Abstract
Background
Radical radiotherapy, with or without concomitant chemotherapy forms the mainstay of organ preservation approaches in mucosal primary head and neck cancer. Despite technical advances in cancer imaging and radiotherapy administration, a significant proportion of patients fail to achieve a complete response to treatment. For those patients who do achieve a complete response, acute and late toxicities remain a cause of morbidity. A critical need therefore exists for imaging biomarkers which are capable of informing patient selection for both treatment intensification and de-escalation strategies.
Methods/design
A prospective imaging study has been initiated, aiming to recruit patients undergoing radical radiotherapy (RT) or chemoradiotherapy (CRT) for mucosal primary head and neck cancer (MPHNC). Eligible patients are imaged using FDG-PET/CT before treatment, at the end of week 3 of treatment and 12 weeks after treatment completion according to local imaging policy. Functional MRI using diffusion weighted (DWI), blood oxygen level-dependent (BOLD) and dynamic contrast enhanced (DCE) sequences is carried out prior to, during and following treatment. Information regarding treatment outcomes will be collected, as well as physician-scored and patient-reported toxicity.
Discussion
The primary objective is to determine the correlation of functional MRI sequences with tumour response as determined by FDG-PET/CT and clinical findings at 12 weeks post-treatment and with local control at 12 months post-treatment. Secondary objectives include prospective correlation of functional MRI and PET imaging with disease-free survival and overall survival, defining the optimal time points for functional MRI assessment of treatment response, and determining the sensitivity and specificity of functional MRI sequences for assessment of potential residual disease following treatment. If the study is able to successfully characterise tumours based on their functional MRI scan characteristics, this would pave the way for further studies refining treatment approaches based on prognostic and predictive imaging data.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12616000534482 (26 April 2016).
Similar content being viewed by others
Background
Mucosal primary head and neck cancer (MPHNC) has an estimated incidence of over 900,000 cases per year, and is associated with significant mortality, causing more than 350,000 deaths per year[1]. Organ preservation strategies involve the administration of a radical dose of radiation therapy, usually with concomitant chemotherapy, producing 5-year overall survival rates of between 30 and 50% [2]. Despite technical improvements in radiotherapy, significant toxicity may result from these treatments [3, 4].
Prognostication and treatment strategies are informed by clinical features [5] and increasingly by tumour biological features, notably preceding infection with oncogenic strains of human papilloma virus (HPV) [6, 7]. Reliable imaging biomarkers may therefore provide useful data to facilitate adaptive approaches including treatment intensification for poor responders and de-intensification for good responders [8]. Such strategies may improve the therapeutic ratio by reducing dose to organs at risk and increasing dose to areas harbouring subvolumes of more resistant tumour.
Positron-emission tomography using 18F–fluorodeoxyglucose (FDG-PET) is currently a standard investigation for initial staging of MPHNC. Retrospective studies have described FDG-PET’s performance in assessing disease following radical radiotherapy [9, 10] and its value in decision-making around surgical salvage has been demonstrated in a prospective trial [11]. Some limitations remain however, with the possibility of false-positive results due to benign tissue inflammation and of false-negative results due to limited spatial resolution.
Magnetic resonance imaging (MRI) has an established role in diagnosis and staging of MPHNC, and its use in radiotherapy planning continues to expand [12]. MRI has superior spatial resolution, better soft tissue definition and does not employ ionising radiation. Furthermore, functional sequences can provide additional information on tissue composition and biology which is not revealed by standard anatomical T1 and T2 weighted scans.
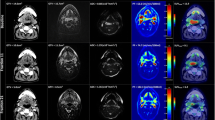
Diffusion-weighted imaging (DWI) characterises tissue based on movement of water within a volume. The diffusivity of water molecules depends on microstructural features of the tissue, such as cellular size and density, and may be quantified with the apparent diffusion coefficient (ADC). Its applications in the upper aerodigestive tract were previously limited by technical factors such as susceptibility artefact from air-tissue interfaces and low signal-to-noise ratio. Modern scanning techniques have been able to overcome many of these issues, and DWI measurements before, during and following RT have been investigated as a diagnostic and prognostic tool [13,14,15,16,17,18].
