Abstract
Background
Male breast cancer (MBC) is rare. Given the paucity of randomized trials, treatment is generally extrapolated from female breast cancer guidelines.
Methods
This is a retrospective analysis of all male patients presenting with MBC at the Department of Oncology at University Federico II of Naples between January 1989 and January 2014. We recorded the following data: baseline characteristics (age, height, weight, body mass index, risk factors, family history), tumor characteristics (side affected, stage, histotype, hormonal and HER2 status, and Ki-67 expression), treatment (type of surgery, chemotherapy, endocrine therapy, and/or radiotherapy), BRCA1/2 mutation status (if available), other tumors, and long-term survival.
Results
Forty-seven patients were analyzed. Median age was 62.0 [55.0–72.0]. Among risk factors, obesity and family history of breast cancer were associated with 21 % and 30 % of MBC cases, respectively. The majority of tumors were diagnosed at an early stage: stage I (34.0 %) and stage II (44.7 %). Infiltrating ductal carcinoma was the most frequent histologic subtype (95.8 %). Hormone receptors were generally positive (88.4 % of cases were Estrogen receptor [ER] positive and 81.4 % Progesteron receptor [PgR] positive). Human epidermal growth factor receptor 2 (HER2) was positive in 26.8 % of cases; 7.0 % of MBCs were triple negative. The tumor had high proliferation index (Ki67 ≥ 20 %) in 64.7 %. Surgery was predominantly mastectomy (85.1 %), whereas quadrantectomy was performed in 14.9 % of patients. Adjuvant chemotherapy was administered to 70.7 % of patients, endocrine therapy to 90.2 %, trastuzumab to 16.7 % and radiotherapy to 32.6 %. BRCA status was available for 17 patients: 10 wild-type, 1 BRCA1 carrier, 5 BRCA2 carriers, 1 unknown variant sequence. The overall estimated long-term survival was about 90 % at 5 years, 80 % at 10 years and 70 % at 20 years. Patients carrying a BRCA mutation had a significantly lower survival than patients with wild-type BRCA (p = 0.04).
Conclusions
Long-term survival was high in MBC patients referred to our clinical unit. Survival was poorer in BRCA-mutated patients than in patients with wild-type BRCA.
Similar content being viewed by others
Background
Male breast cancer (MBC) represents about 1 % of all breast cancers and approximately 0.2 % of all male cancers [1, 2]. Its incidence is estimated at <1 per 100,000 men-years, and it appears to be increasing by 1.1 % yearly [1, 2]. However, given the rarity of this disease, few randomized controlled trials have been conducted, and most of the data about MBC come from retrospective studies [3]. Consequently, treatment of MBC is based on female breast cancer guidelines and trials.
Although MBC shares some features of female breast cancer, it differs significantly in terms of epidemiology and biologic features. The etiology of MBC is unclear although anthropometric and hormonal factors appear to be involved in its development. Clinical disorders, such as Klinefelter’s syndrome, obesity, liver diseases and testicular abnormalities, represent risk factors for MBC. These disorders are associated with an imbalanced estrogen/androgen ratio that result in abnormal estrogen exposure [4]. Other risk factors are race and radiation exposure. Moreover, family history and genetic abnormalities, such as mutations of the BRCA1 and BRCA2 genes, play a relevant role in MBC pathogenesis [5].
About 20 % of patients with MBC have a family history of breast cancer. Subjects with a positive first-degree family history have a 2.0-fold increased risk. The risk of MBC increases to more than 10.0-fold if the number of affected first-degree relatives are two (i.e. mother and sister), thus suggesting that genetic factors plays a relevant role in MBC chance [3, 6]. Accordingly, 2 % of patients with MBC develop a second primary breast cancer and more than 20 % of patients develop tumors at other sites, most frequently prostate, colon or genitourinary cancer [3]. The breast cancer susceptibility genes, BRCA1 and BRCA2, are responsible for a high proportion of cases of hereditable breast cancer. Up to 10 % of all MBCs are caused by inherited germline mutations in either of the two BRCA genes [7, 8], mutations in BRCA2 being more frequently recorded in population-based series [8–10]. The estimated lifetime risk of breast cancer is 1–5 % in male BRCA1-mutation carriers and 5–10 % in male BRCA2-mutation carriers versus 0.1 % in the general population [10]. Mutations in these genes are found in MBC patients with and without a family history of breast and/or ovarian cancer [11, 12]. Therefore, regardless of family history, all men with breast cancer should be routinely screened for BRCA1 and BRCA2 mutations.
