Abstract
Background
People from lower socioeconomic groups have worse survival outcomes for cancer, which in part reflects later-stage disease at diagnosis. The mechanisms underlying delayed cancer symptom presentation in lower socioeconomic groups are not well understood.
Methods
Systematic review of studies of actual or anticipated symptom presentation across all tumour sites. Included studies measured socioeconomic group, symptom presentation and one or more of the following variables: cancer symptom knowledge, beliefs about cancer, barriers/facilitators to symptom presentation.
Results
A total of 60 studies was included. Symptom knowledge overall was lowest and actual presentation time was longest in lower socioeconomic groups. Knowledge for specific symptoms such as lumps and bleeding was good and encouraged timely symptom presentation, in contrast to non-specific symptoms which were not well recognised. The combination of fearful and fatalistic beliefs was typically associated with later presentation, especially in lower socioeconomic groups. Emotional barriers such as ‘worry what the doctor might find’ were more frequently reported in lower socioeconomic groups, and there was evidence to suggest that disclosing symptoms to family/friends could help or hinder early presentation.
Conclusions
Poor symptom knowledge, fearful and fatalistic beliefs about cancer, and emotional barriers combine to prolong symptom presentation among lower socioeconomic groups. Targeted interventions should utilise social networks to improve knowledge of non-specific symptoms, challenge negative beliefs and encourage help-seeking, in order to reduce avoidable delays and minimise socioeconomic group inequalities.
Similar content being viewed by others
Background
Socioeconomic inequalities in cancer survival outcomes exist, but the reasons for this are not fully understood [1–3]. Survival differences are likely to reflect later-stage disease at diagnosis [2, 4, 5] partly as a consequence of delayed cancer symptom presentation in people from lower socioeconomic groups [6]. By eradicating socioeconomic inequalities at stage of diagnosis, it is estimated that 5600 patients in the UK annually could be diagnosed with earlier stage disease [7], and that 11 % of deaths from cancer could be avoided if three-year survival in lower socioeconomic groups matched that in higher socioeconomic groups [1].
‘Patient delay’ is defined as the time between discovery of a cancer symptom and the initial visit to a healthcare professional. It accounts for the greatest proportion of delay time in the pathway from symptom discovery to the start of cancer treatment [8–10] and has been associated with socioeconomic deprivation [6]. Patient delay has been conceptualised in Walter et al.’s Model of Pathways to Treatment, with various stages involving an ‘appraisal interval’ during which the individual detects a bodily change, and a ‘help seeking interval’ in which the individual decides to seek medical help (see Fig. 1 [11]). Evidence suggests that knowledge of cancer symptoms is important during the appraisal stage, with potential misattribution of symptoms attenuating the decision to present [12, 13]. Beliefs about cancer are considered to be important in both the appraisal and help-seeking stages, where emotions such as fear might influence interpretation of symptoms [12] and the decision to seek medical help [6, 14–17]. Barriers such as competing life events and ease of getting a medical appointment are thought to delay symptom presentation during the help-seeking interval [11].
Model of pathways to treatment. Produced with permission of SAGE Publications Ltd., London, Los Angeles, New Delhi, Singapore and Washington DC, from Walter FM, Scott SE, Webster A, Emery JD. ‘The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis’. J Health Services Research & Policy (© Walter, 2012)
The contribution of socioeconomic and other demographic factors to delayed presentation has been highlighted in the Model of Pathways to Treatment, and more recently in the updated National Awareness and Early Diagnosis Initiative (NAEDI) framework designed to conceptualise the route from public awareness and beliefs about cancer to cancer survival outcomes ([18]). Empirical evidence supports associations between lower socioeconomic group and poor cancer symptom knowledge [19], fearful and fatalistic beliefs about cancer [20] and emotional barriers such as worry about what the doctor may find [19]. These findings help to explain why people from lower socioeconomic groups tend to present with more advanced stage cancers, and hence have worse survival outcomes [1–5]. However, a more detailed understanding of psychosocial influences on the relationship between socioeconomic deprivation and cancer symptom presentation is essential to developing behavioural interventions designed to promote timely presentation and reduce socioeconomic inequalities in cancer outcomes.
