Abstract
Background
Cancer is the second leading cause of death in the Caribbean, including the islands of Trinidad and Tobago (TT). The population of TT consists of over 1.3 million people with diverse ancestral and sociocultural backgrounds, both of which may influence cancer incidence and mortality. The objective of this study was to examine incidence and mortality patterns and trends in TT.
Methods
Cancer surveillance data on 29,512 incident cancer cases reported to the Dr. Elizabeth Quamina Cancer Registry (population-based cancer registry of TT) between 1995 and 2009 were analyzed. Age-standardized rates, overall and by sex, ancestry, and geography, were reported.
Results
The highest incidence and mortality rates were observed for cancers related to reproductive organs in women, namely, breast, cervical, and uterine cancers, and prostate, lung and colorectal cancers among men. Average incidence rates were highest in areas covered by the Tobago Regional Health Authority (TRHA) (188 per 100,000), while average mortality rates were highest in areas covered by the North West Regional Health Authority (108 per 100,000). Nationals of African ancestry exhibited the highest rates of cancer incidence (243 per 100,000) and mortality (156 per 100,000) compared to their counterparts who were of East Indian (incidence, 125 per 100,000; mortality, 66 per 100,000) or mixed ancestry (incidence, 119 per 100,000; mortality, 66 per 100,000).
Conclusions
Our findings highlight the need for national investment to improve the understanding of the epidemiology of cancer in Trinidad and Tobago, and to ultimately guide much needed cancer prevention and control initiatives in the near future.
Similar content being viewed by others
Background
Cancer is the second leading cause of death in the Caribbean and has created tremendous challenges for healthcare services and expenditures throughout the region [1]. The World Health Organization (WHO) projects that cancer incidence will increase by 58%, from 84,703 cases in 2015 to 133,937 cases in 2035, and cancer mortality will increase by 67% during this period, from 52,282 to 87,430 deaths [2]. Aging of the population, improvements in healthcare and economic development has led to a higher prevalence of lifestyle-related risk factors for cancer, including reproductive behaviors, dietary patterns, physical inactivity, obesity, and alcohol and tobacco use. In addition, the prevalence of cancer-associated viral infections (e.g., human papillomavirus, human herpesvirus-8 (HHV8), human T-cell lymphotropic virus-1 (HTLV-1), hepatitis B virus (HBV)), may be higher among Caribbean populations compared to United States (US) populations [3, 4].
In the twin island nation of Trinidad and Tobago (TT), cancer is a leading cause of death much like the rest of the Caribbean [5]. These English-speaking islands are unique in terms of their economy and ancestry. TT, located off the northeastern edge of South America, is one of the richest countries by gross domestic product (GDP) per capita in the Americas and is classified as a high-income economy by the World Bank [6]. This is due to the nation’s industrialized economy, which includes petroleum, natural gas, chemical industries, and food and beverage industries [7]. While TT is classified as a developing country by the International Monetary Fund (IMF) [8] and is a member of the United Nations Conference of Small Island Developing States (SIDS) [9], this nation faces major challenges in its efforts to achieve developed nation status in sectors such as healthcare [10]. TT’s estimated population is 1.4 million [11] with an average life expectancy of 74.61 years [12]. From 1990 to 2010, the demographic profile of TT underwent a transition marked by a declining fertility rate, a decrease in the < 15 years age-group, and a doubling of the > 60 years age-group [5]. While the Trinidad population consists of diverse ancestral groups (including African (31.76%), East Indian (37.01%), Mixed ancestry (23.52%), Chinese, White, and Syrian/Lebanese (< 1%)), as well as religious groups (including Christian, Muslim, and Hindu), the population in Tobago is predominantly of African ancestry (85.29%) and Christian [11, 12]. These demographic patterns have resulted in customs and traditions that have marked the sociocultural development of the islands [13]. Hence, research on the epidemiology and etiology of cancers in TT, in relation to the environment, lifestyle and ancestry, is essential for the success of cancer prevention and control programs and policies.
