Abstract
Background
The phase 3 MPACT trial in patients with metastatic pancreatic cancer demonstrated superior efficacy of nab-paclitaxel (nab-P) + gemcitabine (Gem) vs Gem monotherapy for all endpoints examined including overall survival, the primary endpoint. In the MPACT trial, patients were treated until progressive disease (PD) or unacceptable toxicity. The current exploratory analysis investigated outcomes of patients from the MPACT trial who were treated until PD, in order to understand how to maximize treatment benefit from nab-P + Gem.
Methods
The trial design has been described in detail previously. Progressive disease was determined by the investigator on the basis of radiological imaging.
Results
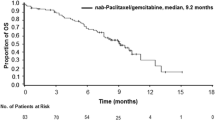
Among patients who were treated until PD, overall survival was significantly longer for those who received nab-P + Gem vs Gem (median, 9.8 vs 7.5 months; P < 0.001). Independently assessed progression-free survival and overall response rate were significantly greater among patients in the treatment-to-PD cohort who received nab-P + Gem compared with Gem (P < 0.001 for each). Although not compared statistically, patients who were treated until PD received greater treatment exposure and experienced more favourable efficacy than the intent-to-treat population of the MPACT trial. Among patients who were treated with nab-P + Gem until PD, > 50 % went on to receive a subsequent therapy. The safety profile for patients treated until PD was similar to what was reported in the overall MPACT trial.
Conclusion
The nab-P + Gem regimen is an active first-line treatment option; most patients were treated until PD, and this exposure was associated with improved efficacy outcomes. Prolonged first-line treatment exposure and ability to receive subsequent therapies likely contributed to the improved survival among these patients. Our data highlight the importance of managing adverse events and indicate that patients should be treated until PD when possible.
Trial registration
ClinicalTrials.gov NCT00844649 (MPACT trial); Registration date of this prospective phase III trial: February 13, 2009; current exploratory subanalysis was conducted retrospectively.
Similar content being viewed by others
Background
Worldwide pancreatic cancer mortality and incidence rates are nearly equal [1]. In the United States and Europe, pancreatic cancer is the fourth leading cause of cancer-related mortality, with a 5-year survival rate of 7 % to 8 % among patients with all disease stages [2–4]. Surgical resection offers the only curative treatment for pancreatic cancer; however, only 15 % to 20 % of patients are candidates for surgery at diagnosis [5]. Even when an R0 resection is achieved, many patients will relapse within 2 years, and it is likely that distant micrometastases have already been established in the ≈ 15 % to 20 % of patients believed to be surgery candidates [6, 7]. According to the Surveillance, Epidemiology, and End Results Program, 52 % of patients with pancreatic cancer are diagnosed with metastatic disease, which portends a 2.6 % 5-year survival rate [4].
At the metastatic stage, the goals of treatment are to palliate symptoms and prolong survival [7]. Since the phase 3 trial nearly 20 years ago [8] that led to the approval of gemcitabine (Gem), numerous phase 3 trials of Gem combination regimens have failed to demonstrate a clinically and statistically significant survival benefit compared with Gem monotherapy in patients with metastatic pancreatic cancer [9–16]. Recently, 2 regimens, FOLFIRINOX (folinic acid + 5-fluorouracil [5-FU] + irinotecan + oxaliplatin) and nab-paclitaxel (nab-P) + Gem, demonstrated significantly longer survival compared with Gem alone [17–19]. The phase 3 MPACT trial (ClinicalTrials.gov NCT00844649) demonstrated superior efficacy of nab-P + Gem compared with Gem alone for all trial endpoints, including the primary endpoint, overall survival (OS; median, 8.7 vs 6.6 months; hazard ratio [HR], 0.72; P < 0.001) in patients with metastatic pancreatic cancer and Karnofsky performance status ≥ 70 [18, 19]. In the MPACT trial, grade ≥ 3 adverse events (AEs) were effectively managed by dose reductions and delays.
