultrasonography, and thorax and abdominal computed tomography. Esophagogastroscopy was performed annually after surgery. The last follow-up date was December 30, 2017.
Data collection and statistical analysis
A low, normal, and high body mass index (BMI) were defined as a BMI of less than 18.5, between 18.5 and 25, and more than 25, respectively. Tumor diameter was gauged at the final pathological examination.
The QOL score was graded following the EORTC Scoring Manual. Higher global health and physical function scores indicate better QOL, while higher scores for symptoms such as pain imply poorer QOL. Overall survival (OS) was defined as the length of time from the date of surgery to the last known living date. Disease-free survival (DFS) was defined as the length of time from the date of surgery to the date of death from any cause or recurrence verified by pathological examination or imaging features.
Propensity score matching was used to balance the clinical characteristics between the two groups. To estimate the propensity score, a multinomial logistic regression model was applied based on age, gender, BMI, Charlson comorbidity index, tumor location, tumor invasion stage, lymph node stage and pathological TNM stage. A 1:1 match was achieved using the nearest neighbor-matching algorithm with a caliper definition of 0.02. Finally, 216 paired cases were matched. This research project was approved by Ethics Committee of the 2nd Affiliated Hospital, School of Medicine, Zhejiang University.
Variables are presented as proportions, means, or medians where appropriate. Data were compared using Student’s t test, χ2 test, one-way ANOVA or the Mann-Whitney U test, as appropriate. The Kaplan-Meier method or Cox proportional hazards method was used to analyze OS and DFS. All statistical analyses were performed using SPSS (SPSS 19.0 for Windows; SPSS Inc., Chicago, IL). A p-value less than 0.05 was defined as statistically significant.
Results
Clinical baseline
After the short acceptance phase for the MIILE early in this study, the number of patients who chose the Sweet approach decreased and eventually became significantly less than that of those who elected MIILE (Fig. 1). As shown in Table 1, the demographic and clinical characteristics of the MIILE and Sweet groups were well balanced. There was no significant difference between patients in these groups in terms of age, gender, BMI, comorbidities, tumor location or TNM stage.
Perioperative outcomes and recurrence
The perioperative comparisons are presented in Table 2. MIILE procedure took longer and resulted in less blood loss than the Sweet approach (200 (150–300) ml vs 300 (250–400) ml, p < 0.001), but the blood transfusion rate was similar. More lymph nodes in the RLN region and in the thoracic and abdominal field were retrieved during MIILE procedure, and consequently the total lymph node number was higher (31(22–40) vs 18(12–28), p < 0.001). Note that the number of patients with RLN lymph node dissection was significantly higher in the MIILE (164 vs 35, p < 0.001). Intraoperative frozen sections were routinely obtained, and R0 resection was obtained for all patients.
The MIILE group began oral intake earlier (6 (5–7) days vs 9 (7–11) days, p < 0.001) and left the hospital earlier (13 (11-16) days vs 18 (16–25) days, p < 0.001). More complications, especially pneumonia, occurred in the Sweet group. The reoperation rate was similar. Nineteen reoperations were performed in patients from the MIILE, namely, six operations for chylothorax, five for wound infections, four for anastomotic leakage, two for intestinal obstructions, one for intrathoracic hemorrhage, and one for abdominal hemorrhage. Seventeen patients in the Sweet group required reoperation. There were four operations for anastomotic leakage, four for chylothorax, five for wound infections, two for intrathoracic hemorrhage, and two for abdominal hemorrhage.
There was no intraoperative mortality. The postoperative 30-day mortality rate did not differ significantly between approaches. Five postoperative deaths occurred in the MIILE group. One patient died from anastomotic leakage, three died from severe pneumonia, and one died from sudden cardiac arrest. Six patients in the Sweet group died. Two patients died from anastomotic leakage, three died from respiratory failure secondary to pulmonary infection, and one died from congestive heart failure.
Recurrence was observed in nearly half of patients (45.4% in the MIILE group vs 46.7% in the Sweet group, p = 0.772). The recurrence pattern was similar between the MIILE group and the Sweet group (locoregional, 42 vs 56, p = 0.108; distant, 47 vs 39, p = 0.335); in half of the patients, recurrence was observed at distant sites, mainly the liver, lungs, and bone.