The literature on the burden of cancer within TT remains relatively barren. Previous studies of cancer in TT have reported site-specific cancer incidence, mortality and survival rates, including for breast, prostate, and gastric cancers [14,15,16,17,18,19]. However, a comprehensive analysis of cancer incidence and mortality has never been reported. Since 1994, the Dr. Elizabeth Quamina Cancer Registry has served as the National Cancer Registry of TT, using standard cancer registry guidelines and statistical methods [20, 21].
Here, we present on cancer incidence and mortality rates and trends in TT for the overall population, and by sex, geography, ancestry, and age. This is the first epidemiologic examination of cancer rates and trends across all cancer sites in TT for the period 1995 to 2009.
Methods
We obtained retrospectively collected cancer surveillance data (incidence and mortality) reported between January 1, 1995 and December 31, 2009 to the National Cancer Registry of TT, which represents all of the most current and available data from the cancer registry. The analytic dataset consisted of the 29,512 incident cancers (pediatric and adult cases) reported during the study period. The source of the registry records was previously described [18]. In brief, the registry abstracts data from private and public hospitals across Trinidad and Tobago including all of the main cancer treatment centers. Abstracted data included place of residence, age, sex, ancestry, stage, grade and method of cancer detection. In the registry data file, cancer histology was coded based on WHO International Classification of Diseases for Oncology (ICD-O) code C61.9. as supplied by the healthcare institutions [22]. The boundaries for the geographic analysis by corporation and Regional Health Authority (RHA) were previously described [18]. In brief, TT is divided into fifteen governmental administrative corporations and five RHAs for healthcare delivery and administration. Self-identification, medical records, and to a lesser extent, imputation by binary logistic regression were used to determine ancestry [18].
Death certification and population data were obtained from the Trinidad and Tobago Central Statistical Office (CSO) 2000 and 2010 census. The population pyramids for 2000 and 2011 were previously described [11, 12]. Populations estimates for the other study years were calculated through interpolation using the “irregular points of year” estimation method [20, 21]. The CSO collects several population measures including age (single year of age, 5- and 10-year age groups), ethnicity, and sex. From these data points, we calculated the age-standardized incidence and mortality rates (per 100,000 TT population) by age (10-year), sex, geography, ancestry, and individual years based on the 1960 world-standardized population [23, 24]. This methodology was selected for ease of comparison with the International Agency for Research on Cancer (IARC) data which uses the same standardization. The TT case fatality rate was calculated by dividing the number of cancer deaths over the study period by the number of incident cases and then multiplying the resultant ratio by 100 to yield a percentage. For the period 1995–2007, the average time between cancer incidence and cancer death was calculated by taking the average length of the time from the year of incidence to the year of death for the 15,279 cancer deaths recorded during the same time period. For the same time period, the mean survival time (among cancer cases reported in the registry as still living at last contact, N = 10,087) was calculated by taking the average of the time from incidence to date of last contact. The geospatial maps were rendered in the R computing environment [25] and Statistical Package of Social Science (SPSS) V.20 (IBM Corporation, Valhalla, NY) was used for analyses.
Results
Cancer incidence and mortality rates among men and women
The number and percentage of cancer cases and deaths, along with age-standardized cancer incidence and mortality rates are shown in Table 1. Between 1995 and 2009, there were a total of 29,512 incident primary cancers and 18,216 cancer deaths in TT, with an overall case-fatality rate of 61.7%. For this time period, the average length of time between diagnosis and death was 1 year (range: less than 1 year to 40 years) and the average survival time among living cases was 1.1 year (range: less than 1 year to 14 years). Several basic metrics of data quality from the registry are provided (Additional file 1: Table S1). Of note, the percent of cases registered only on the basis of the death certificate only (DCO) fluctuated from 12.12% in 1995 to 27.63% in 2000 to 10.48% in 2005 and then to 6.32% in 2009. The average over the study period was 18.44%.
