Abstract
Background
The purpose of this study is to investigate the most suitable first-line approach and the best combination treatment for primary mediastinal large B-cell lymphoma (PMLBCL) as they have been matter of debate for at least two decades.
Methods
Our single centre experience in the treatment of 98 de novo PMLBCL patients over the last 20 years is reviewed. All patients received MACOP-B chemotherapy. Thirty-seven received both rituximab and mediastinal radiotherapy; 30 were irradiated after chemotherapy, although not receiving rituximab and 20 received rituximab without radiotherapy consolidation. Eleven patients received chemotherapy only.
Results
Sixty-one (62.2%) patients achieved a complete response after MACOP-B (with or without rituximab); among the 27 (27.6%) partial responders, 21 obtained a complete response after radiotherapy. At the end of their scheduled treatment, 82 patients (83.7%) had a complete and 6 a partial response (6.1%). Eleven patients relapsed within the first 2 years of follow-up. The 17-year overall survival is 72.0% (15 patients died); progression-free and disease-free survival are 67.6% and 88.4%, respectively. A statistically significant difference in overall and progression-free survival was noted among treatment groups, although no disease-free survival difference was documented.
Conclusions
Our data indicate that a third-generation regimen like MACOP-B could be considered a suitable first-line treatment. Mediastinal consolidation radiotherapy impacts on survival and complete response rates and remains a good strategy to convert partial into complete responses. Data suggest that radiotherapy may be avoided in patients obtaining a complete response after (immuno)chemotherapy, but this requires confirmation with further ad hoc studies.
Similar content being viewed by others
Background
The 1994 Revised European American Lymphoma (REAL) Classification firstly recognizes primary mediastinal large B-cell lymphoma (PMLBCL) as a subtype of diffuse large B-cell lymphoma (DLBCL), although it has been regarded as a specific clinical and biological entity since the 2001 World Health Organization classification [1, 2].
It is a rapidly-growing and progressive neoplasm, normally presenting with bulky masses usually exerting compressive effects on mediastinal structures, giving rise to the possible abrupt onset of dyspnea, dysphagia, thoracic pain, facial, neck, breasts and arms edema and pleuro-pericardial effusions. In this sense, it should be regarded as a hematological emergency, and promptly treated: initial therapy is therefore crucial for the management of this disease.
In the last 15 years, several issues have emerged regarding the treatment of this disease, and in particular: 1) the choice of initial chemotherapy approach, based on either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) and CHOP-like schedules or more intense ones, such as the so-called “third-generation” regimens, perhaps including a high-dose consolidation with autotransplantation for patients in first remission [3]; 2) the value of a rituximab-based immunotherapy in this subset of patients, on the basis of the results obtained in randomized studies involving DLBCL patients [4, 5]; 3) the role of external beam radiotherapy (RT), as an adjuvant strategy through which consolidate a response to chemotherapy and produce an eradication of the disease [6].
In terms of first-line chemotherapy, if on the one hand the CHOP regimen has been mainly adopted by American centers, the European experience has carried out the evidence that MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone) or VACOP-B (same as MACOP-B, with etoposide instead of methotrexate), both weekly-based “third-generation” dose-dense regimens, may be superior to CHOP [7,8,9]. As a consequence of the application of dose-dense regimens, in fact, remission rates and survival functions have appeared to be at least as good as – or probably even better than – those observed for DLBCL patients, thus retracting the initial impression that PMLBCL was per se a prognostically unfavorable subset of DLBCL. Although this conclusion is drawn from existing reports, no randomized clinical trial have been carried on so far. It is clear, however, that an anthracycline-containing regimen should be regarded as the first approach to PMBCL [10].
The addition of rituximab to first line chemotherapy has shown no advantages in terms of overall and relapse-free survival if the monoclonal antibody was added to a third-generation regimen [11], although R-CHOP chemoimmunotherapy seems significantly superior to CHOP alone [12, 13].