Attempts to understand why people might delay seeking medical help for cancer symptoms have examined actual or anticipated symptom presentation behaviour, exploring perceived barriers to symptom presentation. Prospective study designs are difficult due to follow-up of a large sample, so studies frequently use retrospectively recalled or hypothetically anticipated symptom study designs. Previous reviews have focused on tumour site-specific delay factors [15, 16, 21] or common cancers only [6], or have been restricted to qualitative studies [17] and patients with cancer [6, 16, 17]. The purpose of the current systematic review was to explore how knowledge, beliefs and barriers/facilitators to symptom presentation affect actual or anticipated cancer symptom presentation in relation to socioeconomic group and across all tumour sites.
Method
Identification of included studies followed the PRISMA guidelines [22]. The protocol was registered on PROSPERO (CRD42014013220 [23]) and is available on the NIHR HTA programme website (www.hta.ac.uk). At all stages of the search, data extraction and quality appraisal, 10 % of studies were double checked for consistency by a second member of the research team (RR). All discrepancies were resolved through discussion.
Search strategy
The literature was searched up to July 2015 on the electronic databases of MEDLINE, PsychINFO, EMBASE and CINAHL. The de-duplicate function was used on Ovid and CINAHL before reviewing abstracts. Manual searches of reference lists of included studies were performed. A SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) search strategy tool was used for retrieval of studies (see Additional file 1: Appendix 1 [24]). Databases were searched using terms relating to symptom presentation, cancer symptom knowledge, beliefs about cancer, perceived barriers and facilitators to symptom presentation (see Additional file 1: Appendix 1).
Inclusion criteria
Publications that measured and reported data for symptom presentation and socioeconomic group were included. ‘Symptom presentation’ was defined as actual symptom presentation (retrospectively recalled) or anticipated symptom presentation (hypothetically estimated) measured as continuous (time to presentation) or binary (did/did not present) variables. ‘Socioeconomic group’ was defined in terms of individual level socioeconomic indicators including education, income, home/car ownership, occupation and employment, and/or area-level indicators based on postcode. In addition, publications were included if they measured and reported one or more of the following domains of interest:
-
‘Knowledge’: studies which assessed knowledge for the symptoms of cancer through recall e.g. ‘What symptoms of cancer can you list?’ or recognition methods e.g. ‘Which of these are symptoms of cancer?’, or through retrospective recall of symptom interpretation at the time of symptom discovery.
-
‘Beliefs’: studies which explored any positive (e.g. beliefs about the benefits of early diagnosis and curability) or negative (e.g. fear and fatalism) beliefs surrounding cancer.
-
‘Perceived barriers/facilitators’: studies which assessed any anticipated or actual barriers or facilitators to symptom presentation.
There were no restrictions on date of publication or study methodology. Only English language studies from high income countries as classified by Organisation for Economic Co-operation and Development (OECD) membership (OECD, 2014 [25]) were included.
Exclusion criteria
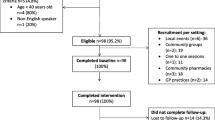
Studies that did not measure and report symptom presentation, socioeconomic group and one or more of the domains of interest were excluded. Studies not relating to cancer, and those examining screening behaviour, self-examination behaviour, efficacy of interventions, genetic risk, healthcare professionals’ perspective, cancer prevention, treatments for cancer or living with cancer and studies involving children were excluded. Studies from low/middle income countries, not written in English, review papers or conference abstracts were excluded (Fig. 2).
PRISMA flow diagram. Produced using a downloadable template available at http://www.prisma-statement.org/ (Moher et al, 2009 [22])
Data extraction and synthesis
Data were extracted onto a template using the following headings: method, sample characteristics, tumour site, symptom presentation, knowledge, beliefs, perceived barriers/facilitators and socioeconomic group measure. A meta-analysis was precluded due to the heterogeneity of included studies and a narrative synthesis was performed [26].