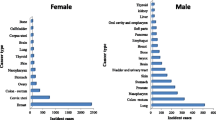
Overall, cancer incidence and mortality rates were 13.4 and 22.3% higher, respectively, among men than women. Cancer incidence and mortality trends by sex in TT are shown in Fig. 1. The overall age-standardized cancer incidence rate among TT men was 159.7 per 100,000, while the overall mortality rate was 103.8 per 100,000. The most commonly diagnosed cancers among men were prostate (64.0 per 100,000), lung and bronchus (15.9 per 100,000), colon (11.6 per 100,000), hematologic (11.4 per 100,000) and stomach (6.5 per 100,000). Similarly, the cancers with the highest mortality rates among men were prostate (37.5 per 100,000), lung and bronchus (12.7 per 100,000), hematologic (8.2 per 100,000), colon (7.6 per 100,000) and stomach cancer (5.3 per 100,000). Among TT women, the overall age-standardized cancer incidence rate was 146.3 per 100,000, while the overall mortality rate was 81.5 per 100,000. The most commonly diagnosed cancers among women were breast (46.6 per 100,000), cervix uteri (18.1 per 100,000), corpus uteri (13.4 per 100,000), hematologic (9.1 per 100,000), colon (9.0 per 100,000) and ovarian cancer (8.9 per 100,000). The cancers with the highest mortality were breast (18.4 per 100,000), cervix uteri (9.7 per 100,000), corpus uteri (6.4 per 100,000), hematologic (6.5 per 100,000) and ovarian (5.8 per 100,000) (Table 1A, B).
Cancer incidence and mortality rates by geography
The geographical area of residence corresponded with Ministry of Health RHAs (Fig. 2). There are five RHAs in TT – North West Regional Health Authority (NWRHA); North Central Regional Health Authority (NCRHA); South West Regional Health Authority (SWRHA); Eastern Regional Health Authority (ERHA); and Tobago Regional Health Authority (TRHA) – responsible for direct provision of healthcare services in their respective catchment area [18]. As shown in Fig. 2, age-standardized cancer incidence and mortality rates varied by RHA. Average incidence rates were highest in areas covered by the TRHA (188 per 100,000), followed by NWRHA (173 per 100,000), and NCRHA (153 per 100,000), compared to ERHA (139 per 100,000) and SWRHA (131 per 100,000). Overall, average mortality rates were highest in areas covered by NWHRA (108 per 100,000), followed by NCRHA and TRHA, (94 and 91 per 100,000, respectively). The NWRHA covers the area that includes the capital city of Port-of-Spain, which had the highest overall age-standardized cancer incidence (238 per 100,000) and mortality (151 per 100,000). Corporations within the SWRHA catchment area, Penal and Debe (110 per 100,000) and Couva, Tabaquite, and Talparo (119 per 100,000) had among the lowest overall cancer incidence rates. Penal and Debe had the lowest cancer mortality (59 per 100,000).
Geospatial maps of cancer incidence and mortality rates in Trinidad and Tobago 1995–2009: (Top panel, left to right) Age-standardized incidence rates for all (a) Regional Health Authorities and (b) Corporations, and age-standardized mortality rates for all (c) Regional Health Authorities and (d) Corporations. Rates are age adjusted to the 1960 world standard population. H, Hospital
Cancer incidence and mortality by ancestry
The highest cancer incidence (243 per 100,000) and mortality (156 per 100,000) rates were observed among individuals of African ancestry compared to Indian or mixed ancestry (Fig. 3). Cancer incidence and mortality rates by sex, ancestry, and age in TT are shown in Fig. 4. The highest burden of cancer for both men and women were observed among those ≥45 years. Among TT men, cancers with the highest incidence and mortality (prostate, colon, hematologic and stomach cancers) were observed among those 65–74 years (Fig. 4a-e). However, the highest lung cancer rates were observed among those aged 55–64 years (Fig. 4b). Among TT women, the highest breast and cervical cancer incidence and mortality rates were observed among those 45–54 years (Fig. 4f-j). Women 55–64 years experienced the highest corpus uteri incidence, while colon cancer and ovarian cancer occurred most frequently among women 65–74 years (Fig. 4h-j). The highest mortality rates for corpus uteri cancer were observed among women 55–64 years, while the highest mortality rates for colon and ovarian cancers were observed for women 65–74 years (Fig. 4h-j). Incidence and mortality data for those under 24 is provided (Additional file 2: Table S2).