Here we report the results of a single-center retrospective analysis of the clinical features, BRCA status, treatments and long-term prognosis of patients affected by MBC.
Methods
This is a retrospective analysis of all male patients presenting with MBC at the Department of Oncology at University Federico II of Naples between January 1989 and January 2014. We recorded the following data: baseline characteristics (age, height, weight, body mass index, risk factors, family history), tumor characteristics (side affected, stage, histotype, hormonal and HER2 status, and Ki-67 expression), treatment (type of surgery, chemotherapy, endocrine therapy, and/or radiotherapy), BRCA1/2 mutation status (if available), other tumors, and long-term survival. Clinico-pathological data were obtained from medical and pathology reports without performing additional tests. BRCA1 and BRCA2 mutation analysis was performed in 17 MBC patients within the framework of a genetic counseling programs ongoing at our center [13]. BRCA1/2 mutations were classified according to their potential functional effect as recorded in the Breast Cancer Information Core (BIC) database [14] and in the Leiden Open Variation Database (LOVD-IARC) [15].
Data regarding estrogen receptor (ER), progesterone receptor (PgR), Ki-67, and HER2 status of breast tumors were extracted from medical, pathology, or tumor registry records or obtained from the results of immunohistochemical analysis of sections of formalin-fixed, paraffin-embedded primary mammary tumor blocks. HER2 status was assessed by fluorescent in situ hybridization analysis in ambiguous cases (immunohistochemistry score = 2+). According to international guidelines [16], ER and PgR were considered positive if ≥ 1 % of tumor cell nuclei were immunoreactive; whereas Ki-67 was considered high at ≥ 20 % cut-off.
Follow-up and additional diagnostic exams were performed according to clinical practice as for females. Patients were followed with clinical visits, every 6 months up to 5 years and thereafter annually. Patients were contacted by phone to update follow-up and survival status.
All the patients included in this retrospective analysis provided their informed consent in the framework of cancer genetic counseling program regulated and approved by the Local Ethic committee (University ‘Federico II’ of Naples, Prot. 80/00 and 63/02).
Statistical analysis
Continuous variables are expressed as median with interquartile range (IQR). Categorial variables are expressed as numbers and percentages. Overall survival was calculated with the Kaplan Meier method. A survival analysis for patients who underwent BRCA testing was performed and statistical significance was considered for p value <0.05 calculated with the log rank test. All statistical analyses were performed with SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
From January 1989 to 1 January 2014, 47 patients received diagnosis and treatment for MBC and were followed at our center (Fig. 1).
Clinical features
The baseline and clinical characteristics of the 47 patients and BRCA mutational status (if available) are reported in Table 1. Age at MBC diagnosis ranged between 33 and 82 years (median: 62.0 years [55.0–72.0]). Most patients were over the age of 50 years (80.9 %) and most were diagnosed in the sixth decade of life (Fig. 2). The median value of the body mass index (BMI) was 26.2 [24.2–28.9] kg/m2. Apart from 10 patients (21.3 %) classified obese (BMI ≥30 kg/m2), no patient had a history of clinical disorders associated with an imbalanced estrogen/androgen ratio such as Klinefelter’s syndrome, liver diseases or testicular abnormalities. Fourteen of the 47 patients (29.7 %) had a family history of breast or ovarian cancer in first-degree relatives. Nine patients (19.1 %) had a second tumor unrelated to MBC (one or two malignancies besides MBC), melanoma and prostate cancer being the most frequent (3 and 2 cases, respectively). In addition, gastric cancer, contralateral breast cancer, thyroid cancer and kidney cancer each occurred in 1 patient.