Critical appraisal
The methodological quality of all included studies was examined using the Critical Appraisal Skills Programme tool (CASP, 2014 [27]) appropriate for the study design. Quality was assessed according to each domain on the CASP checklists: rationale of study, methodology, design, recruitment, data collection, data analysis, ethical issues, reporting of findings and contribution to research. Overall quality was categorised as good, medium or poor.
Results
The search returned a total of 1536 studies after 810 duplicates had been removed. A total of 1309 studies was excluded based on title and abstract, leaving 227 studies to be read in full. A total of 60 studies met the inclusion criteria (see Fig. 2). Eleven of these studies were found through hand searching reference lists.
Included studies employed qualitative methods (n = 15), quantitative methods (n = 42) and mixed methods (n = 3). Quality of studies was good (n = 18), medium (n = 37) and poor (n = 5). Limitations of lower quality studies included measuring but not reporting socioeconomic group differences for all outcome measures, leaving a long period of time between cancer diagnosis and participation in the study and recruitment of samples biased towards higher socioeconomic groups. The overall combined percentage agreement between raters (GM and RR) for inclusion/exclusion of studies, critical appraisal and data extraction was 87 %.
A total of 53 studies examined time to symptom presentation, seven studies reported presentation behaviour (if participants did or did not present or anticipate presenting to their doctor with reported symptoms), 45 studies measured actual symptom presentation, 15 studied anticipated symptom presentation, 46 studies assessed knowledge for cancer symptoms, 32 studies explored beliefs about cancer and 50 studies examined perceived barriers/facilitators to symptom presentation. The numbers of studies by tumour site were as follows: breast (n = 22), any cancer/multiple tumour sites (n = 15), colorectal (n = 7), skin (n = 6), oral and pharyngeal (n = 3), ovarian (n = 3), lung (n = 2), gynaecological (n = 1), and prostate (n = 1) (see Table 1). Results are presented according to domain headings.
Symptom presentation
Studies involving anticipated symptom presentation reported shorter time to symptom presentation compared with studies that examined actual time to symptom presentation. In the former, most participants anticipated seeking medical help within one week [28–30] or within one month [19, 31, 32], in contrast to real-world studies where it was more common for patients to have waited over two months before seeking medical help [33–41]. The most prompt actual and anticipated symptom presentation was reported for lumps [32, 38, 42–47] or bleeding [19, 32, 48–53]. Studies examining participants who reported experiencing a potential symptom of cancer in the past three months found between 59 % and 75 % of participants had consulted a doctor about their symptom [49, 54, 55].
Disparity between actual and anticipated symptom presentation relating to socioeconomic group was observed. In five studies, shorter anticipated time to symptom presentation was observed in lower compared to higher socioeconomic groups [19, 28, 31, 32, 48]. Conversely, in two studies, longer anticipated time to symptom presentation was reported in those from lower socioeconomic groups compared with higher socioeconomic groups [56, 57].
Studies which measured actual time to symptom presentation reported the longest delays in symptom presentation among individuals with lower educational attainment [33, 34, 42, 54, 58–61], lower annual income [61, 62], lower occupation and employment [43, 61, 63] and those from deprived areas [64]. This effect was also observed in studies of actual symptom presentation where multiple socioeconomic indices were reported [34, 42, 44, 61, 65, 66]. Twenty-two studies found no group differences for socioeconomic group indicators and time to symptom presentation [30, 33, 35–37, 45, 46, 49, 50, 52, 60, 67–77].