Age-standardized incidence and mortality rates for the leading cancer sites by sex (a-e, male; f-j, female), ancestry (purple, African ancestry; orange, Indian ancestry; gray, Mixed ancestry), and age groups, Trinidad and Tobago, 1995–2009. All bars are uniformly scaled. Data for persons under 24 are presented in Additional file 2: Table S2
Stage distribution of selected cancers
Figure 5 shows the distribution of stage at diagnosis among the leading cancers by sex and ancestry. Most prostate cancers were diagnosed at localized stage (African 42%, Indian 46%, and mixed ancestry 39%). More than 15% of lung and bronchus (African 21%, Indian 19%, and mixed ancestry 33%) and stomach cancers (African 18%, Indian 19%, and mixed ancestry 28%) occurred in the distant stage. Less than 15% of breast (African 10%, Indian 6%, and mixed ancestry 8%) and cervix uteri cancers (African 10%, Indian 6%, and mixed ancestry 8%) occurred in the distant stage. More than 30% of all ovarian cancers (African 36%, Indian 27%, and mixed ancestry 31%) occurred in the distant stage. Strikingly, across all sites, there was a high percentage (12–57%) of cancers with unknown stage.
Discussion
This is the first epidemiologic study to examine TT cancer rates and trends across all cancer sites, by age, ancestry, geography and sex, with a focus on the cancers with highest incidence rates. We found that incident cases of prostate, lung, colon, stomach, and hematologic cancers were most common among men, while among women, the cancers with the highest incidence were breast, cervical, endometrial, colon, and ovarian. Overall, the incidence rates were highest in Tobago and the area covered by the NWRHA (which contains the capital city of Port-of-Spain), while cancer mortality was highest in NWRHA. The highest incidence and mortality rates were observed among adults aged ≥45 years. Except cervical and breast cancers, most cancers had a significant proportion of cases (> 10%) diagnosed at distant stage and all cancers except breast had > 20% with unknown stage. TT nationals of African ancestry had the highest incidence and mortality rates. The high overall case-fatality rate reflects the need for improved strategies to reduce cancer mortality in TT.
Evidence suggests that many of the most prevalent cancers among men and women in TT are likely attributable to preventable lifestyle factors (e.g., associated with the “westernized lifestyle” of developed countries). Tobacco use, obesity, pathogens, physical inactivity, diet, and alcohol are among the known lifestyle factors associated with increased cancer incidence [26,27,28,29]. A recent study in TT reported that the current prevalence of tobacco use among men (33.5%) is significantly higher than among women (9.4%), which may contribute to the higher rate of lung cancer among men [30]. Additionally, the study found that the overall mean body mass index (BMI) for women and men in TT was 27.4 kg/m2 and 25.6 kg/m2, respectively, and that > 55.7% of the population was overweight or obese (BMI ≥25) [30]. A recent IARC working group comprehensive review of multiple datasets, concluded that excess body fat causes cancer in multiple anatomical sites, including those identified in this study as placing the highest burden in TT [31, 32]. Numerous studies have reported the causal relationship between physical inactivity and cancer [33,34,35], and while the amount of total physical activity (PA) required to lower the risk of specific cancers in a dose-response fashion has not been established, the WHO recommends at least 600 metabolic equivalents of task (MET) minutes/week for health benefits [36]. Strikingly, a recent study in TT reported that the median PA approximated 300.30 (MET minutes/week) (median, 149.1 MET minutes/week) [30]. Focused cancer prevention initiatives in TT targeting obesity reduction and an increase in PA might have value in reducing the risk of cancer.