External RT, conceived as the delivery of radiation on residues of bulky masses at the end of chemotherapy, has shown a great efficacy when it was incorporated after the completion of an induction strategy based on chemotherapy, particularly in converting partial responses into complete responses and in rendering active residual masses negative at gallium scan or positron emission tomography (PET) [14,15,16].
This report presents our 20 years monocentric experience in the first-line treatment of primary mediastinal patients, in accordance with our Institute treatment policy and with the practical guidelines outlined by the Italian Society of Hematology [10].
Methods
To perform this population-based retrospective study, our clinical database was searched to find all the consecutive patients with a diagnosis of PMLBCL, homogeneously treated with a third-generation MACOP-B chemotherapy regimen, regardless they received either chemotherapy alone, immunotherapy, consolidative RT, or a combination of all these strategies. Patients treated with chemotherapy regimens other than MACOP-B were excluded. The study was approved by our institutional board and by our Ethical Committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Patients were consecutively enrolled to avoid selection bias, and all patients provided written informed consent to collect retrospectively their data. We obtained a special permission (for scientific purpose) from our Ethical Committee to collect even data of patients who were deceased or lost to follow up.
Diagnostic and staging procedures
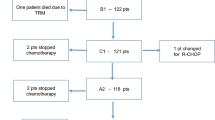
Between October 1989 and April 2010, 98 patients with de novo PMLBCL were diagnosed and subsequently treated in our Institution. Diagnostic material was obtained by supraclavicular or transthoracic lymph node biopsy, thoracotomy or mediastinoscopy. Initial clinical evaluation included physical examination, hematologic and biochemical survey, chest X-ray, computed tomography (CT) scan of neck, chest, abdomen and pelvis, and unilateral bone marrow biopsy. PET scan was also performed at baseline in all patients treated after 2001.
Disease stage was established according to the Ann Arbor staging system. Stage II indicated disease spread within contiguous thoracic, jugular or supraclavicular nodes, whereas the presence of distant, non-contiguous, involved nodes on both sides of the diaphragm was consistent with stage III. Patients with any extranodal involvement apart from mediastinal disease were categorized as stage IV. The extent of mediastinal disease was defined as mediastinal mass ratio (MMR), which was calculated by measuring the maximum single horizontal width of the mass on a standing chest radiograph, and dividing it by the maximum intrathoracic diameter. An MMR exceeding one third or a mass measuring more than 10 cm in its largest diameter as measured by CT scan was considered bulky.
Treatment protocol
All patients were treated with the MACOP-B regimen, given for 12 consecutive weeks, with leucovorin rescue after any methotrexate-containing cycle. The median number of cycles delivered was 12. Rituximab was administered every 21 days (375 mg/m2) along with chemotherapy in 57 patients (58.2%), all treated after 2001, when it became available in Italy [7].
Disease restaging, response assessment and survival analysis
Radiologic restaging was performed by total body CT scan 1 month after the end of immuno/chemotherapy, and then 3 months after the completion of RT. PET scan was done at the same timepoints, whenever available. Bone marrow biopsy was repeated only if positive at baseline. Treatment responses were categorized according to standardized response criteria [17, 18]. Nodal residues larger than 1.5 cm which have regressed by more than 75% in their major diameter were compatible with a complete response (CR), and regarded as residual scar tissue. PET negativity was corroborative of a CR.
Overall survival (OS) was calculated from diagnosis to the last follow-up or death for any cause; progression-free survival (PFS) was calculated from diagnosis to the first disease progression or death; disease-free survival (DFS) was determined in all CR patients as the time between the first documented responses and the first disease relapse, or death as a result of lymphoma or acute treatment toxicity. Survival analysis was conducted according to Kaplan-Meier’s method and log rank test was used for comparisons [19]. Demographics and patients’ characteristics were summarized by descriptive statistics and compared using χ2 test. Statistical analyses were performed with Stata11 (StataCorp LP, TX) and p-values were set at 0.05.
