Abstract
Background
Detecting superficial head and neck squamous cell carcinoma (HNSCC) by endoscopy is challenging because of limited morphological hallmarks, and iodine cannot be applied to head and neck lesions due to severe mucosal irritation. γ-glutamyltranspeptidase (GGT), a cell surface enzyme, is overexpressed in several cancers, and it has been reported that γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG), a fluorescent targeting agent which can be enzymatically activated and becomes fluorescent after cleavage of a GGT-specific sequence, can be activated within a few minutes after application to animal models. We investigated whether early HNSCC can be detected by applying gGlu-HMRG to clinical samples.
Methods
gGlu-HMRG was applied to four HNSCC cell lines, and fluorescence was observed by fluorescence microscopy and flow cytometry. Immunohistological examination was performed in three recent cases of endoscopic submucosal dissection (ESD) to investigate GGT expression. Fluorescence imaging with gGlu-HMRG in eight clinical samples resected by ESD or surgery was performed, and fluorescence intensity of tumor and normal mucosa regions of interest (ROI) was prospectively measured.
Results
All four gGlu-HMRG-applied cell lines emitted green fluorescence. Immunohistological examination demonstrated that GGT was highly expressed in HNSCC of the recent three ESD cases but barely in the normal mucosa. Fluorescence imaging showed that iodine-voiding lesions became fluorescent within a few minutes after application of gGlu-HMRG in all eight resected tumors. Tumor ROI fluorescence intensity was significantly higher than in the normal mucosa five minutes after gGlu-HMRG application.
Conclusions
Fluorescence imaging with gGlu-HMRG would be useful for early detection of HNSCC.
Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world, with about 630,000 new cases diagnosed annually [1]. The prognosis of HNSCC is poor because it is typically detected at the advanced stage [2, 3]. However, patients with early-stage HNSCC, such as stage I and II, achieve a better prognosis with a 70–90 % 5-year survival rate as compared with patients with advanced HNSCC [4]. Therefore, early detection of HNSCC is imperative, particularly for high-risk patients, such as cigarette smokers and alcohol abusers; however, early detection of superficial HNSCC is very difficult because there are few morphological hallmarks to differentiate the disease [5]. Although iodine chromoendoscopy has been widely accepted for detection of early esophageal squamous cell carcinoma (SCC) [6, 7], it cannot be applied to head and neck lesions in conventional endoscopy because iodine causes severe mucosal irritation, which can result in aspiration into the airways [8, 9].
Although narrow band imaging (NBI) and autofluorescence imaging (AFI) have been used to detect early HNSCC, these modalities have not been widely accepted [10, 11]. Therefore, an efficient and reliable method to detect superficial HNSCC is required.
γ-glutamyltranspeptidase (GGT) is a cell surface enzyme involved in cellular glutathione metabolism and has been reported to be overexpressed in several human cancers, such as those of the lung, ovary, liver and bile duct [12–14]. GGT has been reported to play a role in tumor progression, invasion and drug resistance [12, 15]. It has been reported that γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG), a fluorescent targeting agent that can be enzymatically activated, based on the fluorophore rhodamine green, which becomes fluorescent after cleavage of a GGT-specific sequence, was developed and gGlu-HMRG can be activated specifically in seconds to minutes by topical application [16]. It has also been demonstrated that gGlu-HMRG can improve endoscopic detection of colitis-associated cancer with a higher target-to-background ratio than conventional white light colonoscopy in a murine model [17]. However, whether fluorescence imaging with gGlu-HMRG can detect human HNSCC remains to be elucidated.
Accordingly, the aim of this study was to evaluate whether superficial HNSCC can be detected by application of gGlu-HMRG using fresh clinical samples obtained by endoscopic submucosal dissection (ESD) or local surgical resection.
Methods
Enzymatic-activatable fluorescent targeting agent
gGlu-HMRG was synthesized as previously described [18], and resuspended in 10 mM dimethylsulfoxide (DMSO, Sigma-Aldrich, St. Louis, Missouri) and stored at −80 °C. When used, gGlu-HMRG was thawed at room temperature and diluted to 1 or 100 μM using phosphate-buffered saline (PBS, Life Technologies, Carlsbad, California).
Cell culture
HNSCC cell lines—HSC2, HSC3 and HSC4—were obtained from the American Type Culture Collection (ATCC; Manassas, Virginia), and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10 % fetal bovine serum (FBS; Life Technologies), 100 U/mL of penicillin and 100 μg/mL of streptomycin (Wako Pure Chemical Industries, Osaka, Japan). SCC25 cells were obtained from the Japanese Collection of Research Bioresources (JCRB, Osaka, Japan) and cultured in DMEM/F-12 (Nacalai Tesque) supplemented with 10 % FBS, 100 U/mL of penicillin and 100 μg/mL of streptomycin. The culture was maintained at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2.