Survival
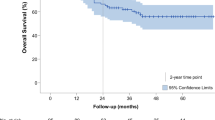
The median follow-up time was 36 months. The 2-year OS and DFS rates for all patients were 59.4 and 35.0%, respectively, and the 5-year OS and DFS rates were 54.8 and 34.0%, respectively. There was no significant difference in OS and DFS between groups (Fig. 2 and Fig. 3). Patients in the MIILE group classified as TNM stage I showed superior OS and DFS.
Overall survival curves by MIILE and the Sweet approach for (a) the entire cohort (p = 0.503) and for patients with (b) TNM stage I (p = 0.029), (c) TNM stage II (p = 0.544), (d) TNM stage III (p = 0.468). Kaplan-Meier, Log-rank. MIILE = minimally invasive Ivor Lewis esophagectomy. TNM = tumor-node-metastasis
Disease-free survival by MIILE and the Sweet approach for (a) the entire group (p = 0.370) and patients with (b) TNM stage I (p = 0.006), (c) TNM stage II (p = 0.582), and (d) TNM stage III (p = 0.459). Kaplan-Meier, Log-rank; MIILE = minimally invasive Ivor Lewis esophagectomy. TNM = tumor-node-metastasis
Table 3 shows the results of the univariate analysis of clinicopathologic variables influencing OS and DFS. The factors tumor diameter (≤3 cm vs > 3 cm), depth of tumor invasion (T1–2 vs T3–4), lymph node metastasis status (N0 vs N1–3), TNM stage (I vs II vs III), and occurrence of postoperative pneumonia and complications affected the OS and DFS of the whole cohort. Further analysis showed that a high BMI was associated with better OS in the MIILE group, and atrial fibrillation was associated with worse DFS in the Sweet group. Regression analysis using a multivariable Cox proportional hazards model revealed that TNM stage and postoperative complications were independent prognostic factors for survival of the whole cohort (Table 4).
RLN lymph node metastasis was associated with poor OS and DFS in the whole cohort and in the MIILE group but had no influence on survival in the Sweet group (Fig. 4).
Overall survival curve and disease-free survival curves stratified by RLN lymph node metastasis status in 199 patients with RLN lymph nodes retrieved in the whole cohort (a p < 0.001 and b p < 0.001, respectively), 164 patients with RLN lymph nodes retrieved in the MIILE group (c p < 0.001 and d p < 0.001, respectively), and 35 patients with RLN lymph nodes retrieved in the Sweet group (e p = 0.776 and f p = 0.816, respectively). MIILE = minimally invasive Ivor Lewis esophagectomy. RLN = recurrent laryngeal nerve. LN = lymph node
Quality of life
The QOL results are shown in Table 5. There was no significant difference in the baseline level of QOL. MIILE group scored significantly higher in the postoperative global health and physical component and lower in symptom categories than the Sweet group. Furthermore, the scores for global health, physical function, role function, emotional function, cognitive function, social function, fatigue, and pain improved faster in the MIILE group.
Discussion
Although left transthoracic esophagectomy is the primary surgical approach for ESCC in China, the debate regarding the left and right thoracic approaches remains unsettled [12, 13]. MIILE has been demonstrated to have advantages over the open Ivor Lewis approach in the treatment of esophageal adenocarcinoma patients [3, 14], but its effect on the long-term survival of patients with ESCC needs further investigation. There have also been few detailed comparisons between MIILE and the left transthoracic approach (Sweet approach) in ESCC patients [15, 16]. To fill this gap in knowledge, we compared the QOL and survival of patients who underwent MIILE and the Sweet procedure.
We found that both surgical procedures were comparable at the clinical baseline. The short-term advantages of MIILE, such as decreased blood loss, reduced hospital stay, and decreased pain were in line with those reported previously [16, 17]. Similar to previous studies [16, 18], there were significantly fewer respiratory complications in patients who underwent MIILE. This finding could be ascribed to reduced pulmonary parenchymal trauma and gentle lung retraction with the thoracoscope. In addition, the minimally invasive incisions made during MIILE procedure resulted in less intercostal nerve injury, which reduced pain and allowed effective expectoration. Compared with the Sweet approach, MIILE approach accelerated the recovery phase, as MIILE patients had decreased ICU stays, accelerated oral intake and shortened postoperative hospital stays. The recurrence pattern was similar between approaches, which needs further investigation [19]. The relatively high recurrence rate was comparable to those in similar studies (47–54.3%) [20, 21] and may be ascribed to the tumor location, undetectable metastasis, depth of invasion and lymph node metastasis status [22, 23]. Thus, close follow-up of patients is recommended.