Clinico-pathological features
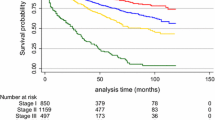
Most tumors were at early TNM stage: stage II (44.7 %) and 16 were stage I (34.0 %). Only one case was bilateral (2.1 %).
Pathologic confirmation was available for all 47 patients. Infiltrating ductal carcinoma was the most common histologic subtype (45 patients; 95.8 %), whereas papillary carcinoma was diagnosed in 1 patient and mixed (tubular-ductal) carcinoma was diagnosed in the remaining patient. Hormonal status was available for 43 patients: 38 (88.4 %) were ER+ and 35 (81.4 %) were PgR+; the distribution of both hormone receptors was as follow: 34 cases were ER+/PgR+ (79.1 %), 4 were ER+/PgR- (9.3 %), 1 was ER-/PgR+ (2.3 %) and 4 were ER-/PgR- (9.3 %). HER2 status was known in 41 patients and was positive in 11 cases (26.8 %). Triple negative phenotype was revealed in 3 cases (7 %). In 22 patients (64.7 %) the tumor had high proliferative activity, namely, Ki67 ≥ 20 %.
Treatments
Data on the surgical approach were available for all patients. The most frequently used surgical approach was mastectomy (40 patients, 85.1 %), whereas the remaining 7 patients (14.9 %) underwent quadrantectomy. Positive axillary nodes were found in 17 of the 44 patients (38.6 %) who underwent axillary dissection (defined as I/II lymph nodes level removal). Fourteen of 43 patients (32.6 %) received post-operative local radiotherapy, while data were not available for 4/47 patients. The dose used for adjuvant irradiation was 50 Grey (Gy) in 25–28 fractions (2 Gy/fraction) with an additional boost of 10 Gy if clinically indicated. The radiotherapy was performed using the linear accelerator with 6 megavolts photons.
Among patient for whom data are available, systemic medical treatment consisting of adjuvant chemotherapy was administered to 29/41 patients (70.7 %): 17 (58.6 %) received antracycline-based treatment (5 patients received both antracycline and taxane); 7 (24.1 %) received CMF (cyclophosphamide, methotrexate, 5-fluoracil) combination chemotherapy and 5 (17.2 %) received only taxane. Adjuvant endocrine therapy was administered in 37/41 patients (90.2 %): tamoxifene alone in 29/37 patients (78.4 %), and 6 patients (16.2 %) were treated with aromatase inhibitors (AIs). The regimen based on tamoxifene followed by AI was used in 2 cases (5.4 %). Ten of 41 patients (24.4 %) received only endocrine therapy as adjuvant; 27 patients (65.9 %) received both chemotherapy and endocrine therapy in a sequential manner. Moreover, 7 of 42 patients (16.7 %) received trastuzumab therapy, in 1 case in a metastatic setting.
All patients completed adjuvant treatment program and no relevant side effects, treatment intolerance or patients’ refusal were recorded.
Follow-up
Median follow up was 89 months (2660 days, 7.3 years) (Table 1). During follow-up, 9 patients of 40 (22.5 %), for whom data about follow-up was available, experienced relapse at the following sites: thoracic wall (n = 4), bone (n = 4), lung (n = 4), breast (n = 1), brain (n = 1), axillary nodes (n = 1) and adrenal gland (n = 1). The BRCA test was performed in 17 patients (36.2 %). Ten patients (58.8 %) were wild-type for BRCA genes, 1 was a carrier of BRCA1 (5.9 %) and 5 (29.4 %) of BRCA2 mutations, while 1 patient (5.9 %) had an unknown variant sequence. The characteristics of these 17 patients are reported in Table 2. The BRCA1 mutation-positive MBC patient was <40 years old at diagnosis and had a first-degree family history of breast and ovarian cancer. No other risk factors were present. He had an invasive ductal carcinoma, stage II, lymph node positive, HER2– and ER+/PgR+, and low Ki-67 levels. The disease relapsed after adjuvant treatment (chemotherapy and endocrine therapy) and the patient developed a second tumor (thyroid cancer). This patient died at 59.8 months of follow-up. The median age at diagnosis of the 5 BRCA2-positive patients was 72.0 [62.0–72.5] years, and 2 had a positive first-degree family history of breast and/or ovarian cancer (Table 2). No other risk factors were present. MBC was an invasive ductal carcinoma in all cases, stage I (3 cases) and III (2 cases); all cases were ER+/PgR+, with high levels of Ki67, and only 1 was HER2+. Nodal involvement was observed in 2 patients. One patient relapsed after adjuvant treatment (chemotherapy and endocrine therapy) and 2 developed a second tumor (one case of prostate cancer, and one of prostate and kidney cancer) (Table 3).