Results
Patients’ characteristics and disposition
The median age at presentation was 34.5 (range 15.7–69.5) years; 58 patients were females and 40 males, with a male-to-female ratio of 1:1.45. Three patients (3.1%) presented with stage I disease, 68 (69.4%) with stage II, 9 (9.2%) with stage III and 18 (18.4%) with stage IV disease, with lung, spleen and kidney involvement. B-symptoms were present in 41 (41.8%) patients; bulky disease was detected in 95 (96.9%) patients, with a superior vena cava syndrome in 43 (43.9%) patients.
Sixty-seven (68.4%) patients received mediastinal RT, 4 to 6 weeks after the completion of immuno/chemotherapy, with tumor doses ranging from 30 to 36 Gy over a 4 to 5 weeks treatment schedule, with fractions of 180 cGy/day for 5 days per week. The decision to use RT was based on era-specific institutional guidelines: it was routinely administered after chemotherapy in all patients since 1993 to 2002; before 1993, it was delivered upon physician’s discretion; after 2002, along with the use of PET in detecting potential residual masses after chemotherapy, RT was spared in those patients with a negative PET-scan and without bulky disease at onset.
Among the 57 patients who received rituximab, 37 (64.9%) underwent RT, whereas among the 41 who did not receive rituximab, RT was delivered in 30 (73.2%) patients. Eleven (11.2%) patients received chemotherapy only, and 37 (37.8%) received both immunotherapy and RT. According to the treatment received, patients were subdivided in 4 subgroups, as outlined in Table 1. Patients’ clinical characteristics are reported in Table 2.
Overall treatment response and survival
After 12 cycles of MACOP-B regimen (with or without rituximab), 61 patients out of 98 (62.2%) achieved a CR and 27 (27.6%) a partial response (PR); a stable disease (SD) was documented in one patient, and 9 showed progression (PD). Among those who were irradiated after immuno/chemotherapy, 21 patients previously in PR could convert their disease status to a CR, with no patients being with residual disease after RT. Upon completion of the scheduled treatment, 82 patients achieved a CR (83.7%) and 6 obtained a PR (6.1%), yielding an overall response rate (ORR) of 89.8%. At the time of writing, 73 (88.4%) patients who achieved a CR are still in continuous CR. Median follow-up duration for the entire cohort of patients is 7.6 years. The projected OS at 17 years for all the patients is 72%, with a PFS of 67.6% and a DFS of 88.4%, with all curves showing a plateau (Fig. 1a-c).
Overall survival (a), progression-free survival (b) and disease-free survival (c) curves plotted for the entire population on study. Subgroup survival analysis is shown underneath: overall survival (d), progression-free survival (e) and disease-free survival (f). Solid black line is for subgroup 1, dashed black line is for subgroup 2, solid grey line is for subgroup 3 and dashed black line is for subgroup 4. Vertical axis shows survival percentages
Analysis of response failures
Eleven patients (11.2%) showed a disease relapse or progression during follow-up, in any case within the first 2 years after treatment completion. Nine patients were in CR after therapy and 2 in PR. Five received a rituximab-based treatment and 6 were irradiated. Salvage therapy for these patients consisted of autologous stem cell transplantation in all but one cases, with rapid disease progression and death in 6 of them. The patient who did not receive any further treatment rapidly died of disease.
Fifteen patients died during follow-up (15.3%), 13 as a consequence of lymphoma persistence after first-line treatment or disease relapse or progression. Two patients died of a secondary neoplasm (colon carcinoma) both in a CR status and more than 10 years after the conclusion of the treatment. Although both patients belonged to subgroup 4, none of the two solid tumors developed inside a previously irradiated field.
Subgroup analysis
Clinical characteristics across the 4 subgroups were comparable, with no statistically significant differences seen at the χ2 test. Overall survival and PFS curves plotted for the four subgroups show a statistically significant difference (p = 0.0003 and p = 0.0006, respectively), although no difference among groups can be seen in terms of DFS (0.2362) (Fig. 1d-f). However, if OS curves for subgroup 1 and 2 are taken together – i.e. considering patients treated with chemotherapy or chemo + immunotherapy without any RT consolidation – no statistically significant difference can be detected (p = 0.0806), thus the discrepancy between these data does not depend either on patients’ selection or on particularly unfavorable clinical characteristics of patients in subgroup 2.