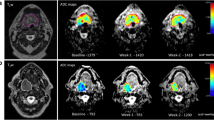
Dynamic contrast-enhanced (DCE) MRI provides information on abnormal tumour vasculature, by assessing the travel of gadolinium contrast agent between intravascular and extracellular extravascular space (EES) by means of sequential T1-weighted scans with high temporal resolution. With knowledge of the pre-contrast T1 tissue values, and the concentration-time course of contrast in feeding blood vessels, pharmacokinetic modelling can generate quantitative parameters for analysis of tumour perfusion [19]. These include Ktrans (the volume transfer constant between blood plasma and EES), Kep (the rate constant between EES and blood plasma) and Ve (the fractional volume of EES). The values of these parameters, as well as their distribution in the volume imaged, have been investigated as potential imaging biomarkers both before and after treatment for MPHNC [20,21,22,23,24,25,26,27,28,29,30].
Blood oxygen level–dependent (BOLD) MRI utilises local changes in magnetic susceptibility due to the presence of paramagnetic deoxyhaemoglobin. An increase in deoxyhaemoglobin shortens transverse relaxation time T2*, giving a qualitative indication of tissue hypoxia [31]. The rate of transverse relaxation R2* (1/T2*) can be measured with a multiple-echo gradient echo sequence and has been shown to correlate with tissue hypoxia as measured by polarographic electrodes and immunohistochemistry [32,33,34]. Pre-clinical and early clinical results suggest that R2* may be a useful biomarker for radiation response and for treatment outcomes [35,36,37].
Our group completed a pilot study of functional MRI and FDG-PET in a cohort of patients undergoing radical radiotherapy for MPHNC, indicating that carrying out sequential DWI and R2* studies before, during and after radiotherapy was feasible. Initial results, including correlations between imaging studies have been published, while mature data regarding clinical outcomes are awaited [18]. Following this we have initiated a prospective observational study named “MRI in MPHNC”, which aims to gather data on the utility of functional imaging sequences with DWI, DCE and BOLD MRI as biomarkers for prediction and assessment of treatment response.
Methods
Study design and consent
This study is a prospective single arm observational trial of patients undergoing curative intent primary radiotherapy, with or without chemotherapy, for MPHNC at Liverpool and Campbelltown Hospitals. Patients are identified and evaluated by the multidisciplinary team. Informed consent is obtained by medically trained personnel as per the trial delegation log. Administrative support, including travel funding for study scans, is provided by clinical trials unit staff.
Hypotheses
-
1.
Functional MRI can be used as a reliable imaging biomarker in prediction of treatment response (radiotherapy ± chemotherapy) in MPHNC
-
2.
Functional MRI can be used as an alternative functional imaging study to FDG-PET scan in assessing response after radiotherapy ± chemotherapy
-
3.
Functional MRI can differentiate between recurrent or residual malignant disease and post-treatment tissue changes following radiotherapy ± chemotherapy
Objectives
Primary objective
To determine the correlation of pre-, during treatment and post-radiotherapy (± chemotherapy) functional MRI with tumour response at 3 months by FDG-PET scan and with local control at 12 months.
Secondary objectives
-
1.
To prospectively correlate different MRI sequences and FDG-PET findings with disease-free survival (DFS) and overall survival (OS)
-
2.
To determine the best time to perform MRI during radiotherapy treatment for assessing tumour response and local control
-
3.
To determine the sensitivity and specificity of functional MRI sequences compared with FDG-PET in assessment of potential residual and recurrent MPHNC following definitive radiotherapy
-
4.
To assess consistency of image registration, signal information and associated analyses over a relevant time frame in a representative subset of patients
Endpoints
Primary endpoints
Correlation of functional MRI sequences with PET-CT at 3 months following treatment and local control at 12 months, including:
-
Measurement of restricted water diffusion as on DWI, which can be a marker of high cellularity within a tumour, using ADC values, ΔADC and parameter maps of ADC.
-
Measurement of tumour perfusion characteristics from DCE-MRI, using parameter maps and calculated values based on inflow and outflow (Ktrans, Kep) as well as degree of contrast enhancement of tissues (Relative Signal Intensity, RSI).
-
Tumour size as defined on anatomical imaging using T2-weighted and/or T1-weighted Volume Interpolated Breathhold Examination (VIBE) Dixon MRI.