Although the OS and DFS results were similar between groups, superior survival of patients with TNM stage I was noted in the MIILE, which may be due to the advantage of this approach in lymph node resection (magnified views, better exposure, and longer instruments), especially in the RLN field. Radical lymph node resection with RLN lymphadenectomy, such as that performed during MIILE, can remove more potential cancer-positive lymph nodes, allow more accurate staging and therapy, and offer a better prognosis in patients with early stage ESCC [24, 25]. Our results also demonstrated that the frequency of lymph node metastasis along the RLN is high, which is consistent with the association of lymph node status with survival benefit [26]. However, RLN lymph node metastasis did not influence the survival of patients in the Sweet group, which may be explained by the limited superior mediastinal lymphadenectomy performed during the Sweet procedure and the omission of RLN lymphadenectomy [27]. Moreover, the lymph nodes along the common hepatic and celiac arteries were not routinely retrieved during the Sweet procedure, which could misguide pathological TNM staging and treatment protocols. Thus, our findings support the recommendation that radical lymphadenectomy, including removal of the bilateral RLN lymph nodes, should be performed during esophagectomy [28].
Among factors affecting survival, the influence of a longer tumor diameter may be ascribed to a more advanced TNM stage [29], and the impact of high BMI may be due to its relationship to a lower pathological stage [30]. The TNM stage and occurrence of postoperative complications were independent prognostic factors, which implies that early diagnosis, prompt treatment, and cautious ward management are essential for improving survival [31, 32].
QOL after esophagectomy is an important factor considering the high morbidity and poor prognosis of esophageal carcinoma patients. QOL was impaired after surgery and gradually recovered within 6–12 months in both groups. A significant difference favoring MIILE was found in the global health, physical component summary, and symptom categories in postoperative patients at three to six months, which is in line with observations made in previous studies [16, 33]. The advantage of MIILE in QOL may be ascribed to decreased surgical trauma and reduced pain [33].
For propensity score matching and minimizing the statistical bias, patients who received neoadjuvant therapy were not enrolled in this study because most of them chose MIILE afterwards. Neoadjuvant therapy has been increasingly adopted in esophageal cancer patients with conflicting results [34, 35]. In addition, the optimal neoadjuvant protocol needs further investigation. The likelihood that the conclusions of this study would be influenced by the downstaging effect of neoadjuvant therapy should not be disregarded.
The strength of this study lies in the large sample size, which is one of the largest for propensity score-matched comparisons between ESCC patients undergoing MIILE and the Sweet procedure. This study has intrinsic limitations regarding statistical bias because it was retrospective and nonrandomized and was also based on the experience of a single surgeon. In addition, it is unclear whether the results of this study were influenced by the postoperative treatments, and the effect of adjuvant therapy on patients was not evaluated. The indications for postoperative therapy in ESCC are still under debate. In addition, the optimal adjuvant protocol for ESCC needs further analysis.
Conclusion
This retrospective study has demonstrated that MIILE could be a safe and effective alternative procedure for ESCC patients. MIILE provides short-term advantages, better postoperative QOL, and favorable long-term oncologic results, especially in patients with early stage. To improve survival, radical lymph node dissection and regular follow-up are recommended.
Change history
21 August 2020
Editors’ Note: Concerns have been raised about the integrity of the data reported in this article and the ethics approval. These are currently being investigated. Further editorial action may be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full..