Seven of 47 patients (15 %) died during follow-up, and the estimated long-term survival was about 90 % at 5 years, 80 % at 10 years and 70 % at 18–20 years (Fig. 3, Table 3).
Among the patients with a known BRCA status, 3 patients died and all were mutation carriers. Survival was significantly lower in BRCA-mutated patients (p = 0.04; Fig. 4).
Discussion
In this single-center retrospective study, we report the characteristics of 47 patients with MBC and analyze the patients’ clinical-pathological features, associated risk factors, oncological and surgical treatments and long-term survival. We also evaluated survival in 17 patients who underwent genetic testing for BRCA1 and BRCA2 mutations.
Median age of our patients at diagnosis was 62 years, which is similar to previously reported data [17]. Regarding the risk factors associated with MBC, 10/47 of our patients (21.3 %) had a history of obesity. Obesity is known to be a risk factor for MBC, and obese men have an increased risk of about 30 % of breast cancer, similar to that of postmenopausal women [18]. In men, obesity is associated with high estrogen levels, and low levels of testosterone and sex hormone binding globulin, thereby leading to greater estrogen bioavailability [19, 20].
No patients in our series was affected by Klinefelter’ syndrome, hormonal alterations or chronic liver disease, which are known risk conditions for MBC [4].
Among genetic risk factors, we found that a family history of breast or ovarian cancer is as relevant in men as in women. A positive family history of breast or ovarian cancer was recorded in 29.7 % of our patients, which is slightly higher percent than reported previously [3]. Mutational analysis for the BRCA1/2 genes was performed in 17/47 patients (32.2 %). BRCA2 mutations were found in five cases (29.4 %) and BRCA1 mutations in only one patient. Despite the low percentage of patients who underwent genetic testing, the greater association with BRCA2 mutations compared to BRCA1 mutations in our MBC patients is in line with a previous report [5].
Carriers of BRCA1 and BRCA2 mutations genes are at an increased risk for cancer at body sites other than breast, such as prostate, stomach, pancreatic cancers and melanoma [3]. According to previous data, about 19 % of our patients developed a second tumor [3].
The role of family history, germinal mutation of BRCA1/2 genes, risk of cancers associated to breast and ovarian cancer syndrome related to BRCA1/2 genes, suggest that cancer genetic counseling for MBC patients should contribute to better clarify genotype-phenotype correlations and to personalize surveillance strategies according to international guidelines (http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp#site).
We found that T stage at diagnosis was heterogeneous, but 44.7 % of our patients were at stage II with axillary lymph node involvement in almost half of these cases (40.5 %). Despite the small sample size, stage at diagnosis was in line with previously described in a larger report [21]. Because of the lower level of awareness among men, and a low index of suspicion for the disease there is often a diagnostic delay. Moreover, a screening program is not available for men because of the low lifetime risk [9].
In the present study, there was a left-sided preponderance over the right side (61.7 % versus 36.2 % respectively) consistent with a previous report [21]. As expected, the most prevalent histological subtype was invasive ductal carcinoma. Our data confirm the high rate of hormone-receptor positivity associated with MBC [22]. In fact, 88.4 % of our MBC patients were ER+ and 81.4 % PgR+. We found that 26.8 % of our patients were HER2 positive. Data on HER2 status in MBC is very heterogeneous, with HER2 overexpression rates ranging from 2 % to 42 % [23–26].