Secondary endpoints
Correlation of different MRI measurements with 1 and 2 year DFS and OS including:
-
Determination of the ability of DWI for detecting residual or recurrent cancer
-
Assessment of functional MRI in correlation with FDG-PET metabolic response at 3 months and local control at 12 months
Subject selection
Inclusion criteria
-
18 years or older
-
Have the ability to give informed consent
-
Histologically-proven invasive mucosal primary squamous cell carcinoma of head and neck region or patients with tumours strongly suspicious for mucosal primary head and neck cancer due to clinical features AND fine needle aspiration (FNA) cytology assessment
-
Primary mucosal head and neck cancer (≥T2 and/or ≥N1) AND no evidence of metastatic disease on staging PET/CT or CT (chest ± abdomen ± pelvis)
-
Patient undergoing curative intent primary radiotherapy ± chemotherapy
Exclusion criteria
-
Contraindication to MRI studies
-
Significant claustrophobia
-
Pacemaker/implantable defibrillator
-
Implanted metals e.g. Intraocular clips
-
Known allergic reaction to gadolinium (Gd)-DTPA
-
-
Previous radiotherapy to the area to be treated
-
Primary cancer surgery to the affected area
-
Other malignancy within 5 years of the current diagnosis, with the exception of successfully treated basal cell or squamous cell skin carcinoma, in situ melanoma, or carcinoma in situ of the cervix
Early withdrawal of subjects
If a patient expresses wishes to withdraw from the trial, site staff will explain the importance of maintaining follow-up. A patient may withdraw, or be withdrawn, from the trial for the following reasons:
-
Unacceptable toxicity
-
Requests or requires early discontinuation for any reason
-
Intercurrent illness which prevents further treatment/follow-up
-
Withdrawal of consent for treatment by patient
-
Develops, during the course of the study, symptoms or conditions listed in the exclusion criteria
-
Investigator discretion
The investigator will also withdraw all subjects from the study if the study is terminated. Subjects are free to withdraw from the study at any time upon their request or the request of their legally acceptable representative.
Patients may re-consent if they change their mind following withdrawal, to resume participation on this trial. The patient will however remain in the trial for the purposes of follow-up and data analysis unless they specifically request otherwise.
Radiotherapy
All participants will receive standard treatment according to locally agreed protocols [38], based on international evidence-based practice guidelines. This study does not involve a treatment intervention or additional ionising radiation exposure. Therefore, there will not be any additional radiation dose or related side effects by participating in this trial. All imaging performed with ionising radiation is part of standard clinical practice.
Chemotherapy
All participants will be treated according to locally agreed protocols [38], based on international evidence-based practice guidelines. Therefore, there will not be any additional chemotherapy related side effects by participating in this trial.
Imaging
Imaging schedule
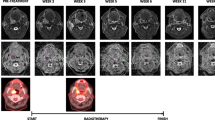
Functional MRI studies are carried out prior to commencing treatment, then weeks 2, 3, 5 and 6 during treatment, followed by 1 month and 3 months post-treatment (Fig. 1).
Imaging procedures
All patients scanned on a 3 T wide bore scanner (Skyra, Siemens, Erlangen, Germany) using a 20-channel head and neck coil (Fig. 2). Morphological and functional sequences are obtained in the same scan session.
Morphological MRI
Sagittal T2 TIRM (turbo inversion recovery magnitude).
Axial T2 TSE DIXON water-only.
Coronal T2 TSE DIXON water-only.
Axial T1 DIXON water-only, pre- and post-contrast.