Although results from the phase 3 PRODIGE and MPACT trials were encouraging [17, 19], the regimens are not recommended for all patients with metastatic pancreatic cancer. A retrospective analysis found that 75 % of real-world patients with metastatic pancreatic cancer did not meet the PRODIGE trial inclusion criteria, with performance status, age, and elevated bilirubin levels being the main reasons for ineligibility [20]. The inclusion criteria of the MPACT trial [18] allowed for nab-P + Gem to be administered to a wider range of patients, including older patients or those with poorer performance status. Because nab-P + Gem has now become the most commonly used first-line chemotherapy option for patients with metastatic pancreatic cancer in the United States [21], it is important to better understand how to achieve the optimal benefit with this regimen. Per protocol, patients in MPACT were treated until progressive disease (PD) or unacceptable toxicity. The current exploratory analysis investigated characteristics and outcomes of patients who were treated until PD as assessed by radiological imaging.
Methods
Study design
Study design and patient eligibility of the phase 3 MPACT trial were described previously [18]. Patients were randomly assigned 1:1 to either intravenous nab-P 125 mg/m2 followed by intravenous Gem 1000 mg/m2 once weekly for the first 3 weeks of a 4-week cycle or Gem 1000 mg/m2 for the first 7 weeks of an 8-week cycle (cycle 1) and subsequently the first 3 weeks of a 4-week cycle (cycle ≥ 2). Per protocol, patients were treated until either PD or an unacceptable level of AEs. Tumour response was evaluated every 8 weeks using spiral computed tomography or magnetic resonance imaging. The aim of the present analysis was to determine the characteristics and outcomes of patients who were treated until PD during the phase 3 MPACT trial.
The PD cohort consisted of patients who experienced disease progression as declared by the investigator on the basis of computed tomography or magnetic resonance imaging and excluded patients who received further therapy. These patients also may have experienced a treatment-limiting toxicity at the time of PD. As a comparator group, patients who discontinued treatment due to AEs in the absence of PD were also analysed.
Subsequent therapy use
Data on subsequent therapies included only the dates and type of treatment administered. For patients who received FOLFOX (folinic acid + 5-FU + oxaliplatin) or OFF (oxaliplatin + folinic acid + 5-FU), data were combined because information regarding dosing and schedule were unknown.
Statistical analyses
The Kaplan-Meier method was used to determine OS, and a stratified log-rank test was used to assess statistical significance. In the case of patients who were lost to follow-up, survival data were censored at the last date at which they were known to be alive. The results presented herein are based on the updated cutoff date for OS analysis, which was 9 May 2013. Progression-free survival (PFS) was compared between the treatment arms using the Kaplan-Meier method, and differences were tested using a stratified log-rank test. For the OS and PFS analysis, the HR and 95 % CI calculation used the proportional hazard assumption. Differences in overall response rate (ORR) were assessed by χ2 test.
Results
Baseline characteristics
In general, the baseline characteristics of patients treated to PD or AEs in the absence of PD were well balanced and similar to those of the intent-to-treat (ITT) population (Table 1). Although differences in baseline characteristics between the cohorts were not compared statistically, some minor imbalances were noted. Among patients treated with nab-P + Gem, those in the treatment-to-AEs cohort were older than those in the treatment-to-PD cohort or ITT population. Patients who received Gem alone in the treatment-to-AEs cohort had a greater metastatic burden compared with all other cohorts. Fewer patients in the treatment-to-AEs cohort underwent a previous Whipple procedure compared with those in the treatment-to-PD cohort and the ITT population. Among patients who were treated with Gem monotherapy, more patients in the treatment-to-AEs cohort had a biliary stent at baseline compared with those in the treatment-to-PD cohort and the ITT population.