All the patients receiving RT – as a consolidation strategy after they had obtained either a CR or a PR – showed an ORR of 100%, with no residual disease being detectable after radiation. This favorable result can be appreciated both in patients receiving rituximab (subgroup 3) and in those with no exposure to the antibody (subgroup 4), with comparable OS rates in the two subgroups at 9 years (p = 0.5103).
DFS rates between subgroup 2 and 3 do not significantly differ (p = 0.55), although the number of patients in CR after treatment is substantially different. This indicates that patients in CR after chemo + immunotherapy behave similarly to those who achieve a CR after receiving mediastinal RT, suggesting that RT has a small consolidative potential in those who obtain a CR status after chemo-immunotherapy only.
Role of rituximab
When patients are subdivided according to whether they have received rituximab or not (Table 3), regardless a subsequent consolidative RT, no statistically significant differences in terms of OS and DFS (p = 0.1 and 0.19, respectively) can be observed (Fig. 2), thus indicating that the addition of the anti-CD20 monoclonal seems to have a limited impact on patients’ survival in our study population. Of note, however, a more favorable trend to better PFS and DFS is observed in those belonging to subgroup 3 compared to patients in subgroup 4, the former also receiving rituximab together with chemotherapy and RT consolidation.
Discussion
The clinical and pathological peculiarities of PMLBCL, the high chances of cure documented in literature in more than 20 years of international experience and the long disease-free life-expectancy of cured patients, have always drawn attention in finding out the most suitable first-line approach and the most convenient combination of treatment modalities, on the one hand trying to maximize the long-term clinical outcomes, while on the other reducing the potential harmful consequences of highly toxic combined treatments [20, 21].
Our monocentric experience over a period of more than 20 years ideally encompasses all the issues met by treating physicians throughout the years, and may virtually suggest some still open points which require clarification with further ad hoc studies. It consists of a series of 98 patients homogeneously treated with a weekly “third-generation” schedule, who in part also received external mediastinal RT and/or anti-CD 20 immunotherapy, depending on our institutional era-specific guidelines.
“Third-generation” regimens, have become the standard of treatment in many European institutions, following the favorable results obtained when compared with the standard CHOP regimen. Lazzarino et al. documented MACOP-B/VACOP-B superiority on CHOP both in terms of CR rates and relapse-free survival (RFS), with 73% CR rate for the former versus 36% for the latter, and a 3-years RFS of 58% versus 38% [22, 23]. A retrospective multicenter Italian experience on 138 patients appeared later again emphasized the difference between the two regimens: patients on MACOP-B/VACOP-B achieved better results than those on CHOP, in terms of complete responses and event-free survival (EFS) rates, with statistically significant difference in low and low-intermediate International Prognostic Index risk groups (into which the majority of patients with PMLBCL generally fall), whereas lacking significance in high-intermediate and high-risk disease [6, 24].
The role of the anti-CD20 monoclonal antibody, rituximab, in a context of a chemo-immunotherapy regimen, still represents a matter of debate: its role in patients with PMLBCL is less well established than in DLBCL, and most of available data derive from retrospective experiences and do not rely on appropriately powered randomized trials. On the one hand, rituximab added to CHOP has demonstrated better EFS and OS rates than CHOP alone (80% and 89% versus 47% and 69% at 5 years, respectively), as well as higher complete response rates and a significant reduction of disease progression [12, 13, 21]. However, in a study from British Columbia, a comparison of rituximab-CHOP and CHOP alone in PMLBCL patients has failed to show any clear survival advantage of the former regimen [25]; moreover, patients treated with MACOP-B/VACOP-B within the same institution showed superior outcomes over CHOP-type treatments, in terms of OS (87%, 82% and 71% for patients treated with M[V]ACOP-B, rituximab-CHOP and CHOP, respectively). Data from a recent experience have also shown that in a subset of 63 PMLBCL patients treated with rituximab-CHOP, with or without radiation, a primary induction failure unacceptably occurred in 21% of the treated patients, particularly in those with increased IPI score, advanced age and stage or multiple extranodal localizations [21, 26].
