Abstract
Background
High-mobility group protein box1 (HMGB1) is a pivotal factor in the development and progression of many types of tumor. Its role in hepatocellular carcinoma (HCC), and especially its correlation with intratumoral and peritumoral macrophage infiltration, remains obscure. We analyzed the potential roles and prognostic value of HMGB1 and explored the correlation between HMGB1 and macrophage infiltration in HCC using clinical samples.
Methods
We reviewed clinicopathological and follow-up data on a cohort of 149 patients with HCC complicated with Hepatitis B-related cirrhosis. We measured the expression of HMGB1 and CD68 in tumoral and peritumoral liver tissues after curative resection and assessed the impacts of the tumor-associated macrophage (TAM) count and HMGB1 expression on clinicopathologic characteristics, overall survival (OS), and recurrence-free survival (RFS).
Results
Ninety-four of the patients had elevated tumoral HMGB1 expression and 59 of the patients had elevated peritumoral HMGB1 expression, compared to only 4 patients with elevated peritumoral HMGB1 expression in 36 pateints with Hepatitis B virus (HBV)-negative HCC without liver cirrhosis (p < 0.001). The peritumoral HMGB1 expression levels were correlated with tumor invasiveness, BCLC stage, and recurrence. The degree of TAM infiltration was higher in peritumoral tissues with high HMGB1 expression than in peritumoral tissues with low HMGB1 expression (p < 0.001). There was no significant difference in TAM infiltration between tumoral tissues with high and low HMGB1 expression. Kaplan-Meier analysis showed that intratumoral HMGB1 overexpression was associated with poor OS, but not with RFS. High peritumoral HMGB1expression and TAM count, which correlated positively with tumor size and BCLC stage, were independent prognostic factors for OS (p < 0.001 and p = 0.017, respectively) and RFS (p = 0.002 and p = 0.024, respectively). Multivariate analyses indicated peritumoral HMGB1 expression (p = 0.014) and TAM count (p = 0.037), as well as tumor differentiation (p = 0.026), to be independent significant prognostic factors for RFS.
Conclusions
High HMGB1 expression in peritumoral liver tissues correlated with peritumoral macrophage infiltration and had prognostic value in HCC, suggesting that peritumoral HMGB1 might show promise as a new biomarker to predict HCC progression.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC), the fifth most common cancer worldwide, ranks as the third leading cause of cancer death globally [1, 2]. Although surgical resection and liver transplantation are the main modalities of curative treatment to provide long-term survival for patients with HCC, a high recurrence rate after surgery is a major problem [3]. Despite progress in molecular biology and cancer therapy in recent years, the overall prognosis for HCC remains dismal, which is mainly attributed to the high incidences of local recurrence, distant metastasis, and therapy resistance [4]. Recently, the tumor microenvironment, which plays an important role in the initiation, progression, recurrence, and metastasis of various tumors, has been intensively studied. Changes of the tumor microenvironment have been closely correlated with cancer-mediated inflammation. Furthermore, an inflammatory response is detectable in tumors that is not causally related to inflammation [5].
High-mobility group box 1 (HMGB1), an evolutionarily conserved, chromatin-binding protein [6, 7], is normally located in the nucleus, where it acts as a DNA chaperon by regulating transcription, replication, recombination, repair, and genome stability [8]. In response to some stimuli, such as hypoxia, it can be released into the extracellular environment [9]. Necrotic cells, particularly those derived from cancer tissues, passively release HMGB1, mediating local inflammation and cancer development [10]. HMGB1 overexpression has been observed in the cells of some cancers such as breast cancer [11], colon cancer [12], gastrointestinal stromal tumors [13], and liver cancer [9]. Through the binding with its receptors, such as RAGE or TLRs, HMGB1 has been associated with tumor-cell survival, progression, and metastasis [14, 15]. Extracellular HMGB1 displays cytokine activity and can promote inflammation by activating macrophages, which represent the main type of inflammatory cells infiltrating tumors [16, 17].