Knowledge
Knowledge of symptoms based on recall methods was generally lower than in studies that used recognition methods. Lump symptoms were the most recalled and well-recognised potential cancer symptom [19, 32, 48, 50, 53, 56, 64, 78]. This was supported by retrospective studies where patients presenting with a lump were most likely to have attributed their lump symptom to cancer [39, 43, 45, 74, 79]. Knowledge was generally poor for non-specific symptoms of cancer. Symptoms such as fatigue or unexplained weight loss were poorly recalled or recognised as potential symptoms of cancer [28, 29, 31, 53, 78]. Poorer cancer symptom knowledge was associated with lower socioeconomic group when measured by educational attainment [28, 32, 40, 50, 54, 56], occupation [53] and multiple indicators [19, 28, 66]. These findings were consistent across site-specific and non site-specific studies, suggesting poor general cancer symptom knowledge in lower socioeconomic groups regardless of cancer type.
In retrospective studies, patients experiencing non-specific symptoms recalled attributing them to other benign causes or life stresses [35, 51, 55, 65, 69, 76, 80, 81] or not recognising the seriousness of their symptoms [9, 33, 35, 37, 40, 42, 43, 45, 47, 51, 54, 55, 57, 60, 65, 68, 76, 77, 81, 82] resulting in patients delaying symptom presentation [35, 39, 51, 76] or later stage at diagnosis [69].
Beliefs about cancer
In most studies, beliefs were formed from participants’ past experiences of cancer, usually witnessing friends or family with the disease [36, 43, 47, 59, 78, 79]. Positive beliefs were identified in nine studies [30, 36, 43, 48, 54, 58, 78, 79] and tended to focus on the effectiveness of modern cancer treatments, where participants expressed trust in doctors and the medical system and endorsed the benefits of early diagnosis [30, 58, 59, 78] or acknowledged that cancer can be cured [78]. Such beliefs tended to encourage timely symptom presentation to a primary care physician [30, 58, 59, 78, 79]. One study found that those with lower educational attainment were less likely to endorse positive beliefs about the benefits of early detection [54].
Negative beliefs tended to manifest in fear or fatalism regarding cancer. Fear was frequently reported across all studies examining beliefs. This included fear of diagnosis [34, 39, 58, 63, 74, 80, 81, 83], fear of treatment [30, 43, 57–59, 68, 78, 83] and fear of dying [59, 78, 83]. Fatalistic beliefs were a common theme throughout studies, but were expressed only by a minority of participants per study [34, 36, 42, 56, 59, 61, 78, 79, 84]. Fearful and fatalistic beliefs about cancer were more likely to be expressed by individuals from lower socioeconomic groups based on educational attainment [36, 50] or multiple indices [42, 71].
When considering time to symptom presentation, fearful beliefs about cancer appeared to operate at the two extremes of immediate or prolonged symptom presentation. For participants whose fearful beliefs encouraged immediate (actual or hypothetical) presentation to doctors [43, 45, 58, 59, 61, 74, 78, 79, 84], a visit to doctors was used to alleviate anxiety associated with the symptom [43, 47, 58, 59, 61, 77, 78]. This was usually coupled with the participant expressing trust in the medical profession and positive beliefs surrounding early diagnosis [43, 59].
For individuals whose fearful beliefs led to prolonged delays (sometimes years) [30, 34, 38, 39, 43, 47, 51, 61, 68, 74, 78, 79], denial of or ignoring symptoms initially alleviated anxiety associated with the symptom [38, 39, 47, 59, 68, 72, 76, 78, 79]. Such beliefs were usually combined with fatalistic beliefs such as ‘cancer cannot be cured’ [59, 61, 79], and were associated with the longest times to symptom presentation or were expressed by those with advanced stage disease [36, 56, 59, 84]. This is likely to reflect a lack of perceived benefit in presenting to doctors due to the belief that ‘nothing can be done’ [59, 78].