Efficacy
Overall survival
Overall survival in the treatment-to-PD cohort was significantly longer for patients who received nab-P + Gem compared with those who received Gem alone (median, 9.8 vs 7.5 months; HR, 0.69; P < 0.001; Fig. 1). Kaplan-Meier estimates of OS rate at 24 months following randomization were 8 % for nab-P + Gem compared with 4 % for Gem alone among patients in the treatment-to-PD cohort. The OS data in the treatment-to-PD cohort were based on 419 events (92 %), including 206 and 213 in the nab-P + Gem (92 %) and Gem-alone (91 %) arms, respectively.
Overall survival in the treatment to AEs cohort was numerically, but not significantly, longer for patients who received nab-P + Gem compared with those who received Gem alone (median, 7.7 vs 6.0 months; HR, 0.87; P = 0.466; Table 2 [based on 136 events; 87 %]). Kaplan-Meier estimates of OS rates at 24 months following randomization were 14 % for nab-P + Gem compared with 11 % for Gem alone among patients in the treatment-to-AEs cohort.
Progression-free survival
In patients treated to PD, PFS was significantly longer for patients treated with nab-P + Gem compared with those who received Gem alone (median, 6.0 vs 3.8 months; HR, 0.62; P < 0.001; Fig. 2). In patients treated to AEs, PFS was numerically longer for patients treated with nab-P + Gem compared with those who received Gem alone, although this difference did not reach statistical significance (median, 5.5 vs 5.0 months; HR, 0.63; P = 0.053).
Overall response rate
In the treatment-to-PD cohort, the independently assessed ORR was significantly higher for patients treated with nab-P + Gem vs those treated with Gem alone (27 % vs 9 %; response rate ratio [RRR], 3.12; P < 0.001; Table 2). One patient (<1 %) in the nab-P + Gem arm and 0 patients in the Gem-alone arm achieved a complete response (CR). The disease control rate (DCR; CR + partial response + stable disease for ≥ 16 weeks) was also significantly higher for patients in this cohort who were treated with nab-P + Gem compared with Gem alone (57 % vs 40 %; RRR, 1.42; P < 0.001).
The independently assessed ORR was numerically higher for patients in the treatment-to-AEs cohort who received nab-P + Gem vs Gem alone (19 % vs 10 %; RRR, 1.87; P = 0.137; Table 2). No patients in either treatment arm achieved a CR in this cohort. The DCR was comparable for patients in this cohort who received nab-P + Gem compared with Gem alone (43 % vs 42 %).
Treatment exposure
The median treatment duration for patients in the treatment-to-PD cohort was 5.3 months (range, 0.16-21.9) for nab-P + Gem and 3.6 months (range, 0.13-21.5) for Gem alone (Table 3). For patients in the treatment-to-AEs cohort, the median treatment durations were 2.9 months (range, 0.13-20.7) and 2.3 months (range, 0.16-25.8), respectively (Table 3).
Among patients treated to PD in the nab-P + Gem arm, 46 % had ≥ 1 nab-P dose reduction and 74 % had ≥ 1 nab-P dose delay (Table 3). Similarly, among patients treated to AEs in the nab-P + Gem arm, 38 % of patients had ≥ 1 nab-P dose reduction and 73 % had ≥ 1 nab-P dose delay (Table 3).
Among patients treated to PD in the nab-P + Gem arm, the percentage of nab-P doses delivered at 125 mg/m2 and Gem doses delivered at 1000 mg/m2 were 72 % and 65 %, respectively; 75 % of Gem doses were delivered at 1000 mg/m2 in the Gem-alone arm (Table 3). Among patients treated to AEs in the nab-P + Gem arm, the percentage of nab-P doses delivered at 125 mg/m2 and Gem doses delivered at 1000 mg/m2 were numerically lower (62 % and 53 %, respectively), and the percentage of Gem doses delivered at 1000 mg/m2 in the Gem-alone arm was numerically higher (81 %; Table 3). Cumulative doses are described in detail in Table 3.
Reasons for treatment discontinuation
The AEs that most commonly led to treatment discontinuation are summarized in Table 4. Among patients in the treatment-to-AEs cohort who received nab-P + Gem, the most common AEs that led to treatment discontinuation were peripheral neuropathy and fatigue. Among patients in the treatment-to-AEs cohort who received Gem alone, the most common AE that lead to treatment discontinuation was thrombocytopenia.