Tumor-associated macrophages (TAMs) are mainly polarized towards an M2 phenotype, which has been associated with angiogenesis, metastasis, and poor prognosis [18]. Accumulating evidence has demonstrated an association between TAM density and unfortunate prognosis in HCC [19–21]. An abundance of CD68 macrophage infiltration in peritumoral liver tissues, but not in tumors, was previously associated with poor prognosis in HCC [22]. Exposure of macrophages to HMGB1 exerts proinflammatory effects by inducing the TLR4-dependent and CD14-dependent release some inflammatory cytokines such as monocyte chemotactic protein 1, interferon gamma-induced protein 10, and macrophage inflammatory protein 1α [23–25].
HMGB1 is associated with clinicopathologic features and has prognostic significance for overall and disease-free survival in patients with HCC after curative hepatectomy [26, 27]. To the best of our knowledge, there are no previous reports of an association between HMGB1 expression and TAM infiltration in tumors and peritumoral tissues in HCC. We therefore aimed to ascertain whether HMGB1 expression is correlated with TAM infiltration in tumors and peritumoral tissues and to determine whether HMGB1 expression correlates with prognosis in patients with HCC.
Methods
Patients, specimens, and follow-up
We collected tumoral and peritumoral specimens from 149 patients (82 men and 67 women; mean age: 67.5 years) with pathologically confirmed HCC complicated with cirrhosis who underwent curative liver resection at our institute (Liver Cancer Institute, Zhongshan Hospital, Fudan University). The median tumor size was 4.2 cm (range: 1.5–11.0 cm). Another 36 patients with HBV-negative HCC without liver cirrhosis were also collected to examine the expression of HMGB1 in peritumoral liver tissues. An experienced pathologist examined haematoxylin and eosin-stained sections from each tumor sample to confirm the histological diagnosis and assess the tumor content. Histological diagnoses including the tumor differentiation and encapsulation were made according to the guidelines proposed by the World Health Organization. Clinical profiles and follow-up records after surgical resection were obtained from the medical records of Zhongshan Hospital of Fudan University.
The follow-up procedures were described in our previous reports [28]. Briefly, the patients were monitored by abdominal ultrasonography, serum a-fetoprotein (AFP), and chest radiography with an interval of 2–6 months according to the postoperative time. If recurrence was suspected, computed tomography scanning or magnetic resonance imaging was performed immediately. Treatment modalities after relapse were administered according to a uniform guideline. Overall survival (OS) and recurrence-free survival (RFS) were defined as the interval between surgery and death or disease recurrence, respectively. If recurrence was not diagnosed, the patients were censored on the date of death or last follow-up.
The study was approved by the Qilu Hospital of Shandong University Ethics Committee, and written informed consent for recording and analysis of data was obtained from all patients.
Immunohistochemical staining and macrophage quantification
HMGB1 expression in paired tumoral and paratumoral liver tissues from the patients was examined by immunohistochemistry. We used CD68 to mark the macrophages in the tissues. Using the semiquantitative scale described previously [29], the HMGB1protein expressions were scored by multiplying the proportion of positive cells and intensity. The proportion of positive cells ranked as follows: 1(≤25 %), 2 (26–50 %), 3 (51–74 %), and 4 (≥75 %). The intensity of nuclear or cytoplasmic staining was evaluated as follows; no staining/background of negative controls (score = 1), weak staining detectable above background (score = 2), moderate staining (score = 3), and intense staining (score = 4). The index was obtained by multiplying the percentage and intensity scores, the score ≤ 8 was defined as low expression, and the score >8 was defined as high expression. Meanwhile, the HMGB1protein expression and CD68+ cells density were also quantified with the method described in our previous study [28]. Under high-power magnification (200×), photographs of two representative fields of each punch were captured by a computerized image system composed of a Leica CCD camera DFC 500 connected to a Leica DM IRE2 microscope and Leica Qwin Plus v3 software (Leica Microsystems Imaging Solution, Cambridge, UK). We measured the area of positive staining in pixels using Image-Pro Plus v6.2 software (Media Cybernetics, Bethesda, MD). The expression of HMGB1protein and the densities of CD68 staining were expressed as the ratio of the positively stained area to the total area of each photograph.