Barriers to symptom presentation
Some participants reported service barriers relating to concerns about wasting doctors’ time [19, 29, 31, 34, 41, 43, 55, 80, 81], lack of continuity with primary care doctor [42, 81] or difficulties with accessing and making an appointment [29, 32, 34, 38, 53, 55, 56, 65, 78, 81]. For others, practical barriers such as being ‘too busy to make an appointment’ were reported and these delayed symptom presentation [31, 39, 40, 43, 49, 74, 77, 78]. Low general health service utilisation for acute or long term conditions lengthened time to cancer symptom presentation [9, 34, 42, 43, 57, 58, 61, 66, 68, 73, 77, 78, 80, 84]. Emotional barriers included embarrassment or fear associated with undergoing intimate diagnostic tests [19, 29, 31, 34, 35, 49, 57, 78, 81].
Practical barriers such as ‘being too busy’ were more frequently reported in high socioeconomic groups [19]. In countries where patients pay for their healthcare, those with lower annual income were more likely to report the cost of a consultation as a barrier to symptomatic presentation [38, 63].
Facilitators to symptom presentation
The most common facilitator of symptom presentation was disclosure of symptoms to a family member or friend [34, 39, 41, 43, 45, 47, 55, 61, 65, 70, 73, 76–79, 81, 84, 85]. In some cases, this reduced time to symptom presentation by half [36] or by six times [45]. The appearance of a new symptom [43, 69, 76, 83] or persistence of the current symptom [45, 69, 76, 81, 84] facilitated decisions to seek medical help. One study found that individuals from a lower socioeconomic group who disclosed their symptom to a family member or friend took longer to seek medical help compared to those from a higher socioeconomic group [65]. In five studies, participants waited until they developed another health complaint or tagged their cancer symptom on to the end of a consultation which provided an opportunity to disclose the cancer symptom during the consultation [42, 45, 68, 81, 82].
Discussion
This review is the first to systematically explore how knowledge, beliefs and barriers/facilitators to symptom presentation affect actual or anticipated cancer symptom presentation in relation to socioeconomic group, across all tumour sites. Poor knowledge of non-specific cancer symptoms such as fatigue and weight loss prolonged presentation due to misattribution of symptoms in lower socioeconomic groups. In contrast, lump and bleeding symptoms were most frequently recalled, recognised and prompted the fastest symptom presentation. A knowledge gradient was observed, where poorer cancer symptom knowledge was associated with lower socioeconomic group based on multiple indices. There was some evidence to suggest that those from a lower socioeconomic group were more likely to hold fearful and fatalistic beliefs about cancer and less likely to endorse positive beliefs about the benefits of early diagnosis. Such combinations of fearful and fatalistic beliefs were associated with prolonged symptom presentation. In addition, emotional barriers to symptom presentation such as worry what the doctor might find were more likely to be endorsed in lower socioeconomic groups. Such poor knowledge and prevalent beliefs might account for the long actual delays and later stage cancers diagnosed in lower socioeconomic groups. Disclosure of a symptom to a family member or friend was a key facilitator in the decision to seek medical help, although there was some evidence to suggest that symptom disclosure acted as a barrier in lower socioeconomic groups.
Most included studies were of medium quality. In many studies, socioeconomic group was measured but not reported for all outcome variables. Most studies only reported socioeconomic group differences for symptom presentation. Twenty-three studies reported socioeconomic group differences for the other outcome measures: knowledge, beliefs and barriers/facilitators to symptom presentation. A further eight studies could have met the inclusion criteria, but were excluded due to non-reporting of any outcomes associated with socioeconomic group [14, 86-92]. Methodological limitations included a long duration between cancer diagnosis and participation in retrospective studies, and samples biased towards higher socioeconomic groups. In some studies, socioeconomic variation was insufficient to perform statistical analysis on all outcomes.
There are methodological limitations associated with retrospective (actual symptom presentation) and hypothetical (anticipated symptom presentation) designs. Whilst retrospective studies are affected by recall bias, hypothetical studies rely on intentions which may not translate into actual presentation behaviour [93]. This was observed in the variation between actual and hypothetical time to symptom presentation, where participants anticipated prompt symptom presentation but in reality reported longer delays. Study designs exploring actual symptom presentation behaviour in a population sample are likely to reduce some of the limitations associated with retrospective and hypothetical symptom presentation study designs. In such study designs, participants disclose actual symptoms experienced in the past three months, usually prompted by a list (without any mention of cancer), and reasons for not consulting a doctor are explored [49, 54, 55, 81].