Subsequent therapy use
Within the treatment-to-PD cohort, the use of subsequent therapy in the nab-P + Gem and Gem-alone arms was 52 % and 57 %, respectively, and these patients had numerically longer OS (median, 11.3 and 9.4 months, respectively; Table 5) than all patients in this cohort (median, 9.8 and 7.5 months, respectively; Fig. 1 and Table 2). In both arms of the treatment-to-PD cohort, 5-FU– or capecitabine-based regimens were the most commonly used subsequent therapies, with the majority of these patients having received a 5-FU–based regimen. Among patients who were treated to AEs, the majority (73 % and 74 % of those in the nab-P + Gem and Gem-alone arms, respectively) did not receive a subsequent therapy; therefore, OS was not reported for these patients.
Safety
Incidences of grade ≥ 3 hematologic AEs in both cohorts were similar to those reported in the MPACT trial, although there were higher rates of anaemia among patients treated to AEs in both treatment arms compared with the treated population of the MPACT trial [18]. Among patients treated to PD, nab-P + Gem, compared with Gem alone, had slightly higher rates of neutropenia (39 % vs 31 %) and thrombocytopenia (13 % vs 9 %) but not anaemia (14 % each; Table 6). Among patients treated to AEs, nab-P + Gem, compared with Gem alone, had higher rates of neutropenia (40 % vs 27 %), but rates of anaemia (19 % vs 18 %) and thrombocytopenia (14 % each) were similar (Table 6). Febrile neutropenia occurred in 2 % of patients in each treatment arm of the treatment-to-PD cohort and in 4 % and 2 % of patients who received nab-P + Gem and Gem alone, respectively, in the treatment-to-AEs cohort (Table 6).
Incidences of grade ≥ 3 nonhematologic AEs in both cohorts were generally similar to those reported in the MPACT trial, although fatigue occurred more frequently among patients who received nab-P + Gem in the treatment-to-AEs cohort vs the treated population of the MPACT trial [18]. In both cohorts, rates of fatigue, peripheral neuropathy, and diarrhoea were higher for nab-P + Gem vs Gem alone (Table 6). The frequency of grade 2 peripheral neuropathy was similar for patients who received nab-P + Gem in the treatment-to-PD cohort, treatment-to-AEs cohort, and overall MPACT treated population (16 %, 13 %, and 15 %, respectively).
Discussion
This subanalysis provides evidence that the nab-P + Gem combination is an active first-line treatment option with significant clinical efficacy for patients with metastatic pancreatic cancer because the majority of patients were treated until PD, which was associated with longer survival compared with the ITT population. Treatment until PD allowed better efficacy (as assessed by OS and disease control), likely due to the longer treatment duration and greater treatment exposure these patients received compared with those in the ITT population [18]. In addition, more than half of the patients who were treated to PD received a subsequent therapy, indicating that nab-P + Gem was also a feasible first-line option on which a treatment plan can be built. Conversely, ≈ 20 % of patients in the study discontinued treatment due to AEs in the absence of PD, which limited their treatment exposure and potential efficacy benefit.
A detailed examination revealed interesting differences in the relationships of reason for discontinuation, treatment exposure, and efficacy between the 2 treatment arms. Among patients who received nab-P + Gem, there was greater efficacy in terms of OS, PFS, and ORR between patients treated until PD vs the ITT population. Conversely, although there was a survival benefit in the Gem-alone arm between patients treated until PD vs the ITT population, the difference in PFS and ORR between the 2 cohorts was modest. The difference in treatment duration between patients treated until PD and the overall treated population was numerically longer for those who received nab-P + Gem compared with Gem alone (1.4 vs 0.8 months). The cumulative Gem dose in the PD cohort was 14 % and 11 % higher than that in the overall treated population for the nab-P + Gem and Gem-alone arms, respectively; however, the cumulative nab-P dose was 22 % higher for the PD cohort than the overall treated cohort for the combination arm (Table 3). These data raise the intriguing but speculative question of whether exposure to nab-P vs Gem imparts a greater relative treatment benefit.