The most frequently used surgical procedure for loco-regional treatment of MBC is modified radical mastectomy (MRM) and it was performed in 40 of our patients (80.5 %), which is consistent with a previous study [27]. This approach is probably preferred given the anatomic characteristics of male breast tissue, the limited surgical sequelae and the better cosmetic outcome compared to other procedures [3, 28]. Most of our patients underwent axillary dissection, as suggested in the literature [5]. In recent years, the technique of sentinel lymph node (SLN) is a reliable tool in MBC patients, as shown in recent experiences [29–31].
The criteria for administration of post-surgical radiation are usually extrapolated from data obtained in women, due to the absence of controlled trials [32]. In the present series, postoperative radiotherapy was performed in 14 of 43 patients (32.6 %) and 7 of them received post-mastectomy radiotherapy. Several studies showed that radiation reduces the post-operative loco-regional recurrence rate [33–36], but only one study demonstrated a survival benefit [36].
In our study, tamoxifen was the most frequent adjuvant hormone therapy and AIs alone were used in 6 (16.2 %) patients. Endocrine therapy was found to be beneficial in small retrospective studies [37–39]. In the study by Goss et al. [39] adjuvant hormone treatment with tamoxifen significantly improved disease-free and overall survival, thereby representing the standard of care. The role of AIs in male patients is not as clear as it is for women. Monotherapy with AIs does not completely restrain estrogen production because they do not inhibit the testicular production of estrogen, which represents 20 % of circulating estrogen [40]. Moreover, AI administration causes an increase in the levels of luteinizing hormone and follicle stimulating hormone that could lead to an increase in the substrate for aromatization, suggesting that AIs could be used in combination with a luteinizing hormone-releasing hormone analog [41]. Aromatase inhibitors are mainly used in metastatic patients who are resistant to tamoxifen or with contraindications to tamoxifen therapy [41].
The role of chemotherapy in MBC is not well defined and only CMF has been prospectively evaluated in the adjuvant setting [42, 43]. In the study conducted by Giordano et al., 63 % of patients undergoing systemic treatment received chemotherapy (alone or in combination with hormone therapy) and anthracycline-based chemotherapy, more frequently used than CMF (81 % versus 16 %), had a reduced risk of death [44]. In our series, adjuvant chemotherapy was administered to 29 patients (70.7 %) and antracycline-based treatment (with or without taxane) was the preferred regimen. The patient’s evaluation was performed considering different clinical and pathological features such as tumor stage, ER/PgR and Ki-67 level, HER-2 status, age and comorbidities, as commonly done in female BC patients, in order to select those who may benefit from systemic treatment. The high percentage of patients undergoing chemotherapy could be explained by the following considerations: 1) 40 % of patients had nodal positive disease; 2) patients with hormone-receptors negative disease were considered at high-risk and received chemotherapy, particularly, those patients with triple negative phenotype (7 % of patients); 3) patients with HER-2 positive disease were treated with trastuzumab plus chemotherapy (roughly 27 % of patients); 4) 65 % of patients were found to have high levels of Ki-67; 5) no patients refused treatment or presented age-related comorbidities contraindicating chemotherapy; 6) the majority of the patients included in our series received diagnosis of MBC after 2000s (Fig. 1).