14 April 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s12885-022-09471-x
Abbreviations
- BMI:
-
Body Mass Index
- DFS:
-
disease-free survival
- EORTC:
-
European Organization for Research and Treatment of Cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- ICU:
-
Intensive care unit
- MIILE:
-
Minimally invasive Ivor Lewis esophagectomy
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- QOL:
-
Quality of life
- RLN:
-
Recurrent laryngeal nerve
- TNM:
-
Tumor-node-metastasis
References
Mao YS, He J, Xue Q, Shao K, Su K, Li N, et al. Nationwide speaking tour of standardized diagnosis and treatment for esophageal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16(9):801–4.
Takeuchi H, Miyata H, Ozawa S, Udagawa H, Osugi H, Matsubara H, et al. Comparison of short-term outcomes between open and minimally invasive Esophagectomy for esophageal Cancer using a Nationwide database in Japan. Ann Surg Oncol. 2017;24(7):1821–7.
Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally invasive versus open Esophagectomy for esophageal Cancer: a comparison of early surgical outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2016;101(4):1281–9.
Holscher AH, Bollschweiler E, Schneider PM, Siewert JR. Prognosis of early esophageal cancer. Comparison between adeno- and squamous cell carcinoma. Cancer. 1995;76(2):178–86.
Eroglu A, Aydin Y, Yilmaz O, Ulas AB. An outcome comparison of adenocarcinoma of the esophagus to squamous cell carcinoma after transthoracic esophagectomy. Turkish Journal of Thoracic and Cardiovascular Surgery. 2013;21(2):402–7.
NCCN Clinical Practice Guidelines in Oncology for Esophageal and Esophagogastric Junction Cancers. Washington, PA, USA: National Comprehensive Cancer Network [first cited 20 Sept 2010 and updated online ]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf/.
Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis Esophagectomy. Ann Thorac Surg. 2010;89(6):S2159–62.
Churchill ED, Sweet RH. Transthoracic resection of tumors of the esophagus and stomach. Ann Surg. 1942;116:566–73.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer staging manual: esophagus and Esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European-organization-for-research-and-treatment-of-Cancer Qlq-C30 - a quality-of-life instrument for use in international clinical-trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezer O, Koller M, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39(10):1384–94.
Ma J, Zhan C, Wang L, Jiang W, Zhang YX, Shi Y, et al. The Sweet approach is still worthwhile in modern Esophagectomy. Ann Thorac Surg. 2014;97(5):1728–33.
Li B, Hu H, Zhang YW, Zhang J, Miao LS, Ma LF, et al. Extended right thoracic approach compared with limited left thoracic approach for patients with middle and lower esophageal squamous cell carcinoma three-year survival of a prospective, randomized, open-label trial. Ann Surg. 2018;267(5):826–32.
Tapias LF, Mathisen DJ, Wright CD, Wain JC, Gaissert HA, Muniappan A, et al. Outcomes with open and minimally invasive Ivor Lewis Esophagectomy after neoadjuvant therapy. Ann Thorac Surg. 2016;101(3):1097–103.
Li B, Xiang JQ, Zhang YW, Li HC, Zhang J, Sun YH, et al. Comparison of Ivor-Lewis vs Sweet Esophagectomy for esophageal squamous cell carcinoma a randomized clinical trial. Jama Surg. 2015;150(4):292–8.
Wang H, Shen YX, Feng MX, Zhang Y, Jiang W, Xu ST, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: a propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg. 2015;149(4):1006–14.
Biere SSAY, Henegouwen MIV, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–92.
Sihag S, Wright CD, Wain JC, Gaissert HA, Lanuti M, Allan JS, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume Centre. Eur J Cardiothorac Surg. 2012;42(3):430–7.
Hamai Y, Hihara J, Emi M, Furukawa T, Ibuki Y, Yamakita I, et al. Treatment outcomes and prognostic factors after recurrence of esophageal squamous cell carcinoma. World J Surg. 2018;42(7):2190–8.
Chen JQ, Pan JJ, Liu J, Li JC, Zhu KS, Zheng XW, et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;86(4):671–7.
Heroor A, Fujita H, Sueyoshi S, Tanaka T, Toh U, Mine T, et al. Adjuvant chemotherapy after radical resection of squamous cell carcinoma in the thoracic esophagus: who benefits? A retrospective study. Dig Surg. 2003;20(3):229–35; discussion 36-7.