Functional MRI
Coronal BOLD
-
Gradient echo imaging with multiple echoes
-
5 mm slice thickness, 2 averages, 8 slices through region of interest
-
In-plane resolution 1.7 × 1.7 mm
-
TR 500 ms, TE 2.46 to 56.58 ms (12 echoes, 4.92 ms spacing)
Axial DWI
-
Readout segmentation of long variable echo-trains (RESOLVE) sequence
-
3 mm slice thickness
-
In-plane resolution 0.4 × 0.4 mm
-
3-scan trace, monopolar
-
b = 50 s/mm2 with one average, b = 800 s/mm2 with 3 averages, calculated image at b = 1400 s/mm2
-
Bandwidth 868 Hz/Px
Repeat coronal BOLD
-
Gradient echo imaging with multiple echoes
-
5 mm slice thickness, 2 averages, 8 slices through region of interest
-
In-plane resolution 1.7 × 1.7 mm
-
TR 500 ms, TE 2.46 to 56.58 ms (12 echoes, 4.92 ms spacing)
DCE scans
-
2 x 3D coronal T1 VIBE (volumetric interpolated breath-hold examination) scans for T1-mapping
-
2° and 15° flip angles
-
3 mm slice thickness
-
In-plane resolution 1 × 1 mm
-
Acquisition time 1 m 21 s
-
Bandwidth 440 Hz/Px
-
-
TWIST (Time-resolved angiography With Interleaved Stochastic Trajectories)
-
Slice thickness 3 mm
-
In-plane resolution 1 × 1 mm
-
TR 3.06 ms, TE 1.35 ms
-
Bandwidth 800 Hz/Px
-
i.v. gadobutrol (Gadovist, Bayer) 0.1 mL/kg, capped at a maximum of 7.5 mL; injection of contrast immediately before 4th measurement, 4 ml/s with 20 ml saline flush
-
Scan for total 60 timepoints, temporal resolution 5.64 s
-
Data acquired for 5 m 33 s
-
Hop on hop off sub-study scans (week −3 to 0 only)
-
Patients who consent to the sub-study get up from the couch and are repositioned a few minutes later
-
Axial T1, T2 and DWI scans are repeated
Statistical plan
Sample size determination
Based on our previous study using week 3 FDG-PET scan in head and neck cancer patients, the following sample size calculation is made. To test a significant FDG-PET complete metabolic response (CMR) rate of 87% in the MRI-positive group versus an FDG-PET CMR rate of 51% in the MRI-negative group, the study will need to recruit 55 patients in total to reach 80% power (at 5% significance level). This number is based on an assumption that the MRI-positive group and MRI-negative group will be balanced (e.g. 50%:50% but is powered to allow up to a 40%:60% biased ratio). It is anticipated that recruitment will be completed in 24 months. Patients will be offered participation in the “hop on hop off” sub-study, with a recruitment goal of 20.
Assessment of MRI sequences
MRI scans will be determined as a good response (e.g. positive) or poor response (e.g. negative) based on the following definitions for endpoint analysis:
DWI
High ADC values will be considered a good response (positive MRI) and low ADC values a poor response (negative MRI).
DCE MRI
A reduction in blood inflow and outflow (e.g. Ktrans and Kep) will be considered a good response (positive MRI) and either no change or an increase will be considered a poor response (negative MRI).
BOLD
A reduction in R2* values will be considered a good response (positive MRI) and either no change or increase in R2* values will be considered a poor response (negative MRI).
Definition of local control
FDG-PET response at 12 weeks will be defined as a complete metabolic response, partial metabolic response, stable metabolic disease or progressive metabolic disease based on visual assessment by the nuclear medicine physician’s report. Local control for patients will be assessed at 12 months as indicated by having no evidence of local disease on clinical (including endoscopic) examinations and imaging studies at 12 months. Tumour response will be assessed using Response Evaluation Criteria In Solid Tumors (RECIST) criteria version 1.1[39] which is the internationally recognised gold standard assessment method on the evaluation of therapeutic response in solid tumours.
Statistical analysis
Statistical analyses will be performed by using software SPSS and p < 0.05 will be considered statistically significant. Chi-square test will be used to compare the prevalence of FDG-PET CMR between MRI-positive and negative groups. Comparisons of the ADC values/maps of pre-, intra- and post-treatment DWI images will be performed by using one way analysis of variance (ANOVA) test. Comparisons of the DCE parameters such as Ktrans, Kep, area under the curve (AUC) at initial 60 and 90 s of pre-, intra- and post-treatment will be performed by using the Kruskal-Wallis test. To determine the sensitivity and specificity of DWI and DCE parameters for FDG-PET CMR and 12 month local control, Kappa association statistics will be used. Receiver operator characteristics (ROC) will be used to obtain an optimal threshold for individual MRI parameters.