The TT population of African ancestry suffers a higher cancer burden across all cancer sites. This is similar to studies reporting that members of the African diaspora suffer a disproportionate cancer burden compared to other groups [37, 38]. Interestingly, we found that in Tobago, with its relatively homogeneous African ancestry population, there was a higher overall cancer incidence rate (187.7 per 100,000), driven primarily by the incidence rates of breast and prostate cancers. Surprisingly, while the incidence rates are high, mortality rates are relatively low (91.3 per 100,000). Further exploration is needed to evaluate the causes of the excess cancer burden, which may include genetic variation, tumor biology, and additional factors that have been understudied in the TT population. The contribution of tumor biology, genomics, comorbidities and patterns of care to the higher cancer burden and the observed disparities is not clear and warrant further exploration.
There are no national cancer screening programs in TT. However, the results from a population-based prostate cancer screening in Tobago between September 1997 and June 2001 are particularly worth noting. Here screening for prostate cancer using serum prostate-specific antigen (PSA) and digital rectal exam (DRE) revealed a very high prevalence of clinically-detected prostate cancer [39]. While it is true that increased prostate cancer screening leads to higher incidence, data support the hypothesis that populations of African descent share genetic and/or lifestyle factors that underlie an elevated prostate cancer risk [40, 41]. A recent study compared the effect of birthplace on prostate cancer risk comparing US-born men and men from two Caribbean countries (Guyana and TT) [19]. This study found that Caribbean-born men were diagnosed at an older age and had worse 5-year survival than US-born men, although among Caribbean-born men who immigrated to the US, 5-year survival was similar to that of US-born African American men [19]. A similar study of breast cancer reported lower survival among Caribbean women of African ancestry living in the Caribbean compared to those who migrated to the US, compared to US-born African American women [15]. This gap could be due to the intersection of screening, health literacy, tumor biology, genomics, and patterns of care issues [42].
This study has certain limitations, perhaps the greatest of which was the limited quality of cancer surveillance data currently available on the TT population. For example, large proportions of data on cancer stage were missing and molecular subtype for breast cancer, for example was not reported, which precludes interpretation of some of the study’s findings. There are issues related to the data validity that can be addressed by an increase in the quality of the data collected and improved steps to have its data included in Cancer Incidence in Five Continents. While the average DCO cases of 18.44% falls below the threshold set by IARC for inclusion in Cancer Incidence in Five Continents [43], it still reflects a need for the TT cancer registry to improve data validity. Fluctuations in data quality over the study period might have impacted some of the trends we report. This further highlights the need to strengthen the capacity of the registry. Another limitation was the inability to access and examine cancer screening data, which would be an important consideration in terms of the mortality disparities reported herein. It is plausible that disparities in cancer mortality were associated with disparities in access and utilization of cancer screening tests, because of differences in socioeconomic status, geography and/or other factors. Furthermore, under the equal access to care model in TT, initiation and receipt of optimal cancer treatment, as well as cancer care overall, may be dependent upon a patient’s place of residence, and therefore related to differences in resource allocation by RHAs. Another limitation was the lack of information on where patients sought cancer care (i.e., within the catchment area of their assigned RHA or elsewhere). Cancer surveillance data collected in TT is not routinely linked to tumor clinicopathological data leading to compromised data accuracy, utility and quality. Additionally, TT does not have electronic health records, further limiting the availability for this detailed cancer data. Similar to National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registries, data on behavioral characteristics of cancer cases are not included in the TT cancer registry. This highlights the need for improved cancer surveillance that can accurately inform and support cancer prevention and control initiatives [44]. Despite these limitations, this study highlights the need for national strategic investment in cancer epidemiology, prevention and control.