Fluorescence microscopy
Cells were cultured in 35-mm dishes; next, once the cells had been reached at around 80 % confluence, cells were washed with PBS, 1 μM of gGlu-HMRG was added and cells were incubated in the dark for 20 min at 37 °C. Fluorescence microscopy was performed using a Biorevo BZ-9000 microscope (Keyence, Osaka, Japan), equipped with the following filters: excitation wavelength, 450–490 nm; emission wavelength, 500–550 nm. Phase contrast images were also developed.
Flow cytometry
Cultured cells were treated with 0.25 % trypsin/ethylenediaminetetraacetic acid (Life Technologies), harvested and resuspended in PBS. Cells (1 × 106) were incubated in the dark with 1 μM of gGlu-HMRG for 20 min at 37 °C and analysed using a flow cytometer (FACSCanto II; Becton, Dickinson and Company, Franklin Lakes, New Jersey).
Time course of fluorescence intensity in cultured cell lines
Cells (2 × 104) were cultured on a black 96-well plate overnight and incubated with 1 μM of gGlu-HMRG with or without 10 μM of GGT inhibitor (GGsTop®, Wako Pure Chemical Industries). The time course of the fluorescence intensity was analysed using a microplate reader (505–555 nm; Tecan, Mannedorf, Switzerland).
Patient studies
This study prospectively reviewed eight consecutive HNSCC tumors treated by ESD and local surgical resection in seven patients at the Department of Gastroenterology and Hepatology and the Department of Otolaryngology-Head and Neck Surgery of Hokkaido University Hospital between June 2014 and February 2016.
The indication of ESD or local surgical resection for HNSCC are as follows; 1) within slight invasion in the subepithelium and 2) no lymph node metastasis by computed tomography. ESD was performed using a single-channel gastrointestinal endoscope with a transparent attachment hood fitted to the tip using a needle knife (KD-10Q-1, Olympus, Tokyo, Japan) and insulation tip (IT knife, Olympus) under general anesthesia [19]. All ESD procedures were performed by an experienced endoscopist who had performed over 100 esophageal ESD procedures.
Local surgical resection was performed using a Colorado microdissection needle (Stryker, Kalamazoo, Michigan) and an electrosurgical generator (Force FX, Covidien, Dublin, Ireland) under general anesthesia.
The resected specimen was immediately extended on a black rubber and fixed with pins. Next, 100 μM of gGlu-HMRG was sprayed onto the specimen. Fluorescence imaging was performed using a handheld fluorescent imaging system (Discovery; INDEC Medical Systems, Santa Clara, California), which enables the capture of white-light images and fluorescent images with 450–490 nm blue excitation light. Fluorescence images were recorded 0, 0.5, 2, 5, 7, 9, 11 and 13 min after gGlu-HMRG administration. Subsequently, specimens were washed with PBS and observed using an endoscope (H260Z, Olympus) under a white light with 1.5 % iodine staining.
Fluorescence intensities were measured with Image J software (National Institutes of Health, Rockville, Maryland). Tumor and normal squamous mucosa regions of interest (ROI) were manually traced on each image. The ROI of the tumor was determined according to the iodine staining images. The mean fluorescence intensity of each ROI was calculated as pixel intensity values ranging from 0 to 255.
Histopathology
Specimens were fixed in 40 g/L of formaldehyde saline, embedded in paraffin and cut into 5-μm sections. Tissue sections were stained with hematoxylin and eosin (H&E) and microscopically examined for histological type, tumor size, depth of invasion, lymphovascular invasion and resected margin by experienced pathologists, according to the World Health Organization (WHO) classification. Immunohistochemical analysis of GGT expression was performed using an anti-GGT1 antibody (dilution, 1:600; Abcam, Cambridge, UK).
Statistical analysis
Data were expressed as means ± SEM. Parameters were compared between the groups using a paired t-test. Differences were considered significant at a P value < 0.05. All analyses were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, California).
Results
Evaluation of gGlu-HMRG in HNSCC cell lines
To investigate GGT expression in HNSCC cells, gGlu-HMRG fluorescence was examined using four cell lines of HNSCC (HSC2, HSC3, HSC4 and SCC25). All tumor cell lines emitted fluorescence following gGlu-HMRG administration as evidenced by fluorescence microscopy (Fig. 1a) and flow cytometry (Fig. 1b). Fluorescence intensity was increased over time in all cell lines; however, when cultured with a GGT inhibitor, fluorescence emission was completely blocked (Fig. 1c). These results suggest that GGT is expressed in HNSCC cell lines and that gGlu-HMRG is activated by GGT.