Data collection
General baseline demographic data will be collected. Other data collection can be defined by the 6 general categories below:
Quality of life
-
Baseline
-
Final week of chemoradiotherapy
-
Follow up visits 1, 3, 6, 12, 18, 24, 30 and 36 months post treatment
-
Assessed using University of Washington Quality of Life Questionnaire (UW-QOL v4)[40]
Blood tests
-
Baseline
-
Weeks 2, 3, 5, 6
-
Follow up visits 1 and 3 months post-treatment
-
Consist of full blood count and basic biochemistry panel including urea, creatinine, sodium, potassium and chloride
Toxicity
-
Acute toxicities – dry mouth, dysphagia, pain, weight loss, oral mucositis, radiation dermatitis, dygeusia,
-
Baseline, weekly during treatment
-
Follow up visits 1 and 3 months post treatments
-
-
Late toxicities – dry mouth, skin induration, osteonecrosis of jaw, voice alteration, oral mucositis, dysphagia, hearing, tracheostomy, laryngectomy, tube feeding
-
At follow up visits 3, 6, 12, 18, 24, 30 and 36 months post treatment
-
-
Using CTCAE version 4.0
-
Serious adverse events to be reported to the HREC for review
Swallowing assessment
-
Baseline
-
Final week of chemoradiotherapy
-
Follow up visits 1, 3, 6, 12, 18, 24, 30 and 36 months post treatment
Scans
-
Functional MRI scans as per Fig. 1 and Imaging procedures above
-
As per standard of care, a PET/CT will be carried out at week 3 of chemoradiotherapy treatment and then at 3 months following completion of chemoradiotherapy treatment
Treatment data
-
The dose and duration of treatment will be recorded.
Data handling and record keeping
All imaging datasets will be de-identified and stored in a secure research drive at the Cancer Therapy Centre, Liverpool Hospital after recruiting patients to the study. Contours will be performed by radiation oncologists/fellows and saved on the research drive. Datasets will be imported into MATLAB (MathWorks Inc. Massachusetts, United States) and similar mathematical analysis programs for data-analysis. Data analysis will be in line with study aims.
The investigator site file and patient study file will be kept at the Ingham Institute Liverpool and will be accessible only by clinical trials staff.
Confidentiality
Subject confidentiality is strictly held in trust by the participating investigators, research staff, the sponsoring institution and their agents. The study documentation, data and all other information generated will be held in strict confidence. No information concerning the study or the data will be released to any unauthorized third party, without prior written approval of the HREC and the principal investigator. Clinical information will not be released without written permission of the subject, except as necessary for monitoring by Human Research Ethics Committee (HREC) or regulatory agencies.
Records retention
It is the investigators’ responsibility to retain study essential documents for at least 15 years after the completion of this clinical trial. All information will be stored in the Radiation Oncology research office located at the Ingham Institute, Liverpool Hospital, either on a password protected computer or in files kept in a locked room. Access to this information will be limited to the principal investigator, research assistants and statistician as authorized by the delegation log.
Timelines/milestones
The study started recruitment after ethics approval was granted. A sample size of 55 has been selected for this study for the reasons discussed above. Initial projections suggested this could be achieved within 24 months, however this timeframe will be reviewed as recruitment progresses.
Publication and dissemination
The results of the study will be submitted for publication in peer-reviewed journals and for presentation at scientific meetings. Participants in the study will be notified of study results in writing.
Discussion
In recent years intensity-modulated RT, along with advanced diagnostic and on-treatment imaging technologies, have improved the therapeutic ratio in head and neck cancer [41]. The enhanced ability to dose-paint and the recognition of heterogeneity within tumours gives rise to the promise of functional image-guided RT (fIGRT), where subvolumes may be treated to differing doses according to imaging-derived parameters. Longitudinal diffusion-weighted imaging has already been shown to be feasible in MRI-guided cobalt radiotherapy systems [42], suggesting that the addition of functional as well as anatomical imaging may serve to improve outcomes with these systems, as well as in the MRI-guided linear accelerators in development.