Conclusion
In conclusion, the findings of this study demonstrate that in TT, the highest incidence and mortality rates were observed for cancers related to reproductive organs in women, and prostate, lung and colorectal cancers among men, with differences observed by geography and ancestry. In developed countries, prostate and breast cancer rates are decreasing, unlike in TT, where the rates are increasing. Thus, the findings reported herein highlight the need for national investment to improve the understanding of the epidemiology of cancer in Trinidad and Tobago, and to ultimately guide much needed cancer prevention and control initiatives in the near future. Cancer prevention efforts should be strategically increased, particularly among those cancers that are attributable to lifestyle choices. The high proportion of cancers diagnosed at distant and unknown stages, also highlights the need for improvements in cancer screening and treatment initiatives in TT. Considering the high burden of cancer in TT, we expect that findings from this study will inform future policies, particularly related to resource allocation across the cancer care continuum in TT. Additionally, it is clear that capacity-building within the cancer registry (e.g., to mandate standardized data collection and routine molecular subtyping of tumors) is essential for improved cancer surveillance. This will undoubtedly improve the quality of data available for future research and will play an instrumental role in improving cancer care in TT.
Abbreviations
- BMI:
-
Body Mass Index
- CSO:
-
Central Statistical Office
- DCO:
-
Death Certificate Only
- DRE:
-
Digital Rectal Exam
- ERHA:
-
Eastern Regional Health Authority
- GDP:
-
Gross Domestic Product
- HBV:
-
Hepatitis B Virus
- HHV8:
-
Human Herpesvirus-8
- HTLV-1:
-
Human T-Cell Lymphotropic Virus-1
- IARC:
-
International Agency for Research on Cancer
- ICD-O:
-
International Classification of Diseases for Oncology
- IMF:
-
International Monetary Fund
- MET:
-
Metabolic Equivalents of Task
- NCRHA:
-
North Central Regional Health Authority
- NWRHA:
-
North West Regional Health Authority
- PA:
-
Physical Activity
- PSA:
-
Prostate-Specific Antigen
- RHA:
-
Regional Health Authority
- SEER:
-
Surveillance, Epidemiology and End Results
- SIDS:
-
United Nations Conference of Small Island Developing States
- SPSS:
-
Statistical Package of Social Science
- SWRHA:
-
South West Regional Health Authority
- TRHA:
-
Tobago Regional Health Authority
- TT:
-
Trinidad and Tobago
- US:
-
United States
- WHO:
-
World Health Organization
References
Regional Health Observatory - Regional Mortality Information System. https://www.paho.org/salud-en-las-americas-2017/?p=1457. Accessed 10 Dec 2017.
Globocan 2012 V1.0 Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 2013. http://globocan.iarc.fr/Default.aspx. Accessed 10 Dec 2017.
Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J et al: Human Papillomavirus and Related Diseases in Trinidad & Tobago Summary Report 2015-03-20 In, 2015.
Ragin C, Edwards R, Heron DE, Kuo J, Wentzel E, Gollin SM, Taioli E. Prevalence of cancer-associated viral infections in healthy afro-Caribbean populations: a review of the literature. Cancer Investig. 2008;26(9):936–47.
Health Report Card for Trinidad and Tobago, 2011. http://www.health.gov.tt/downloads/DownloadDetails.aspx?id=223. Accessed 10 Dec 2017.
World Development Indicators - Trinidad and Tobago. http://data.worldbank.org/country/trinidad-and-tobago. Accessed 10 Dec 2017.
Trinidad and Tobago GDP Annual Growth Rate 1991-2015. http://www.tradingeconomics.com/trinidad-and-tobago/gdp-growth-annual. Accessed 10 Dec 2017.
IMF World Economic Outlook (WEO). http://www.imf.org/external/pubs/ft/weo/2013/02/index.htm. Accessed 10 Dec 2017.
UN SIDS: Small Islands Bigger Stakes. http://unohrlls.org/custom-content/uploads/2013/08/SIDS-Small-Islands-Bigger-Stakes.pdf. Accessed 10 Dec 2017.