Statistical analysis
We performed all statistical analyses using SPSS16.0 for Windows (SPSS). Correlations between clinicopathologic characteristics and HMGB1 expression were assessed using the χ2 test. We used the Pearsonχ2 test or the Fisher exact test to compare qualitative variables and the Student t test or the Spearman correlation test to compare quantitative variables. We used a Kaplan-Meier analysis to determine the survival curves and the log-rank test to compare the survival curves between subgroups. In the survival analyses, we used death from disease and recurrence/metastasis as the endpoints for OS and RFS, respectively. We tested the associations between the variables and survival using univariate and multivariate analyses with the Cox proportional hazard model. The difference between survival curves was analyzed by the log rank test. p < 0.05 was considered to be statistically significant.
Results
Correlation between HMGB1 expression and clinicopathologic characteristics
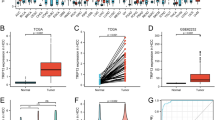
HMGB1 immunohistochemical expression showed predominant cytoplasmic staining and little nuclear staining in the tumoral and paratumoral liver tissues (Fig. 1). High HMGB1 expression was detected in 94 (63.1 %) of the tumor tissues and in 59 (39.6 %) of the paired paratumoral tissues. The correlations between clinicopathologic characteristics and HMGB1 expression in the tumoral and paratumoral liver tissues are shown in Table 1. High peritumoral HMGB1 expression was positively correlated with recurrence (p = 0.001), tumor encapsulation (p = 0.019) (Fig. 2), and advanced BCLC stage (p = 0.036). In contrast, intratumoral HMGB1 expression had no statistically significant correlation with tumor encapsulation (Table 2).
Peritumoral HMGB1 expression was upregulated in HBV-positive HCC, but not in HBV-negative HCC
We examined the expression of HMGB1 in peritumoral liver tissues from 36 patients with HBV-negative HCC without liver cirrhosis. Only four of those samples displayed high HMGB1 expression, while the other 32 displayed low HMGB1 expression. Overall, the peritumoral expression of HMGB1 was lower in the patients with HBV-negative HCC than in the patients with HBV-positive HCC (p = 0.004), which might be attributed to the background of cirrhosis (Additional file 1: Figure S1).
HMGB1 expression was associated with peritumoral TAM infiltration
Because HMGB1 could activate human peripheral blood monocytes and induce the production of proinflammatory cytokines, we examined whether macrophage infiltration was associated with HMGB1expression in tumoral or peritumoral liver tissues. As shown in Fig. 3, there was significantly more TAM infiltration in the peritumoral liver tissues with high HMGB1 expression than in the peritumoral liver tissues with low HMGB1 expression. TAM infiltration was high in all of the tumor tissues, however, regardless of the HMGB1 expression level, and there was no significant difference in TAM infiltration in the tumor tissues based on HMGB1 expression. As shown in Fig. 3, HMGB1 overexpression was correlated positively with CD68+ macrophage density in the peritumoral liver tissues (p < 0.001).