Our protocol builds on our earlier work which established the feasibility of serial functional MRI during a course of treatment for MPHNC. Refinements to the imaging protocol include the addition of DCE sequences, which may be expected to add further value [43], and the expansion of the BOLD sequence to acquire multiple slices across a volume, an imaging technique which may be more reliable than BOLD imaging carried out on single slices [44]. The additional information gained from the enhanced suite of imaging sequences in the current study will help to define which sequences, at which time points, can be used to adapt and personalise radiotherapy treatment. This information will then feed into the design of interventional studies of adaptive RT.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- BOLD:
-
Blood oxygen level-dependent
- CMR:
-
Complete metabolic response
- CRT:
-
Chemoradiotherapy
- CT:
-
Computerised tomography
- CTCAE:
-
Common terminology criteria for adverse events
- DCE:
-
Dynamic contrast-enhanced
- DFS:
-
Disease free survival
- DWI:
-
Diffusion-weighted imaging
- EES:
-
Extracellular extravascular space
- FDG-PET:
-
Fluoro-deoxy glucose positron emission tomography
- fIGRT:
-
Functional image-guided radiotherapy
- FNA:
-
Fine needle aspiration
- Gd:
-
Gadolinium
- HPV:
-
Human papilloma virus
- HREC:
-
Human research ethics committee
- IV:
-
Intravenous
- Kep :
-
Rate constant between extracellular extravascular space (EES) and blood plasma
- Ktrans :
-
Volume transfer constant between blood plasma and extracellular extravascular space (EES)
- MPHNC:
-
Mucosal primary head and neck cancer
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- QoL:
-
Quality of life
- R2*:
-
Relaxation rate for T2* (1/T2*)
- RESOLVE:
-
Readout segmentation of long variable echo-trains
- ROC:
-
Receiver operator characteristic
- RSI:
-
Relative signal intensity
- RT:
-
Radiotherapy
- TIRM:
-
Turbo inversion recovery magnitude
- TSE:
-
Turbo spin echo
- TWIST:
-
Time-resolved angiography with interleaved stochastic trajectories
- Ve :
-
Fractional volume of extracellular extravascular space (EES)
- VIBE:
-
Volume interpolated breath-hold examination
References
Global Burden of Disease Cancer C: Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2016.
Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, Pignon J-P. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33–40.
Chen AM, Daly ME, Farwell DG, Vazquez E, Courquin J, Lau DH, Purdy JA. Quality of life among long-term survivors of head and neck cancer treated by intensity-modulated radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140(2):129–33.
Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36.
Leoncini E, Vukovic V, Cadoni G, Pastorino R, Arzani D, Bosetti C, Canova C, Garavello W, La Vecchia C, Maule M, et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015;39(3):367–74.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Chakravarthy A, Henderson S, Thirdborough SM, Ottensmeier CH, Su X, Lechner M, Feber A, Thomas GJ, Fenton TR. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated With an Immune Response Largely Restricted to the Oropharynx. J Clin Oncol. 2016;0(0):JCO682955.
Bhatnagar P, Subesinghe M, Patel C, Prestwich R, Scarsbrook AF. Functional Imaging for Radiation Treatment Planning, Response Assessment, and Adaptive Therapy in Head and Neck Cancer. Radiographics 2013;33(7):1909–29.
Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33(3):210–22.
Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, Murthy V, Budrukkar A. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38(11):2083–95.
Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, Nutting C, Powell N, Al-Booz H, Robinson M, et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. N Engl J Med. 2016;374(15):1444–54.
Metcalfe P, Liney GP, Holloway L, Walker A, Barton M, Delaney GP, Vinod S, Tome W. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12(5):429–46.
Driessen JP, Caldas-Magalhaes J, Janssen LM, Pameijer FA, Kooij N, Terhaard CH, Grolman W, Philippens ME. Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: association between apparent diffusion coefficient and histologic findings. Radiol. 2014;272(2):456–63.
King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, Bhatia KS, Vlantis AC, Ahuja AT. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiol. 2013;266(2):531–8.
King AD, Thoeny HC: Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: Potential and limitations. Cancer imaging: the official publication of the International Cancer Imaging Society. 2016;16(1).
Yun TJ, Kim JH, Kim KH, Sohn CH, Park SW. Head and neck squamous cell carcinoma: differentiation of histologic grade with standard- and high-b-value diffusion-weighted MRI. Head Neck. 2013;35(5):626–31.
Lambrecht M, Van Herck H, De Keyzer F, Vandecaveye V, Slagmolen P, Suetens P, Hermans R, Nuyts S. Redefining the target early during treatment. Can we visualize regional differences within the target volume using sequential diffusion weighted MRI?. Radiother Oncol: J Eur Soc Ther Radiol Oncol. 2014;110(2):329–34.