In our series, the estimated long-term survival was about 90 % at 5 years, 80 % at 10 years and 70 % at 18–20 years. Percentage of long-term survival is higher for our patients compared to data reported in literature. MBC has a worse overall prognosis than female breast cancer, namely, an overall 5-year survival rate of 40–65 % versus 85 % in women [45]. However, when matched for stage, age and prognostic factors, the prognosis is similar [45]. The worse outcome in men seems primarily due to the presence of more advanced disease at diagnosis, and gender itself does not seem to be a prognostic factor [46]. Evidence on survival in MBC is quite small compared with female BC and the wide range of survival described likely reflects the heterogeneity of disease stage and different treatments strategies across time. Our high rates of survival might be related to several aspects such as patient characteristics, tumor features, and treatments strategies adopted. To better put our results into the context of previous literature, the following considerations should be taken into account: 1) the most important prognostic factors in MBC seem to be patient age, tumor stage and lymph node status. Fentiman et al. reported 5-year survival rates of 75–100 % for stage I, 50–80 % for stage II, and 30–60 % for stage III [47]. Additionally, some data showed that 40 % of patients with MBC will die for other causes [3], likely reflecting the influence of comorbidities and older mean age at diagnosis. Most of our patients were diagnosed in the sixth decade of life and at early TNM stage (approximately 79 % stage I-II), without significant age-related comorbidities contraindicating the treatment; 2) 90 % of our patients received hormone therapy, mainly tamoxifen which demonstrated to decrease recurrence and improve overall survival [39]; 3) 70 % of our patients received adjuvant chemotherapy and previous studies showed that patients undergoing chemotherapy have survival benefits compared to those without chemotherapy [45, 46]. Moreover, most of our patients received antracycline and/or taxanes rather than CMF, and this was previously found to be associated with better survival in female disease; 4) trastuzumab was used in all HER2 positive disease and it is well-known to improve survival in female BC.
Obviously, we cannot draw definitive conclusions on the impact of each factor on the overall survival, however, this experience could be useful for the current limited knowledge on MBC and for driving future prospective studies.
In women, BRCA-associated BC tends to manifest specific genotype–phenotype correlations [48], whereas little is known about the phenotype characteristics of BRCA-associated MBC. A recent study identified more high-grade, progesterone-receptor negative, HER2-positive disease in male patients who carried BRCA2 mutations [9], and earlier research found poorer prognosis in men with BRCA2-associated tumors [9]. Interestingly, we found that patients with BRCA mutation showed a low survival compared with BRCA wild-type. Despite small size, this is the second study on association with prognosis and BRCA status. Our data are consistent with the poorer prognosis previously reported [9].
One of the limits of this study is its retrospective nature and the small number of patients enrolled. However, it is difficult to conduct randomized trials or large multicenter studies because of the rarity of MBC. Other limitations are: 1) the presence of some missing data; and 2) the availability of BRCA status just for 17 patients. However, many of our patients have a long-term follow-up and some were referred to our center in the 1990s when BRCA testing was not a routine procedure.
Conclusion
This study has shown a high long-term survival rate in patients with MBC compared with other studies. A significantly reduced survival rate was registered in the subgroup of patients carrying BRCA1/2 mutations in line with the poorer prognosis previously reported in the same setting. MBC remains a challenge and future larger studies are warranted. However, given the low incidence of the disease, prospective studies are difficult to plan, and retrospective data collections such as our series play an important role in acquiring information about MBC.
Abbreviations
AIs, aromatase inhibitors; BIC, breast cancer information core database; BMI, body mass index; CMF, cyclophosphamide, methotrexate, 5-fluoracil; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IQR, interquartile range; Ki-67, proliferation index; LOVD-IARC, Leiden Open Variation Database; MBC, male breast cancer; MRM, radical mastectomy; PgR, progesterone receptor; SLN, sentinel lymph node
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073.
Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918–26. doi:10.1002/ijc.27841.
Korde LA, Zujewwski JA Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114–22. doi:10.1200/JCO.2009.25.5729.
Brinton LA, Cook MB, McCormack V, Johnson KC, Olsson H, Casagrande JT, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst. 2014;106(3):djt465. doi:10.1093/jnci/djt465.
Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24:1434–43. doi:10.1093/annonc/mdt025.
Thompson D, Easton D. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia. 2004;9:221–36.
Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, et al. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast cancer research: BCR. 2002;4(1):R2.
Sverdlov RS, Barshack I, Bar Sade RB, Baruch RG, Hirsh-Yehezkel G, Dagan E, et al. Genetic analyses of male breast cancer in Israel. Genet Test. 2000;4:313–7.
Ottini L, Silvestri V, Rizzolo P, Falchetti M, Zanna I, Saieva C, et al. Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: results from a collaborative multicenter study in Italy. Breast Cancer Res Treat. 2012;134(1):411–8. doi:10.1007/s10549-012-2062-0.