A multicenter Italian experience has demonstrated that the combination of rituximab with a “third-generation” regimen is not significantly different from rituximab-CHOP, rituximab-EPOCH (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone) or M[V]ACOP-B therapy alone in terms of EFS: this means that the addition of rituximab may improve outcomes if a CHOP-like regimen is used as induction therapy, but it confers little benefit if it is added to a more intense treatment strategy [11]. Data from our series indicate no substantial improvement from the addition of rituximab to MACOP-B (subgroups 1 and 2, Table 3 and Fig. 2) in terms of OS (p = 0.0806 between subgroups 1 and 2; p = 0.10 between R-MACOP-B and MACOP-B only). The better OS and PFS outcomes seen in subgroups 3 and 4, therefore, presumably rely more on the use of RT than of rituximab.
It is clear, in fact, that external mediastinal RT, delivered after immuno/chemotherapy as a consolidative strategy, still impacts markedly on global survival and on CR rates, being able to convert PRs to CRs in the majority of cases [6, 14, 27]. In a retrospective multinational study on 426 untreated patients, data from patients treated with CHOP/CHOP-like, M[V]ACOP-B regimens and high-dose therapy/autotransplantation were compared: CR rates obtained upon completion of the three chemotherapy arms were similar (49%, 51% and 53%, respectively), but they significantly differed after mediastinal RT (67%, 84% and 77%, respectively) and in terms of 10-years OS (44%, 71% and 77%, respectively) and PFS (35%, 67% and 78%, respectively) [28].
Concerns exist, however, regarding the incidence of second malignancies and late side effects after chest irradiation, mainly on the cardiovascular system [29,30,31]: risks and benefits should be thoroughly balanced at the moment of treatment planning, although more experience is required in identifying patients in whom RT can be spared. Dunleavy et al. have recently demonstrated that the use of a dose-adjusted chemotherapy based on EPOCH and containing rituximab could obviate the need for radiotherapy in PMLBCL patients, with EFS and OS rates of 93% and 97%, respectively, and no relapsing patients over a median follow-up of more than 5 years [32]. However, a post-chemotherapy PET evaluation may represent a tool to guide the RT usage, reasonably sparing mediastinal irradiation in those who show a PET-negativity – i.e. Deauville score 1–2, and possibly 3 – after the completion of the chemo/immunotherapy induction [21, 33, 34]. Data in this sense are from Savage et al., where the PET-guided RT approach was applied in rituximab-CHOP-treated patients [35]. Similarly, we have recently published an experience from our institution involving 74 patients – belonging to an independent data base from the one we have described in this paper – all treated with a rituximab-MACOP-B induction therapy [36]. In both reported series, patients with a PET-documented CR were observed, whereas those with a positive PET-scan received consolidative RT [35, 36]. OS and time-to-progression seen in the first series were 89% and 83% at 5 years, respectively, whereas in our recent experience OS and DFS rates were 82% and 91% at 10 years, respectively, without any significant difference between irradiated and not-irradiated patients [35, 36]. A similar trend can be appreciated after comparing the DFS rates for subgroups 2 and 3 in the present study (90.0% and 94.4%, respectively, at 5 years, p = 0.55).
PET functional parameters measured at disease diagnosis may also represent useful tools in order to determine which patients will do worse with standard treatment schedules, therefore requiring a more intense approach since the beginning [37]. In addition, the detection of a truncating deletion of the NFKBIE gene, which encodes IκBε, a negative feedback regulator of NF-κB, and which is frequently observed in PMLBCL patients, may help segregate patients with a more aggressive disease and with therapy refractoriness [38]. It has been demonstrated that NFKBIE-deleted patients, in fact, display inferior outcomes compared to wild-type ones, but apparently benefit greatly from RT and rituximab [38].