Min M, Lee MT, Lin P, Holloway L, Wijesekera D, Gooneratne D, Rai R, Xuan W, Fowler A, Forstner D, et al. Assessment of serial multi-parametric functional MRI (diffusion-weighted imaging and R2*) with (18)F-FDG-PET in patients with head and neck cancer treated with radiation therapy. Br J Radiol. 2016;89(1058):20,150,530.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32.
Newbold K, Castellano I, Charles-Edwards E, Mears D, Sohaib A, Leach M, Rhys-Evans P, Clarke P, Fisher C, Harrington K, et al. An exploratory study into the role of dynamic contrast-enhanced magnetic resonance imaging or perfusion computed tomography for detection of intratumoral hypoxia in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74(1):29–37.
Jansen JF, Schoder H, Lee NY, Wang Y, Pfister DG, Fury MG, Stambuk HE, Humm JL, Koutcher JA, Shukla-Dave A. Noninvasive assessment of tumor microenvironment using dynamic contrast-enhanced magnetic resonance imaging and 18F–fluoromisonidazole positron emission tomography imaging in neck nodal metastases. Int J Radiat Oncol Biol Phys. 2010;77(5):1403–10.
Chawla S, Kim S, Loevner LA, Hwang WT, Weinstein G, Chalian A, Quon H, Poptani H. Prediction of disease-free survival in patients with squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. Am J Neuroradiol. 2011;32(4):778–84.
Agrawal S, Awasthi R, Singh A, Haris M, Gupta RK, Rathore RK. An exploratory study into the role of dynamic contrast-enhanced (DCE) MRI metrics as predictors of response in head and neck cancers. Clin Radiol. 2012;67(9):e1–5.
Chikui T, Kitamoto E, Kawano S, Sugiura T, Obara M, Simonetti AW, Hatakenaka M, Matsuo Y, Koga S, Ohga M, et al. Pharmacokinetic analysis based on dynamic contrast-enhanced MRI for evaluating tumor response to preoperative therapy for oral cancer. J Magn Reson Imaging. 2012;36(3):589–97.
Jansen JF, Carlson DL, Lu Y, Stambuk HE, Moreira AL, Singh B, Patel SG, Kraus DH, Wong RJ, Shaha AR, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48(8):717–22.
Jansen JF, Schoder H, Lee NY, Stambuk HE, Wang Y, Fury MG, Patel SG, Pfister DG, Shah JP, Koutcher JA, et al. Tumor metabolism and perfusion in head and neck squamous cell carcinoma: pretreatment multimodality imaging with 1H magnetic resonance spectroscopy, dynamic contrast-enhanced MRI, and [18F]FDG-PET. Int J Radiat Oncol Biol Phys. 2012;82(1):299–307.
Shukla-Dave A, Lee NY, Jansen JF, Thaler HT, Stambuk HE, Fury MG, Patel SG, Moreira AL, Sherman E, Karimi S, et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1837–44.
Wang P, Popovtzer A, Eisbruch A, Cao Y. An approach to identify, from DCE MRI, significant subvolumes of tumors related to outcomes in advanced head-and-neck cancer. Med Phys. 2012;39(8):5277–85.
Chawla S, Kim S, Dougherty L, Wang S, Loevner LA, Quon H, Poptani H. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. Am J Roentgenol. 2013;200(1):35–43.
King AD, Chow SKK, Yu K-H, Mo FKF, Yeung DKW, Yuan J, Law BKH, Bhatia KS, Vlantis AC, Ahuja AT. DCE-MRI for Pre-Treatment Prediction and Post-Treatment Assessment of Treatment Response in Sites of Squamous Cell Carcinoma in the Head and Neck. PLoS ONE. 2015;10(12):e0144770.
Krishna MC, Subramanian S, Kuppusamy P, Mitchell JB. Magnetic resonance imaging for in vivo assessment of tissue oxygen concentration. Semin Radiat Oncol. 2001;11(1):58–69.
Chopra S, Foltz WD, Milosevic MF, Toi A, Bristow RG, Menard C, Haider MA. Comparing oxygen-sensitive MRI (BOLD R2*) with oxygen electrode measurements: a pilot study in men with prostate cancer. Int J Radiat Biol. 2009;85(9):805–13.
Hoskin PJ, Carnell DM, Taylor NJ, Smith RE, Stirling JJ, Daley FM, Saunders MI, Bentzen SM, Collins DJ, d’Arcy JA, et al. Hypoxia in prostate cancer: correlation of BOLD-MRI with pimonidazole immunohistochemistry-initial observations. Int J Radiat Oncol Biol Phys. 2007;68(4):1065–71.