Pan American Health Organization (PAHO). PAHO/WHO country cooperation strategy, Trinidad and Tobago. Pan American Health Organization; 2006.
Central Statistical Office (Trinidad and Tobago). Trinidad & Tobago 2011 Housing and Population Census, vol. 2017. Trinidad: Ministry of Planning and Development; 2017.
The Central Statistical Office. Trinidad and Tobago 2011 Population and Housing Census Demographic Report. Trinidad and Tobago. Trinidad and Tobago Ministry of Planning and Sustainable Development; 2012.
Pan American Health Organization (PAHO). Health in the Americas, Book 636. Washington, DC: Pan American Health Organization; 2012.
Naraynsingh V, Hariharan S, Dan D, Bhola S, Bhola S, Nagee K. Trends in breast cancer mortality in Trinidad and Tobago--a 35-year study. Cancer Epidemiol. 2010;34(1):20–3.
Taioli E, Attong-Rogers A, Layne P, Roach V, Ragin C. Breast cancer survival in women of African descent living in the US and in the Caribbean: effect of place of birth. Breast Cancer Res Treat. 2010;122(2):515–20.
Naraynsingh V. Gastric carcinoma in the West Indies: a Trinidad study. Cancer. 1985;56(8):2117–9.
Luciani S, Cabanes A, Prieto-Lara E, Gawryszewski V. Cervical and female breast cancers in the Americas: current situation and opportunities for action. Bull World Health Organ. 2013;91(9):640–9.
Warner WA, Morrison RL, Lee TY, Williams TM, Ramnarine S, Roach V, Slovacek S, Maharaj R, Bascombe N, Bondy ML, et al. Associations among ancestry, geography and breast cancer incidence, mortality, and survival in Trinidad and Tobago. Cancer Med. 2015;4(11):1742–53.
Mutetwa B, Taioli E, Attong-Rogers A, Layne P, Roach V, Ragin C. Prostate cancer characteristics and survival in males of African ancestry according to place of birth: data from Brooklyn-New York, Guyana, Tobago and Trinidad. Prostate. 2010;70(10):1102–9.
Jensen O, McLeannan R, Muir C, Skeet G. Cancer registration: principles and methods. IARC Sci Publ. 1991;95(17):p127-8.
Boyle P, Parkin DM. Cancer registration: principles and methods. Statistical Methods for registries IARC Sci Publ. 1991;95:126–58.
Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization; 2000.
Segi M. Cancer mortality for selected sites in 24 countries (1950-1957): Sendai Department of Public Health. Tohoku University of Medicine; 1960.
Segi M, Kurihara M. Cancer mortality for selected sites in 24 countries (1966-1967) vol. no 6. Nagoya: Japan Cancer Society; 1972.
Team. RC: R. A language and environment for statistical computing. In: R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e1S–29S.
Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–116.
Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362(9387):847–52.
Wardwell NR, Massion PP. Novel strategies for the early detection and prevention of lung cancer. Semin Oncol. 2005;32(3):259–68.
Caribbean Epidemiology Centre (CAREC). Central Statistical Office (Trinidad and Tobago), Ministry of Health (Trinidad and Tobago), Pan American Health Organization (PAHO), University of the West Indies: Trinidad and Tobago Chronic Non-Communicable Disease Risk Factor Survey. Trinidad: Port-of-Spain; 2011.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and Cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8.
Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–60.
Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(10010):2287–323.
Bull FC, Armstrong TP, Dixon T. Physical inactivity. In: Ezzati M, Lopez AD, Rodgers A, CJL M, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Diseases Attributable to Selected Major Risk Factors. Volume 847, EDN. World Health Organization; 2004.
World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. http://www.who.int/chp/steps/GPAQ/en/]. Accessed 10 Dec 2017.
Churpek JE, Walsh T, Zheng Y, Moton Z, Thornton AM, Lee MK, Casadei S, Watts A, Neistadt B, Churpek MM, et al. Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015;149(1):31–9.