Fluorescent imaging of HNSCC cell lines in vitro. a gGlu-HMRG fluorescence was detected by fluorescence microscopy. Phase contrast images (left column), gGlu-HMRG fluorescence images (right column), Scale bars, 100 μm. b Flow cytometric analysis of GGT expression. Open area; no gGlu-HMRG, Closed area; with gGlu-HMRG. c GGT inhibition in cell lines shows decreasing GGT activity over time, resulting in low fluorescence intensity
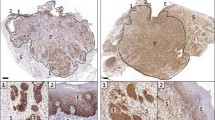
Expression of GGT in recent HNSCC cases treated with ESD
To confirm tumor-specific expression of GGT in HNSCC, we performed immunohistological examination of the tumors of three recent cases that had been treated with ESD. As shown in Fig. 2, GGT was expressed specifically in the tumor and barely expressed in the basal layer of the normal counterpart in all cases examined.
Ex vivo fluorescent imaging with gGlu-HMRG of HNSCC cases treated with ESD or local surgical resection
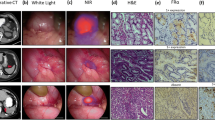
We next evaluated whether early HNSCC can be detected by spraying gGlu-HMRG using dissected specimens. ESD and local surgical resection were performed in seven patients with eight lesions. (Table 1). It was difficult to detect the superficial tumors with white light (Fig. 3a), and all cases were barely detected using narrow band imaging (NBI, Fig. 3b). Iodine staining was performed both before resection under general anesthesia (Fig. 3c) and after resection (Fig. 3d). Resected specimens were also sprayed with gGlu-HMRG and fluorescence images were obtained (Fig. 3e). In all cases, tumor lesions became fluorescent within a few minutes corresponding to an area almost exactly identical to the iodine-unstained lesion. In several cases, the subsites of the resected mucosa became fluorescent even before applying gGlu-HMRG, and immunohistological analysis did not show any positive staining for GGT in the subsites of the resected mucosa. Therefore, we speculate that autofluorescence was emitted by the burning effect [20]. Histological analysis confirmed that the iodine-unstained and fluorescent lesion were early SCC expressing GGT in all cases (Fig. 3f–h).
Ex vivo fluorescent imaging with gGlu-HMRG of two representative HNSCC cases (cases #1 and #5). a Endoscopic imaging with white light. b Narrow band imaging (NBI). c Iodine staining performed under general anesthesia. d Resected specimen observed with iodine staining. e Fluorescent imaging after spraying gGlu-HMRG. f Resected specimen mapping for tumor region. SCC was shown in red line. g Hematoxylin and eosin staining of the tumor and normal component. h Immunohistochemical examination investigating GGT expression in the tumor and normal component. Square lines in f correspond to the upper figures in g and h. Dotted square lines in f correspond to the lower figures in g and h. Scale bars of d-f, 1 mm (case#1) and 5 mm (case#5). Scale bars of g and h, 200 μm
Fluorescence intensity of tumor and normal epithelium after spraying gGlu-HMRG
We finally measured the fluorescence intensity of both tumor and normal epithelium of all eight cases for 13 min. The tumor lesion was traced according to the iodine staining (Fig. 4a). The fluorescence intensity of the tumor lesion increased immediately after gGlu-HMRG spraying and rose to a mean intensity of 7 at 13 min, while that of normal mucosa remained <2 (Fig. 4b). The matched rate between iodine-unstained and gGlu-HMRG-induced fluorescent area was 74 %.
Discussion
In this study, we evaluated the use of gGlu-HMRG for the detection of early HNSCC and found that (1) all HNSCC cell lines examined emitted fluorescence following gGlu-HMRG exposure; (2) HNSCC, but not normal tissue, expressed GGT in three recent ESD cases; and (3) tumor lesions became fluorescent immediately after gGlu-HMRG being applied to all eight HNSCC cases.
It is difficult to detect superficial HNSCC using conventional endoscopy with white light because mucosal changes are usually very subtle [5, 21]. Recently, NBI and AFI have been used to detect early cancers of the upper gastrointestinal tract and have been reported to be superior to white-light endoscopy in terms of sensitivity, specificity and accuracy for the diagnosis of HNSCC and esophageal SCC [10, 11, 21–26]. However, these modalities were reported from a limited number of institutes and hospitals and require remarkable expert skills to be successfully employed. In addition, it has been reported that the detection rate of early esophageal SCC using NBI was 10–13 % for SCC high-risk groups [24, 25], suggesting that it requires a sharp learning curve for the detection of HNSCC with NBI [27]. Furthermore, inflammatory changes in the larynx have been reported to cause false positive results in AFI [23]. Therefore, the development of a novel method with which even a non-expert gastroenterologist or otolaryngologist can detect early HNSCC is warranted.