Ottini L. Male breast cancer: a rare disease that might uncover underlying pathways of breast cancer. Nat Rev Cancer. 2014;14(10):643.
Friedman LS, Gayther SA, Kurosaki T, Gordon D, Noble B, Casey G, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60:313–9.
Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, Borg A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58:1367–71.
Contegiacomo A, Pensabene M, Capuano I, Tauchmanova L, Federico M, Turchetti D, et al. An oncologist-based model of cancer genetic counseling for hereditary breast and ovarian cancer. Ann Oncol. 2004;15(5):726–32.
https://research.nhgri.nih.gov/bic/ [Last Modified: 08 Feb 2016].
http://chromium.lovd.nl/LOVD2/cancer/home.php?select_db=BRCA2 [Last Modified: 06 Sept 2012].
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8-30. doi:10.1093/annonc/mdv298.
Contractor KB, Kaur K, Rodrigues GS. Kulkarni DM, Singhal H. Male breast cancer: Is the scenario changing? World J Surg Oncol. 2008; doi:10.1186/1477-7819-6-58.
Ahn J, Schatzkin A, Lacey Jr JV, Albanes D, Ballard-Barbash R, Adams KF, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091–102.
Rohrmann S, Shiels MS, Lopez DS, Rifai N, Nelson WG, Kanarek N, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control. 2011;22(8):1141–51. doi:10.1007/s10552-011-9790-z.
Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, Rinaldi S, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121(6):1331–8.
Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: A population based study. Cancer. 2004;101:51–7.
Cutuli B, Le-Nir CC, Serin D, Kirova Y, Gaci Z, Lemanski C, et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol. 2010;73:246–54. doi:10.1016/j.critrevonc.2009.04.002.
Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Molecular subtyping of male breast cancer by immunohistochemistry. Mol Pathol. 2012;25(3):398–404. doi:10.1038/modpathol. 2011.
Rudlowski C, Friedrichs N, Faridi A, Füzesi L, Moll R, Bastert G, et al. Her-2/neu gene amplification and protein expression in primary male breast cancer. Breast Cancer Res Treat. 2004;84(3):215–23.
Ge Y, Sneige N, Eltorky MA, Wang Z, Lin E, Gong Y, et al. Immunohistochemical characterization of subtypes of male breast carcinoma. Breast Cancer Res. 2009;11(3):R28. doi:10.1186/bcr2258.
Shaaban AM, Ball GR, Brannan RA, Cserni G, Di Benedetto A, Dent J, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012;133(3):949–58. doi:10.1007/s10549-011-1856-9.
Zhou FF, Xia LP, Guo GF, Wang X, Yuan ZY, Zhang B, et al. Changes in therapeutic strategies in Chinese male patients with breast cancer: 40 years of experience in a single institute. Breast. 2010;19(6):450–5. doi:10.1016/j.breast.2010.04.007.
Gómez-Raposo C, Zambrana Tévar F, Sereno Moyano M, López Gómez M, Casado E. Male breast cancer. Cancer Treat Rev. 2010;36:451–7. doi:10.1016/j.ctrv.2010.02.002.
Gentilini O, Chagas E, Zurrida S, Intra M, De Cicco C, Gatti G, et al. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist. 2007;12(5):512–5.
Boughey JC, Bedrosian I, Meric-Bernstam F, Ross MI, Kuerer HM, Akins JS, et al. Comparative analysis of sentinel lymph node operation in male and female breast cancer patients. J Am Coll Surg. 2006;203(4):475–80.
Flynn LW, Park J, Patil SM, Cody 3rd HS, Port ER. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. J Am Coll Surg. 2008;206(4):616–21. doi:10.1016/j.jamcollsurg.2007.11.005.
Bratman SV, Kapp DS, Horst KC. Evolving trends in the initial locoregional management of male breast cancer. Breast. 2012;21(3):296–302. doi:10.1016/j.breast.2012.01.008.