Conclusions
In conclusion, data we have gathered over a 20-year experience in the treatment of PMLBCL patients clearly indicate that: 1) a “third-generation” chemotherapy regimen such as MACOP-B is feasible and easily deliverable on an outpatient basis; 2) no statistically significant difference is seen in our series between those who received rituximab and those who did not, although a trend to better DFS rates is appreciated in patients treated with chemo + immunotherapy and RT; 3) radiotherapy in this context remains a powerful strategy to convert PRs to CRs, but it may be spared in patients obtaining a PET-documented CR after chemo-immunotherapy without any harmful prognostic consequences. Hopefully, future prospective trials – such as the ongoing International Extranodal Lymphoma Study Group 37 study – will further investigate the role of consolidation radiation therapy in PET-negative patients after induction treatment.
Abbreviations
- CHOP:
-
Cyclophospamide, doxorubicin, vincristine and prednisone
- CHT:
-
Chemotherapy
- CR:
-
Complete response
- CT:
-
Computed tomography
- DFS:
-
Disease free survival
- DLBCL:
-
Diffuse large B-cell lymphoma
- EFS:
-
Event-free survival
- EPOCH:
-
Etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone
- LDH:
-
Lactate dehydrogenase
- MACOP-B:
-
Methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone
- MMR:
-
Mediastinal mass ratio
- NF-κB:
-
nuclear factor κB
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PET:
-
Positron emission tomography
- PFS:
-
Progression free survival
- PMLBCL:
-
Primary mediastinal large B-cell lymphoma
- PR:
-
Partial response
- REAL:
-
Revised European American Lymphoma
- RFS:
-
Relapse-free survival
- RT:
-
Radiotherapy
- SD:
-
Stable disease
- SVC:
-
Superior vena cava
- VACOP-B:
-
Etoposide, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone
References
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92.
Banks PM, Warnke RA. Mediastinal (thymic) large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization classification of tumors. Pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 175–6.
Rodríguez J, Conde E, Gutiérrez A, García JC, Lahuerta JJ, Varela MR, et al. Primary mediastinal large cell lymphoma (PMBL): frontline treatment with autologous stem cell transplantation (ASCT). The GEL-TAMO experience. Hematol Oncol. 2008;26:171–8.
Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab compared with CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large B-cell lymphoma: a randomized controlled trial by the Mabthera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Todeschini G, Secchi S, Morra E, Vitolo U, Orlandi E, Pasini F, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long term results from a retrospective multicenter Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Brit J Cancer. 2004;90:372–6.
Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large B-cell lymphoma. Ann Intern Med. 1985;102:596–601.
Bertini M, Orsucci L, Vitolo U, Levis A, Todeschini G, Meneghini V, et al. Stage II large B-cell lymphoma with sclerosis treated with MACOP-B. Ann Oncol. 1991;2:733–7.
Zinzani PL, Bendandi M, Frezza G, Gherlinzoni F, Merla E, Salvucci M, et al. Primary mediastinal B-cell lymphoma with sclerosis: clinical and therapeutic evaluation of 22 patients. Leuk Lymphoma. 1996;21:311–6.
Zinzani PL, Martelli M, Poletti V, Vitolo U, Gobbi PG, Chisesi T, et al. Practice guidelines for the management of extranodal non-Hodgkin’s lymphomas of adult non-immunodeficient patients. Part I: primary lung and mediastinal lymphomas. A project of the Italian Society of Hematology, the Italian Society of experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica. 2008;93:1364–71.
Zinzani PL, Stefoni V, Finolezzi E, Brusamolino E, Cabras MG, Chiappella A, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma. 2009;9:381–5.
Vassilakopoulos TP, Pangalis GA, Katsigiannis A, Papageorgiou SG, Constantinou N, Terpos E, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist. 2012;17:239–49.
Rieger M, Österborg A, Pettengell R, White D, Gill D, Walewski J, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–70.
Zinzani PL, Martelli M, Bendandi M, De Renzo A, Zaccaria A, Pavone E, et al. Primary mediastinal large B-cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica. 2001;86:187–91.