Adverse events associated with chemotherapy are routinely managed by dose modification. A post hoc analysis of patients who underwent dose reductions or delays in the MPACT trial demonstrated that OS was significantly longer for those with vs without dose modifications [22]. Thus, mitigating AEs associated with nab-P + Gem through dose modification is not detrimental to treatment efficacy. The present analysis reveals that patients who were treated until PD had more dose reductions and delays compared with those in the ITT population, which suggests that effective treatment management may have allowed them to continue to receive and benefit from therapy.
The most common reasons for discontinuation due to AEs in the combination arm were peripheral neuropathy, fatigue, and thrombocytopenia. Rates of grade 3 peripheral neuropathy were relatively similar among patients treated to PD or AEs and the overall treated population, as were rates of grade 2 peripheral neuropathy. Management of peripheral neuropathy is accomplished by pausing treatment or reducing the treatment dose. Interestingly, in the MPACT trial, OS was significantly longer among patients who developed grade 3 peripheral neuropathy vs those who did not develop peripheral neuropathy [23]. Furthermore, dose modification was frequently used for patients who developed grade 3 peripheral neuropathy (≥1 dose delay [80 %] and/or reduction [41 %]). This approach for the management of peripheral neuropathy led to longer treatment duration, and ultimately, greater treatment exposure, which likely influenced survival outcomes. Collectively, these results underscore the importance of AE management through dose modification.
This subanalysis revealed that among patients who received nab-P + Gem, not only were the rates of grade ≥ 3 peripheral neuropathy similar in patients treated to PD vs AEs (19 % vs 21 %, respectively), but so were the rates of grade ≥ 3 neutropenia (39 % vs 40 %) and thrombocytopenia (13 % vs 14 %). Thus, grade ≥ 3 AEs were no less likely in patients who discontinued due to PD than in those who discontinued due to AEs, which may further underscore the importance of managing toxicity to maximize treatment duration.
Analysis of treatment effect by ORR is a direct measurement of antitumour activity and, unlike OS, which can be confounded by subsequent therapies, improvements in ORR can be directly attributed to the ongoing treatment [24]. Progression-free survival encompasses time to disease progression or death [24] and represents an important aspect of palliative treatment pancreatic cancer. Patients treated to AEs in the absence of PD still experienced treatment benefit, as evidenced by ORR and PFS analyses, which suggests that management of AEs before the need for discontinuation may have prolonged treatment and potentially increased survival. The shorter OS in patients treated to AEs is likely due to the shorter treatment duration and infrequent use of subsequent therapies among patients in this cohort. It also remains unanswered whether any of these patients could have resumed therapy outside of a protocol requirement, in which strict rules apply for AE management and treatment discontinuation.
Baseline characteristics were uninformative regarding identification of patients who may have developed treatment-limiting AEs during therapy. Compared with the other cohorts, fewer patients treated to AEs had a previous Whipple procedure, indicating a more advanced disease stage at diagnosis for these patients. Among patients who were treated until AEs, those in the nab-P + Gem arm were older while those in the Gem arm had a greater metastatic burden compared with those in the treatment-to-PD cohort as well as the ITT population. However, at baseline, the performance status of these patients was similar to that of the ITT population. At this point, whether these imbalances influenced survival outcomes is speculative. A future biomarker analysis may provide information regarding which patients are likely to benefit from treatment vs develop unacceptable AEs.