Hiyoshi Y, Yoshida N, Watanabe M, Kurashige J, Karashima R, Iwagami S, et al. Late recurrence after radical resection of esophageal Cancer. World J Surg. 2016;40(4):913–20.
Wu SG, Dai MM, He ZY, Sun JY, Lin HX, Lin H, et al. Patterns of regional lymph node recurrence after radical surgery for thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2016;101(2):551–7.
Guo JC, Lin CC, Huang TC, Huang PM, Kuo HY, Chang CH, et al. Number of resected lymph nodes and survival of patients with locally advanced esophageal squamous cell carcinoma receiving preoperative Chemoradiotherapy. Anticancer Res. 2018;38(3):1569–77.
Lin CS, Cheng CT, Liu CY, Lee MY, Hsiao MC, Shih CH, et al. Radical lymph node dissection in primary Esophagectomy for esophageal squamous cell carcinoma. Ann Thorac Surg. 2015;100(1):278–86.
Taniyama Y, Nakamura T, Mitamura A, Teshima J, Katsura K, Abe S, et al. A strategy for supraclavicular lymph node dissection using recurrent laryngeal nerve lymph node status in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2013;95(6):1930–7.
Wang Q. The left thoracotomy approach for oncologic esophageal resection is still relevant for the modern surgical trainee Reply Ann Thorac Surg 2015;100(4):1516–1516.
Tan ZH, Ma GW, Zhao JM, Bella AE, Rong TH, Fu JH, et al. Impact of thoracic recurrent laryngeal node dissection: 508 patients with tri-incisional Esophagectomy. J Gastrointest Surg. 2014;18(1):187–93.
Worrell SG, Alicuben ET, Oh DS, Hagen JA, DeMeester SR. Accuracy of clinical staging and outcome with primary resection for local-regionally limited esophageal adenocarcinoma. Ann Surg. 2018;267(3):484–8.
Miao LS, Chen HQ, Xiang JQ, Zhang YW. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol. 2015;141(5):941–50.
Cummings LC, Kou TD, Schluchter MD, Chak A, Cooper GS. Outcomes after endoscopic versus surgical therapy for early esophageal cancers in an older population. Gastrointest Endosc. 2016;84(2):232–40e1.
Yamashita K, Makino T, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal Cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23(6):2106–14.
Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, et al. Quality of life and late complications after minimally invasive compared to open Esophagectomy: results of a randomized trial. World J Surg. 2015;39(8):1986–93.
Murakami Y, Hamai Y, Emi M, Hihara J, Imano N, Takeuchi Y, et al. Long-term results of neoadjuvant chemoradiotherapy using cisplatin and 5-fluorouracil followed by esophagectomy for resectable, locally advanced esophageal squamous cell carcinoma. J Radiat Res. 2018;59:616–24.
Hamai Y, Hihara J, Emi M, Furukawa T, Murakami Y, Nishibuchi I, et al. Evaluation of prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant Chemoradiotherapy followed by surgery. World J Surg. 2018;42(5):1496–505.
Acknowledgments
None.
Funding
This work was supported by a grant of the Zhejiang Provincial Natural Science Foundation (grant number LY16H010004, LY18H170001, LQ17H010002) and a grant of Zhejiang Provincial Department of Education (Y201635465). The sponsors played no role in the study design, data collection or interpretation, or analysis, or decision to submit the article for publication.
Availability of data and materials
The data that support the findings of this study are available upon request from the corresponding author Wu Ming. The data are not publicly available as individuals were not consented for the release of their information into a public database.
Author information
Authors and Affiliations
Contributions
WQ, WZX, ZTW, and FS collected the data, performed the statistical analysis, analyzed data, and wrote the manuscript. ZS, SG, and WM analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research project was approved by Ethics Committee of the 2nd Affiliated Hospital, School of Medicine, Zhejiang University. In this study, written informed consent was obtained from the patients or their families, and patient anonymity was preserved.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, Q., Wu, Z., Zhan, T. et al. RETRACTED ARTICLE: Comparison of minimally invasive Ivor Lewis esophagectomy and left transthoracic esophagectomy in esophageal squamous cell carcinoma patients: a propensity score-matched analysis. BMC Cancer 19, 500 (2019). https://doi.org/10.1186/s12885-019-5656-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5656-7