Odedina FT, Akinremi TO, Chinegwundoh F, Roberts R, Yu D, Reams RR, Freedman ML, Rivers B, Green BL, Kumar N. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009;4(1):S2.
Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among afro-Caribbeans: the Tobago prostate Cancer survey. Cancer Epidemiol Biomark Prev. 2002;11(8):726–9.
Rebbeck TR, Devesa SS, Chang BL, Bunker CH, Cheng I, Cooney K, Eeles R, Fernandez P, Giri VN, Gueye SM, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer. 2013;2013:560857.
Zeigler-Johnson CM, Spangler E, Jalloh M, Gueye SM, Rennert H, Rebbeck TR. Genetic susceptibility to prostate cancer in men of African descent: implications for global disparities in incidence and outcomes. Can J Urol. 2008;15(1):3872–82.
Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38.
IARC. Data comparability and quality. In: Forman D, Bray F, Brewster D, Gombe Mbalawa C, Kohler B, Pineros M, Steliarova-Foucher E, Swaminathan R, Lyon FJ, editors. Cancer Incidence in Five Continents Vol X. EDN, vol. 164. France: IARC Scientific Publication No; 2014.
Warner WA, Martin DN, Lee TY, Badal K, Williams TM, Bajracharya S, Sundaram V, Bascombe NA, Maharaj R, Roach V, et al. Patterns of Cancer Incidence and Mortality Rates and Trends in Trinidad and Tobago. In: The 5th Annual Glob Health and Infectious Disease Conference, Science to Solutions: 2017. St. Louis: Institute for Public Health, Washington University; 2017.
Acknowledgements
The authors would like to acknowledge Stephan Samuell (Central Statistical Office, Trinidad and Tobago) for assistance with census data acquisition, Arica White (Centers for Disease Control, GA, USA) and Kim Lipsey (Becker Medical Library, Washington University School of Medicine, St. Louis, MO, USA) for helpful discussions.
Funding
This work was partially supported by Washington University School of Medicine Grant Number GSAS/CGFP Fund 94028C (WAW), by Cancer Center Support Grant Number P30CA072720 from the National Cancer Institute (AAML), and the Public Health Cubed (PH3), Institute for Public Health, Washington University-St. Louis, MO (ATT and JL). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding sources.
Availability of data and materials
The data that supports the findings of this study is available from the Trinidad and Tobago Cancer Registry and the TT Central Statistical Office upon request.
Author information
Authors and Affiliations
Contributions
Project supervision, concept and design: WAW, AAML, data collection/acquisition: WAW, MLG, data analysis and/or interpretation: WAW, TYL, KB, TMW, SB, VS, NAB, RM, AR, MB, MJE, TRR, SS, JL, ATT, AAML. All authors assisted with the drafting the manuscript, revising the manuscript critically for important intellectual content, and approved the final version to be published. All authors also agree to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
There were two data sources included in this study. It is the policy of the Trinidad and Tobago Cancer Registry to provide de-identified data to investigators for research purposes. As such we requested and obtained the cancer surveillance data (de-identified data) used in this study from the registry. The Census data used is from a publicly-available source, which is found on the website of the Trinidad and Tobago Central Statistical Office. This study received ethical review exemptions from the Institutional Review Boards of Washington University School of Medicine, Rutgers Biomedical and Health Sciences and California State University, Los Angeles as only de-identified data were used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Basis of diagnosis for all cancer cases recorded in the National Cancer Registry of TT, 1995–2009. (DOCX 14 kb)
Additional file 2:
Table S2. Age-standardized incidence and mortality rates for two of the ten leading cancer sites by sex, and ancestry, for persons less than 24 years old, Trinidad and Tobago, 1995–2009. (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Warner, W.A., Lee, T.Y., Badal, K. et al. Cancer incidence and mortality rates and trends in Trinidad and Tobago. BMC Cancer 18, 712 (2018). https://doi.org/10.1186/s12885-018-4625-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4625-x