Historically, treatment beyond first line has been an option for a subset of patients with metastatic pancreatic cancer [25, 26]. Several randomized phase 2 clinical trials have explored second-line chemotherapy use in patients with metastatic pancreatic cancer [27–30]. Patients enrolled in these trials were all previously treated with Gem or Gem-based regimens, and efficacy results were modest. The current analysis, although not specifically designed to test this hypothesis, shows that, in the treatment-to-PD cohort, OS was numerically longer among patients who received a subsequent therapy compared with those who did not, regardless of treatment arm. The longest OS was achieved by those who received first-line nab-P + Gem followed by a subsequent therapy. A hypothetical explanation might be that first-line treatment with nab-P + Gem reduced tumor burden, which decreased cancer-related symptoms and ultimately allowed greater use of second-line therapies. These types of comparisons must be interpreted cautiously given the possibility of differences in patient characteristics, such as performance status, at the end of first-line treatment. However, > 50 % of patients who were treated to PD were able to receive subsequent therapy, which supports the suitability of nab-P + Gem as a first-line treatment for metastatic pancreatic cancer.
Conclusions
The results presented herein emphasize that first-line treatment with nab-P + Gem can be optimized for maximum treatment benefit. As revealed by previous subanalyses of the MPACT trial, effective AE management (ie, by treatment delay or dose reduction) allows for longer treatment duration, which, in turn, increases treatment exposure [22, 23]. Therefore, physicians should pay close attention to and promptly address AEs, when possible, to allow treatment to PD. This MPACT subanalysis reveals that most patients treated to PD were able to achieve adequate treatment exposure while managing AEs, which translated to improved disease control and longer survival.
Abbreviations
- 5-FU:
-
5-fluorouracil
- AE:
-
Adverse event
- CR:
-
Complete response
- DCR:
-
Disease control rate
- FOLFIRINOX:
-
Folinic acid + 5-fluorouracil + irinotecan + oxaliplatin
- FOLFOX:
-
Folinic acid + 5-fluorouracil + oxaliplatin
- Gem:
-
Gemcitabine
- HR:
-
Hazard ratio
- ITT:
-
Intent to treat
- KPS:
-
Karnofsky performance status
- MPACT:
-
Metastatic Pancreatic Adenocarcinoma Clinical Trial
- nab-P:
-
nab-paclitaxel
- OFF:
-
Oxaliplatin + folinic acid + 5-fluorouracil
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PRODIGE:
-
Partenarait de Recherche en Oncologie Digestive
- RRR:
-
Response rate ratio
References
World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. 2015.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403.
American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Published 2016. Accessed 1 Jul 2016.
SEER Stat Fact Sheets: pancreatic cancer.http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed 6 Jul 2016.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20.
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zulke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81.
Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40(9):1039–47.
Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolo M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F, Gruppo Oncologico Italia Meridionale (GOIM), Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD), Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC). Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28(10):1645–51.
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–22.
Goncalves A, Gilabert M, Francois E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, Esterni B, Genre D, Moureau-Zabotto L, Giovannini M, Seitz JF, Delpero JR, Turrini O, Viens P, Raoul JL. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23(11):2799–805.
Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, Falk S, Crellin A, Adab F, Thompson J, Leonard P, Ostrowski J, Eatock M, Scheithauer W, Herrmann R, Neoptolemos JP. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513–8.
Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, Cella D, Benson III AB. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-min infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(23):3778–85.
Abou-Alfa GK, Letourneau R, Harker G, Modiano M, Hurwitz H, Tchekmedyian NS, Feit K, Ackerman J, De Jager RL, Eckhardt SG, O’Reilly EM. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006;24(27):4441–7.
Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J, Androulakis N, Kotsakis A, Samonis G, Georgoulias V. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95(5):587–92.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2). 10.1093/jnci/dju413.
Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill S, Ruan JY, Cheung WY: Eligibility of Metastatic Pancreatic Cancer Patients for First-Line Palliative Intent nab-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am J Clin Oncol 2015. [Epub ahead of print]
Abrams TA, Meyer G, Moloney J, Meyerhardt JA, Wolpin BM, Schrag D, Fuchs CS: Patterns of chemotherapy (CT) use in a population-based US-wide cohort of patients (pts) with metastatic pancreatic cancer (MPC). J Clin Oncol 32:5s (Meeting Abstracts; suppl; abstr 4131) 2014.