Correlation of HMGB1 expression with CD68+ macrophage infiltration in peritumoral and tumoral liver tissues in patients with HCC. Two-demissional plot showed HMGB1 overexpression correlated positively with CD68+ macrophage density in peritumoral liver tissue (a), while modest correlation was found between HMGB1 expression and CD68+ macrophage density in tumor tissues (b)
Prognostic relevance of HMGB1 expression and TAM infiltration in HCC
We examined whether the expression of HMGB1 in tumoral and peritumoral liver tissues had value in predicting clinical outcomes or prognosis in HCC. The Kaplan-Meier analysis (log-rank test) showed that HMGB1 overexpression in both the peritumoral liver tissues and the tumor tissues was associated with worse OS (p < 0.001 and p = 0.028, respectively). Only HMGB1 overexpression in the peritumoral liver tissues was associated with worse RFS, however (p = 0.002). The TAM count in the peritumoral liver tissues was significantly associated with both OS and RFS (p = 0.017 and p = 0.024, respectively), whereas the TAM count in the tumor tissues was associated with neither OS nor RFS (Fig. 4). We also performed the analysis of prognosis and HMGB1. 149 patients were categorized into two groups according to their survival time. Of the two groups, good prognosis,who survived longer than median survival time(MST), were involved in group One, and another group was matched with bad prognosis who survived shorter than MST. We compared the HMGB1 level in two groups. Peritumoral HMGB1 expression in group of bad prognosis was significantly higher than those in group of good prognosis, while intratumoral HMGB1 expression in group of bad prognosis was modestly higher than those in group of good prognosis (Fig. 5).
Cumulative overall survival (OS) and recurrence-free survival (RFS) curves of patients with high and low expression of HMGB1 (panels a, b, e, and f) and high and low densities of CD68+ cell infiltration (panels c, d, g, and h) in tumoral and peritumoral liver tissues. In peritumoral liver tissues, the expression of HMGB1 and the density of CD68+ cells were both associated with poor OS and RFS (panels a, c, e, and g). In tumor tissues, the expression of HMGB1 was associated with poor OS but not RFS (panels b and f), while the density of CD68+ cells could not discriminate patients with different OS or RFS (panels d and h)
For further analysis, we investigated whether the HMGB1 expression in the peritumoral or tumoral liver tissues was an independent prognostic variable. We constructed a Cox proportional hazards model using established prognostic factors, HMGB1 expression, and TAM count. The univariate analysis revealed that tumor size, tumor differentiation, BCLC stage, peritumoral and intratumoral HMGB1 expression, and peritumoral TAM count, but not intratumoral TAM count, were independent prognostic factors for OS. In addition, patients with high peritumoral HMGB1 expression had a worse RFS prognosis than those with low peritumoral HMGB1 expression (p = 0.002). Univariate analyses also indicated tumor size, tumor differentiation, BCLC stage, peritumoral HMGB1 expression, and peritumoral TAM count to be prognostic factors for RFS.
Multivariate analyses indicated peritumoral HMGB1 expression, peritumoral TAM count, and BCLC stage to be independent prognostic factors for OS. In addition, multivariate analyses indicated peritumoral HMGB1 expression, peritumoral TAM count, and tumor differentiation to be independent prognostic factors for RFS. The multivariate analyses showed no significant association between intratumoral TAM count and either RFS or OS (Table 3).
Discussion
Previously, we found that the density of peritumoral CD68+ cells was associated with poor RFS and OS [28]. Therefore, we further analyzed the potential roles and prognostic value of HMGB1 and explored the correlation between HMGB1 and macrophage infiltration in patients with HBV-related HCC complicated with liver cirrhosis. High HMGB1 expression in peritumoral liver tissues was correlated with peritumoral macrophage infiltration, was significantly associated with recurrence, incomplete tumor encapsulation, and advanced BCLC stage, and predicted poorer survival for patients with HCC with liver cirrhosis after curative hepatectomy.
HMGB1 is constitutively expressed in the nucleus of tumor cells and can be released by inflammatory cells and by tumor cells [30]. Once released, HMGB1 works as a damage-associated molecular pattern molecule, binding to receptors such as RAGE and TLRs [31]. There is a growing body of evidence that HMGB1 plays pivotal roles in the development and progression of many types of tumors, including HCC [32]. Most of that evidence is based on the direct effects of HMGB1 on tumor cells [33]. The contribution of immune cells in the tumor microenvironment, and particularly the correlation with macrophages, remains elusive. In the immune system, HMGB1 increases the secretion of inflammatory cytokines (IL-1β, IFN-γ, and TNF-α) [34] and acts as a late inflammatory cytokine by activating macrophages in response to LPS [35]. HMGB1 could enhance tumor-cell invasion and promote angiogenesis by supporting the protumoral functions of TAMs by a RAGE-dependent mechanism [36]. Thus, HMGB1 could act as an autocrine/paracrine tumor-growth factor in cancer. A compelling body of evidence has shown the contribution of HMGB1 to the malignant progression of HCC, and high HMGB1 expression predicts a poor prognosis for patients with HCC [27, 37]. To the best of our knowledge, an association between HMGB1 expression and TAM infiltration has not been reported in HCC.