Yu E, Suzuki H, Younus J, Elfiki T, Stitt L, Yau G, et al. The impact of post-mastectomy radiation therapy on male breast cancer patients—a case series. Int J Radiat Oncol Biol Phys. 2012;82(2):696–700. doi:10.1016/j.ijrobp.2011.01.010.
Yoney A, Kucuk A, Unsal M. Male breast cancer: a retrospective analysis. Cancer Radiother. 2009;13(2):103–7. doi:10.1016/j.canrad.2008.11.011.
Atahan L, Yildiz F, Selek U, Sari S, Gurkaynak M. Postoperative radiotherapy in the treatment of male breast carcinoma: a single institute experience. J Natl Med Assoc. 2006;98(4):559–63.
Atalay C, Kanlioz M, Altinok M. Prognostic factors affecting survival in male breast cancer. J Exp Clin Cancer Res. 2003;22(1):29–33.
Fogh S, Hirsch AE, Langmead JP, Goldberg SI, Rosenberg CL, Taghian AG, et al. Use of tamoxifen with post surgical irradiation may improve survival in estrogen and progesterone receptor-positive male breast cancer. Clin Breast Cancer. 2011;11(1):39–45. doi:10.3816/CBC.2011.n.007.
Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer. 1992;65(2):252–4.
Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer. 1999;85(3):629–39.
Handesman D. Androgen actions and pharmacologic uses. In: Detroit IJ, editor. Endocrinology. Philadelphia, PA: WB Saunders; 2001. p. 2232–42.
Doyen J, Italiano A, Largillier R, Ferrero JM, Fontana X, Thyss A. Aromatase inhibition in male breast cancer patients: biological and clinical implications. Ann Oncol. 2010;21:1243–5. doi:10.1093/annonc/mdp450.
Bagley CS, Wesley MN, Young RC, Lippman ME. Adjuvant chemotherapy in males with cancer of the breast. Am J Clin Oncol. 1987;10:55–60.
Walshe JM, Berman AW, Vatas U, Steinberg SM, Anderson WF, Lippman ME, et al. A prospective study of adjuvant CMF in males with node positive breast cancer: 20 year follow-up. Breast Canc Res Treat. 2007;103:177–83.
Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, et al. Adjuvant systemic therapy for male breast cancer. Cancer. 2005;104:2359–64.
Field KM, Campbell B, De Boer R. Male breast cancer: Progress, prognosis and future pathways. Asia–Pacific Journal of Clinical Oncology. 2008;4:6–17.
Macdonald G, Paltiel C, Olivotto IA, Tyldesley S. A comparative analysis of radiotherapy use and patient outcome in males and females with breast cancer. Ann Oncol. 2005;16:1442–8.
Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367(9510):595–604.
Vargas AC, Reis-Filho JS, Lakhani SR. Phenotype-genotype correlation in familial breast cancer. J Mammary Gland Biol Neoplasia. 2011;16(1):27–40. doi:10.1007/s10911-011-9204-6.
Acknowledgements
The authors thank Jean Gilder for her support in editing the manuscript and dr. Giuseppe Gargiulo (Department of Advanced Biomedical Sciences, Federico II University) for his support in graphics.
Funding
None.
Availability of data and materials
Authors can be directly contacted for collaboration or data sharing.
Authors’ contributions
PG conceived and designed the study, carried out the acquisition, analysis and interpretation of data, drafted the manuscript; MP carried out the acquisition, analysis and interpretation of data, revised critically the manuscript; MM, VF, GA, MG, CC carried out the acquisition and interpretation of data and revised critically the manuscript; RL, SDP participated at study design, and interpretation of data and revised the manuscript critically for intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All the patients included in this retrospective analysis provided their written informed consent in the framework of cancer genetic counseling program regulated and approved by the Local Ethic committee (University ‘Federico II’ of Naples, Prot. 80/00 and 63/02).
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient/parent/guardian/relative of the patient. A copy of the consent form is available for review by the Editor of this journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gargiulo, P., Pensabene, M., Milano, M. et al. Long-term survival and BRCA status in male breast cancer: a retrospective single-center analysis. BMC Cancer 16, 375 (2016). https://doi.org/10.1186/s12885-016-2414-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2414-y