Scheithauer W, Ramanathan RK, Moore M, Macarulla T, Goldstein D, Hammel P, Kunzmann V, Liu H, McGovern D, Romano A, Von Hoff DD. Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial. J Gastrointest Oncol. 2016;7(3):469–78.
Goldstein D, Von Hoff DD, Moore M, Greeno E, Tortora G, Ramanathan RK, Macarulla T, Liu H, Pilot R, Ferrara S, Lu B. Development of peripheral neuropathy and its association with survival during treatment with nab-paclitaxel plus gemcitabine for patients with metastatic adenocarcinoma of the pancreas: a subset analysis from a randomised phase III trial (MPACT). Eur J Cancer. 2016;52:85–91.
Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13 Suppl 2:19–21.
Smyth EN, Bapat B, Ball DE, Andre T, Kaye JA. Metastatic pancreatic adenocarcinoma treatment patterns, health care resource use, and outcomes in France and the United Kingdom between 2009 and 2012: A Retrospective Study. Clin Ther. 2015;37(6):1301–16.
Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol. 2015;7(2):68–84.
Astsaturov IA, Meropol NJ, Alpaugh RK, Burtness BA, Cheng JD, McLaughlin S, Rogatko A, Xu Z, Watson JC, Weiner LM, Cohen SJ. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2011;34(1):70–5.
Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30(3):1216–23.
Ge F, Xu N, Bai Y, Ba Y, Zhang Y, Li F, Xu H, Jia R, Wang Y, Lin L, Xu J. S-1 as monotherapy or in combination with leucovorin as second-line treatment in gemcitabine-refractory advanced pancreatic cancer: a randomized, open-label, multicenter, phase II study. Oncologist. 2014;19(11):1133–4.
Ulrich-Pur H, Raderer M, Verena Kornek G, Schull B, Schmid K, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F, Scheithauer W. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer. 2003;88(8):1180–4.
Acknowledgments
Writing assistance was provided by Aaron Runkle, PhD, MediTech Media, through funding by Celgene Corporation. Biostatistical support was provided by Helen Liu, PhD, Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript.
Funding
This analysis was funded by Celgene Corporation, Summit, New Jersey, USA.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is (are) included within the article.
Authors’ contributions
(I) Conception and design: AR (II) Administrative support: None; (III) Provision of study materials or patients: AV, JRZ, JSL, DM, AR, MS (IV) Collection and assembly of data: JSL, DM, AR; (V) Data analysis and interpretation: AV, JRZ, JSL, DM, AR, MS; (VI) Manuscript writing: AV, JRZ, JSL, DM, AR, MS; (VII) Final approval of manuscript: AV, JRZ, JSL, DM, AR, MS. All authors read and approved the final manuscript.
Competing interests
AV: Honoraria for advisory boards and speaker activity from Celgene, Roche, and Baxalta. JRZ: Celgene employee and stock ownership. JSL: Celgene employee and stock ownership. DM: Celgene employee and stock ownership. AR: Celgene employee and stock ownership. MS: Consultant and receipt of honoraria from Celgene.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The MPACT trial was approved by the Institutional Review Board or Independent Ethics Committee at each participating institution and was conducted in accordance with the International Conference on Harmonisation E6 requirements for Good Clinical Practice and with the ethical principles outlined in the Declaration of Helsinki. Please see Additional file 1 for a complete list of Institutional Review Board and Independent Ethics Committee locations. All patients provided written informed consent before initiation of the study.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
List of Independent Ethics Committees and Institutional Review Boards for MPACT. (DOCX 39 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vogel, A., Römmler-Zehrer, J., Li, J.S. et al. Efficacy and safety profile of nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic cancer treated to disease progression: a subanalysis from a phase 3 trial (MPACT). BMC Cancer 16, 817 (2016). https://doi.org/10.1186/s12885-016-2798-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2798-8