When the gGlu-HMRG as targeting agent encounters GGT on the surface of cancer cells, it is hydrolysed by GGT, becoming highly fluorescent HMRG. HMRG is immediately taken up by cancer cell lysosomes through the cell membrane [16]. Therefore, HMRG is expected to emit strong fluorescence in cancer lesions. Accordingly, it has been demonstrated that topical spraying of gGlu-HMRG could provide immediate and specific enhancement of cells overexpressing GGT in animal models [16, 17]. In addition, it has been recently demonstrated, in a pilot study of fluorescence imaging of endoscopically resected colorectal tumors, that topical spraying of gGlu-HMRG enabled rapid and selective fluorescence imaging of 54 % and 76 % of adenomas and carcinomas in adenoma, respectively [28]. Although the authors used 50 or 500 μM of gGlu-HMRG, our results suggested that using 100 μM of gGlu-HMRG is sufficient for tumor detection.
Immunohistological examination demonstrated that GGT was highly expressed in tumor tissue but barely expressed at the basal lamina of the normal epithelium. The fluorescence intensity of the normal epithelium was very weak; however, it gradually became stronger with time. This is probably because it took a longer time for gGlu-HMRG to reach the basal lamina, which only weakly expresses GGT, than to reach the tumor cells.
There are several limitations to this study. Because the study was performed ex vivo, GGT activity may have decreased to some extent following tumor resection. It took 10–20 min to initiate fluorescence imaging after tumor resection, and imaging was performed at room temperature rather than at 37 °C. Therefore, it is expected that gGlu-HMRG would react faster in an in vivo clinical study than in an ex vivo study. In addition, because this is a pilot study investigating only eight cases, it remains to be elucidated whether all superficial HNSCC and precancer lesion can be detected with gGlu-HMRG. Future clinical trials studying a larger number of HNSCC cases would clarify these concerns.
Conclusions
In conclusion, topical spraying of gGlu-HMRG enabled rapid and specific fluorescence imaging of superficial HNSCC, and appears to be useful in the early detection of HNSCC.
Abbreviations
AFI, autofluorescence imaging; DMEM, Dulbecco’s Modified Eagle Medium; DMSO, dimethylsulfoxide; ESD, endoscopic submucosal dissection; FBS, fetal bovine serum; GGT, γ-glutamyltranspeptidase; gGlu-HMRG, γ-glutamyl hydroxymethyl rhodamine green; HNSCC, head and neck squamous cell carcinoma; NBI, narrow band imaging; PBS, phosphate-buffered saline; ROI, regions of interest; SCC, squamous cell carcinoma
References
Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–41.
Johansen LV, Grau C, Overgaard J. Hypopharyngeal squamous cell carcinoma--treatment results in 138 consecutively admitted patients. Acta Oncol. 2000;39(4):529–36.
Eckel HE, Staar S, Volling P, Sittel C, Damm M, Jungehuelsing M. Surgical treatment for hypopharynx carcinoma: feasibility, mortality, and results. Otolaryngol Head Neck Surg. 2001;124(5):561–9.
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. The Lancet. 2008;371(9625):1695–709.
Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101(6):1375–81.
Carvalho R, Areia M, Brito D, Saraiva S, Alves S, Cadime AT. Diagnostic accuracy of lugol chromoendoscopy in the oesophagus in patients with head and neck cancer. Rev Esp Enferm Dig. 2013;105(2):79–83.
Fagundes RB, de Barros SG, Putten AC, Mello ES, Wagner M, Bassi LA, et al. Occult dysplasia is disclosed by Lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the esophagus. Endoscopy. 1999;31(4):281–5.
Hori K, Okada H, Kawahara Y, Takenaka R, Shimizu S, Ohno Y, et al. Lugol-voiding lesions are an important risk factor for a second primary squamous cell carcinoma in patients with esosphageal cancer or head and neck cancer. Am J Gastroenterol. 2011;106(5):858–66.
Hanaoka N, Ishihara R, Takeuchi Y, Suzuki M, Uemura H, Fujii T, et al. Clinical outcomes of endoscopic mucosal resection and endoscopic submucosal dissection as a transoral treatment for superficial pharyngeal cancer. Head Neck. 2013;35(9):1248–54.
Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28(9):1566–72.
Suzuki H, Saito Y, Ikehara H, Oda I. Evaluation of visualization of squamous cell carcinoma of esophagus and pharynx using an autofluorescence imaging videoendoscope system. J Gastroenterol Hepatol. 2009;24(12):1834–9.
Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71(3):231–8.
Schafer C, Fels C, Brucke M, Holzhausen HJ, Bahn H, Wellman M, et al. Gamma-glutamyl transferase expression in higher-grade astrocytic glioma. Acta Oncol. 2001;40(4):529–35.
Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, et al. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88(4):761–9.
Hanigan MH, Frierson Jr HF, Brown JE, Lovell MA, Taylor PT. Human ovarian tumors express gamma-glutamyl transpeptidase. Cancer Res. 1994;54(1):286–90.
Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, et al. Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3(110):110ra19.
Mitsunaga M, Kosaka N, Choyke PL, Young MR, Dextras CR, Saud SM, et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut. 2013;62(8):1179–86.
Kamiya M, Asanuma D, Kuranaga E, Takeishi A, Sakabe M, Miura M, et al. beta-Galactosidase fluorescence probe with improved cellular accumulation based on a spirocyclized rhodol scaffold. J Am Chem Soc. 2011;133(33):12960–3.
Shimizu Y, Yamamoto J, Kato M, Yoshida T, Hirota J, Ono Y, et al. Endoscopic submucosal dissection for treatment of early stage hypopharyngeal carcinoma. Gastrointest Endosc. 2006;64(2):255–9. discussion 60–2.
Devgan L, Bhat S, Aylward S, Spence RJ. Modalities for the assessment of burn wound depth. J Burns Wounds. 2006;5:e2.
Watanabe A, Taniguchi M, Tsujie H, Hosokawa M, Fujita M, Sasaki S. The value of narrow band imaging for early detection of laryngeal cancer. Eur Arch Otorhinolaryngol. 2009;266(7):1017–23.
Goda K, Dobashi A, Tajiri H. Perspectives on narrow-band imaging endoscopy for superficial squamous neoplasms of the orohypopharynx and esophagus. Dig Endosc. 2014;26 Suppl 1:1–11.
Dobre M, Poenaru M, Balica NC, Doros CI. Detection of early laryngeal cancer and its precursor lesions by a real-time autofluorescence imaging system. Rom J Morphol Embryol. 2014;55(4):1377–81.
Nonaka S, Saito Y, Oda I, Kozu T, Saito D. Narrow-band imaging endoscopy with magnification is useful for detecting metachronous superficial pharyngeal cancer in patients with esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2010;25(2):264–9.
Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, et al. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42(3):185–90.
Arens C, Reussner D, Woenkhaus J, Leunig A, Betz CS, Glanz H. Indirect fluorescence laryngoscopy in the diagnosis of precancerous and cancerous laryngeal lesions. Eur Arch Otorhinolaryngol. 2007;264(6):621–6.
Ishihara R, Takeuchi Y, Chatani R, Kidu T, Inoue T, Hanaoka N, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23(6):480–6.
Sato C, Abe S, Saito Y, So Tsuruki E, Takamaru H, Makazu M, et al. A pilot study of fluorescent imaging of colorectal tumors using a gamma-glutamyl-transpeptidase-activatable fluorescent probe. Digestion. 2015;91(1):70–6.
Acknowledgements
We are grateful to all patients participated in this study.
Funding
This study was funded by a Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (JSPS, 26461043) and by The Japanese Foundation for Research and Promotion of Endoscopy (JFE) Grant.
Availability of data and materials
Publication of our data does not comprise anonymity or confidentiality, and informed consent was obtained for publication of patient data.
Authors’ contributions
TM and SOh performed the experiments and analyses and drafted the manuscript, YS performed ESD, AH carried out the surgery, YH and KCH performed pathological examinations, HH, YK and MN performed in vitro experiments, MKam prepared gGlu-HMRG, SOn performed analyses, MKat, NS and YU supervised the entire project. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The ex vivo clinical study protocol was approved by the ethical review board of Hokkaido University Hospital. All patients provided informed consent to participate this study under the ‘Ethics, consent and permissions’ heading, and under the ‘Consent to publish’ heading confirming that we have obtained consent to publish from the participant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mizushima, T., Ohnishi, S., Shimizu, Y. et al. Fluorescent imaging of superficial head and neck squamous cell carcinoma using a γ-glutamyltranspeptidase-activated targeting agent: a pilot study. BMC Cancer 16, 411 (2016). https://doi.org/10.1186/s12885-016-2421-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2421-z