We found that the HMGB1 expression in peritumoral and tumoral liver tissues was correlated with the TAM count and with several conventional clinicopathological parameters. It is therefore worth determining whether HMGB1 expression and TAM count are accurate and reliable prognostic factors in HCC. HMGB1 expression showed predominant cytoplasmic staining and sparse nuclear staining in tumor and paratumoral liver tissues. HMGB1 expression in the peritumoral liver tissues was strongly correlated with higher histological grades and tumor invasiveness, whereas HMGB1 expression in the tumors was only correlated marginally with recurrence and higher BCLC stage. High HMGB1 expression in the peritumoral liver tissues was correlated with peritumoral macrophage infiltration, whereas that in the tumors was not.
Jiang et al. found that HMGB1 overexpression in tumor tissues, rather than that in paratumoral and normal tissues, was correlated with advanced TNM stage, vascular invasion, and capsule invasion [27]. Our results also demonstrated a correlation between HMGB1 overexpression in HCC tumor tissues and poor OS. In contrast to previous results, however, our study also indicated HMGB1 overexpression in paratumoral tissues that was correlated positively with macrophage infiltration and negatively survival. The fact that our study included only patients with HCC complicated with liver cirrhosis might account for the discrepancy with other studies.
HMGB1 acts as a molecular link between hepatocyte death and liver fibrogenesis [38]. Its release has been observed from the hepatocytes of patients suffering from various kinds of liver diseases [39] and was involved in the activation of hepatic stellate cells, which increases extracellular matrix overproduction [40]. Moreover, the serum HMGB1 concentration increased significantly in chronic CCl4 intoxication-induced hepatic fibrogenesis [41]. We identified peritumoral HMGB1 overexpression in only 11 % of patients with HBV-negative HCC without liver cirrhosis, probably because of the close association between HMGB1 expression and liver fibrosis, suggesting that HCC and liver fibrosis are both influenced by HMGB1-mediated macrophage stimulation. Further studies are warranted to investigate of the role and regulation of HMGB1 in liver cirrhosis and HCC.
Conclusions
In summary, we observed high HMGB1 expression levels in peritumoral liver tissues correlated with peritumoral macrophage infiltration. Peritumoral HMGB1 expression had prognostic value in HCC with cirrhosis, suggesting that peritumoral HMGB1 might show promise as new biomarker to predict HCC progression and potential therapeutic targets in HCC and liver fibrosis.
Abbreviations
- AFP:
-
A-fetoprotein
- BCLC:
-
Barcelona-clinic liver cancer
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HMGB1:
-
High-mobility group box 1
- MTS:
-
Median survival time
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- TAMs:
-
Tumor-associated macrophages
References
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76.
Rampone B, Schiavone B, Confuorto G. Current management of hepatocellular cancer. Curr Oncol Rep. 2010;12(3):186–92.
Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–59.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Read CM, Cary PD, Crane-Robinson C, Driscoll PC, Norman DG. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 1993;21(15):3427–36.
Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12(4):1311–9.
Liu Y, Prasad R, Wilson SH. HMGB1: roles in base excision repair and related function. Biochim Biophys Acta. 2010;1799(1–2):119–30.
Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55(6):1863–75.
Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer. 2013;49(3):741–51.
Flohr AM, Rogalla P, Meiboom M, Borrmann L, Krohn M, Thode-Halle B, Bullerdiek J. Variation of HMGB1 expression in breast cancer. Anticancer Res. 2001;21(6A):3881–5.
Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, Joos S, Zornig M. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55(2):234–42.
Choi YR, Kim H, Kang HJ, Kim NG, Kim JJ, Park KS, Paik YK, Kim HO, Kim H. Overexpression of high mobility group box 1 in gastrointestinal stromal tumors with KIT mutation. Cancer Res. 2003;63(9):2188–93.
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14(7–8):476–84.
Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17(3):189–201.
Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol. 2008;20(5):518–23.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–73.
Roderfeld M, Rath T, Lammert F, Dierkes C, Graf J, Roeb E. Innovative immunohistochemistry identifies MMP-9 expressing macrophages at the invasive front of murine HCC. World J Hepatol. 2010;2(5):175–9.
Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, Zheng L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381–9.
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37.
Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. International journal of cancer Journal international du cancer. 2009;125(7):1640–8.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(16):2707–16.
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7.
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–70.
Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98.
Tang D, Kang R, Livesey KM, Zeh 3rd HJ, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal. 2011;15(8):2185–95.
Jiang W, Wang Z, Li X, Fan X, Duan Y. High-mobility group box 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Pathology oncology research : POR. 2012;18(2):293–8.
Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L, Wang WQ, Zhang QB, Wu WZ, Wang L, Fan J, et al. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS One. 2013;8(3):e59771.
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5.
Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010;107(28):12611–6.
Tang D, Kang R, Zeh 3rd HJ, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799(1–2):131–40.
Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31(3):334–41.
Kang R, Zhang Q, Zeh 3rd HJ, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(15):4046–57.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51.
Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA binding and cytokine. J Endotoxin Res. 2001;7(4):315–21.
Rojas A, Delgado-Lopez F, Perez-Castro R, Gonzalez I, Romero J, Rojas I, Araya P, Anazco C, Morales E, Llanos J. HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumour biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;37(3):3321–9.
Liu F, Zhang Y, Peng Z, Gao H, Xu L, Chen M. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135.
Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6(8):1062–74.
Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, Li N, Liu HB, Wang H, Fan XG. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21.
Kao YH, Lin YC, Tsai MS, Sun CK, Yuan SS, Chang CY, Jawan B, Lee PH. Involvement of the nuclear high mobility group B1 peptides released from injured hepatocytes in murine hepatic fibrogenesis. Biochim Biophys Acta. 2014;1842(9):1720–32.
Choi HS, Kang JW, Lee SM. Melatonin attenuates carbon tetrachloride-induced liver fibrosis via inhibition of necroptosis. Translational research: the journal of laboratory and clinical medicine. 2015;166(3):292–303.
Acknowledgements
The specimens and clinical data used in this study were kindly provided by Liver Cancer Institute, Zhongshan Hospital, Fudan University.
Funding
This work was supported by the National Natural Science Foundation of China, No. 81401972 and Natural Science Foundation of Shandong Province, NO. ZR2015HM063.
Availability of data and materials
The data should not be shared due to patients’ privacy consent.
Authors’ contributions
ZLZ and RDJ conceived and designed the study. Experiments were performed by QBZ, QAJ, HW, DS, CXH, and RDJ. Data were analyzed by RDJ, QAJ and QBZ. The first two authors (QBZ and QAJ) contributed equally to this work. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
“Not applicable” in this section.
Ethics approval and consent to participate
The study was approved by the Qilu Hospital Research Ethics Committee. All participants gave written informed consent in accordance with the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Additional file 1: Figure S1.
Immunohistochemical staining demonstrated that peritumoral expression of HMGB1 was higher in the patients with HBV-positive HCC (A, B) than in the patients with HBV-negative HCC (C, D). (TIF 16359 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, QB., Jia, Qa., Wang, H. et al. High-mobility group protein box1 expression correlates with peritumoral macrophage infiltration and unfavorable prognosis in patients with hepatocellular carcinoma and cirrhosis. BMC Cancer 16, 880 (2016). https://doi.org/10.1186/s12885-016-2883-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2883-z