Zinzani PL, Martelli M, Magagnoli M, Pescarmona E, Scaramucci L, Palombi F, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood. 1999;94:3289–93.
Mazzarotto R, Boso C, Vianello F, Aversa MS, Chiarion-Sileni V, Trentin L, et al. Primary mediastinal large B-cell lymphoma: results of intensive chemotherapy regimens (MACOP-B/VACOP-B) plus involved field radiotherapy on 53 patients. A single institution experience. Int J Radiat Oncol Biol Phys. 2007;68:823–9.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardized response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–9.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Zinzani PL, Piccaluga PP. Primary mediastinal DLBCL: evolving biologic understanding and therapeutic strategies. Curr Oncol Rep. 2011;13:407–15.
Zinzani PL, Broccoli A. Optimizing outcomes in primary mediastinal B-cell lymphoma. Hematol Oncol Clin North Am. 2016;30:1261–75.
Lazzarino M, Orlandi E, Paulli M, Boveri E, Morra E, Brusamolino E, et al. Primary mediastinal B-cell lymphoma with sclerosis: an aggressive tumor with distinctive clinical and pathologic features. J Clin Oncol. 1993;11:2306–13.
Lazzarino M, Orlandi E, Paulli M. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol. 1997;15:1646–53.
The International non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17:123–30.
Soumerai JD, Hellmann MD, Feng Y, Sohani AR, Toomey CE, Barnes JA, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55:538–43.
De Sanctis V, Finolezzi E, Osti MF, Grapulin L, Alfò M, Pescarmona E, et al. MACOP-B and involved-field radiotherapy is an effective and safe therapy for primary mediastinal large B cell lymphoma. Int J Radiat Oncol Biol Phys. 2008;72:1154–60.
Zinzani PL, Martelli M, Bertini M, Gianni AM, Devizzi L, Federico M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87:1258–64.
Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–33.
Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75.
Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112:1688–96.
Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408–16.
Meignan M, Barrington S, Itti E, Gallamini A, Haioun C, Polliack A. Report on the 4th International Workshop on positron emission tomography in lymphoma held in Menton, France, 3-5 October 2012. Leuk Lymphoma. 2014;55:31–7.
Martelli M, Ceriani L, Zucca E, Zinzani PL, Ferreri AJ, Vitolo U, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014;32:1769–75.
Savage K, Yenson PR, Shenkier T, et al. The outcome of primary mediastinal large B-cell lymphoma (PMBCL) in the R-CHOP treatment era. Blood (ASH annual meeting abstracts) 2012;120:Abstract 303.
Zinzani PL, Broccoli A, Casadei B, Stefoni V, Pellegrini C, Gandolfi L, et al. The role of rituximab and positron emission tomography in the treatment of primary mediastinal large B-cell lymphoma: experience on 74 patients. Hematol Oncol. 2015;33(4):145–50.
Ceriani L, Martelli M, Zinzani PL, Ferreri AJ, Botto B, Stelitano C, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood. 2015;126:950–6.
Mansouri L, Noerenberg D, Young E, Mylonas E, Abdulla M, Frick M, et al. Frequent NFKBIE deletions are associated with poor outcome in primary mediastinal B-cell lymphoma. Blood. 2016;128:2666–70.
Acknowledgments
None.
Funding
None.
Availability of data and materials
Not applicable.
Authors’ contributions
AB, BC, VS, CP, FQ, LT, AM, MM, LA and PLZ made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; AB, LA and PLZ have been involved in drafting the manuscript or revising it critically for important intellectual content. All the authors have read and approved this manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by our institutional review board (Comitato Etico del Policlinico S.Orsola-Malpighi di Bologna) and written informed consent was obtained from each patient.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Broccoli, A., Casadei, B., Stefoni, V. et al. The treatment of primary mediastinal large B-cell lymphoma: a two decades monocentric experience with 98 patients. BMC Cancer 17, 276 (2017). https://doi.org/10.1186/s12885-017-3269-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-017-3269-6