Liu M, Guo X, Wang S, Jin M, Wang Y, Li J, Liu J. BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1alpha. Eur Radiol. 2013;23(12):3221–7.
Li XS, Fan HX, Fang H, Song YL, Zhou CW. Value of R2* obtained from T2*-weighted imaging in predicting the prognosis of advanced cervical squamous carcinoma treated with concurrent chemoradiotherapy. J Magn Reson Imaging. 2015.
Kim CK, Park SY, Park BK, Park W, Huh SJ. Blood oxygenation level-dependent MR imaging as a predictor of therapeutic response to concurrent chemoradiotherapy in cervical cancer: a preliminary experience. Eur Radiol. 2014;24(7):1514–20.
Hallac RR, Zhou H, Pidikiti R, Song K, Stojadinovic S, Zhao D, Solberg T, Peschke P, Mason RP. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn Reson Med: J Soc Magn Reson Med/Soc Magn Reson Med. 2014;71(5):1863–73.
eviQ Cancer Treatments Online. [https://www.eviq.org.au/].
Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990). 2009;45.
Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15(6):485–96.
Grégoire V, Langendijk JA, Nuyts S. Advances in Radiotherapy for Head and Neck Cancer. J Clin Oncol. 2015;33(29):3277–84.
Yang Y, Cao M, Sheng K, Gao Y, Chen A, Kamrava M, Lee P, Agazaryan N, Lamb J, Thomas D, et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys. 2016;43(3):1369–73.
Noij DP, de Jong MC, Mulders LG, Marcus JT, de Bree R, Lavini C, de Graaf P, Castelijns JA. Contrast-enhanced perfusion magnetic resonance imaging for head and neck squamous cell carcinoma: a systematic review. Oral Oncol. 2015;51(2):124–38.
Wu G-y. Suo S-t, Lu Q, Zhang J, Zhu W-q, Xu J-r. The Value of Blood Oxygenation Level-Dependent (BOLD) MR Imaging in Differentiation of Renal Solid Mass and Grading of Renal Cell Carcinoma (RCC): Analysis Based on the Largest Cross-Sectional Area versus the Entire Whole Tumour. PLoS ONE. 2015;10(4):e0123431.
Acknowledgements
We would like to thank the following: Ms. Mel Grand, Ms. Elise O’Reilly, Ms. Doaa Elwadia, Dr. Wei Xuan, Dr. Michael Jameson, Prof Michael Barton.
Ethical approval and consent to participate
The study protocol, including the Participant Information Sheet and the Informed Consent Form were submitted for approval by HREC prior to the study commencement, with approval granted in March 2016. The study was prospectively registered on the Australian New Zealand Clinical Trials Registry (ANZCTR) in April 2016. Any alteration to this protocol will be documented and submitted to the HREC for approval prior to incorporation into study procedures, with any updated documents then sent to all study personnel.
Funding
This study, including the cost of MRI scans and MRI radiographers, is funded by the Department of Radiation Oncology, Liverpool Hospital. Additional support has come from a competitive research grant for $20,000 from the Royal Australian and New Zealand College of Radiologists (RANZCR). All funding sources will be acknowledged in any publication. The funding bodies were not directly involved in the study design, nor are they directly involved in data collection, analysis or interpretation.
Availability of data and materials
Not applicable at this stage.
Author information
Authors and Affiliations
Contributions
Study conception and design – MTL, LH, RR, MM, DF, AF, GL. Patient recruitment – CNR, MTL, MM, DF, AF. Data acquisition – CNR, RR, GL. Data analysis and interpretation – CNR, MTL, LH, RR, GL. Drafting and revision of the manuscript – CNR, MTL, LH, RR, MM, GL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
This manuscript does not contain any patient details. Fig. 2 contains an image of a staff member who volunteered to be photographed for illustration purposes and who has given consent for the images to be published.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rumley, C.N., Lee, M.T., Holloway, L. et al. Multiparametric magnetic resonance imaging in mucosal primary head and neck cancer: a prospective imaging biomarker study. BMC Cancer 17, 475 (2017). https://doi.org/10.1186/s12885-017-3448-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-017-3448-5