The limitations of this review include problems relating to retrieval of studies and analysis of the evidence. Due to poor indexing of studies in this topic area under the MeSH indexing in this topic area, a high proportion of studies (n = 11) was found through hand-searching. Additionally, meta-analysis was precluded by the wide range of qualitative and quantitative data collection methods of included studies. Finally, other factors such as age, gender and ethnicity can affect symptom presentation [6, 18]; however, interactions between these variables and socioeconomic group were not addressed in the current review.
The findings of the current review confirm that failure to appreciate the seriousness of symptoms [6, 16] and non-disclosure of symptoms [6, 15] lengthened time to symptom presentation. Our findings accord with previous studies in which negative beliefs [20], longer time to actual symptom presentation [6] and low suspicion for cancer symptoms [94] were associated with low socioeconomic group [6]. The current findings support Mitchell et al.’s (2008) [16] review of colorectal cancer patients, in which fear of cancer either lengthened or shortened time to symptom presentation. Such findings might be explained by Type I and Type II information processing systems. Type I processing is a fast and automatic system, which represents an individual’s ‘gut reaction’ to an event, whereas Type II is a slower, more thoughtful and deliberative system [95]. Whilst most people initially experience fear in reaction to a worrying symptom (Type I processing), cognitions during Type II processing may influence the decision to seek medical help since these are slower and may help someone to rationalise the situation [96]. If an individual has had time to consider the benefits of seeking medical help, and based upon their previous beliefs about early diagnosis, such beliefs may override the Type 1 fear response. We found evidence to suggest a higher prevalence of fearful and fatalistic beliefs in lower socioeconomic groups and some evidence for fewer positive beliefs surrounding the benefits of early diagnosis in lower socioeconomic groups. This suggests that Type I beliefs may not be overridden by Type 2 responses relating to the benefits of early diagnosis due to lower knowledge or higher emotive responses. As a consequence this may delay symptom presentation. Findings relating to symptom disclosure suggest that people use the ‘lay system’ of healthcare (consulting family and friends) before making the decision to access formal healthcare [13, 97, 98]. However, among individuals from low socioeconomic groups, disclosing symptoms to someone with equally poor knowledge and Type I negative automatic beliefs about cancer may encourage false reassurance in the benign nature of symptoms and consequently no urgency to seek medical help.
Cancer awareness interventions should be carefully developed to target those who are most likely to present with advanced stage disease: lower socioeconomic groups with low symptom knowledge and fearful and fatalistic beliefs about cancer. Such an intervention should utilise an individual’s social networks to facilitate distribution of information [97], highlighting the significance of non-lump symptoms as potentially indicative of cancer, along with advice on an appropriate time in which an individual should seek medical help and how to access such help [99]. This should be coupled with information outlining the benefits of early diagnosis and improved effectiveness of modern treatments for cancer, countering negative beliefs surrounding cancer. Future research should evaluate the effectiveness of such interventions in lower socioeconomic groups.
Conclusion
Knowledge of potential cancer symptoms, beliefs about cancer and barriers to symptom presentation work in combination to influence symptom presentation: knowledge is necessary for accurate symptom appraisal, but beliefs about cancer and barriers to symptom presentation influence the decision to seek medical help or not. This is especially important in the context of socioeconomic deprivation, where lower knowledge, higher negative beliefs about cancer and perceived barriers may lead to avoidable delays, later stage of diagnosis and ultimately poorer survival outcomes. Targeted interventions should not only educate people about symptoms for cancer, but also work to break down unhelpful myths surrounding cancer survival and treatment options. They should address the barriers that people in lower socio-economic groups experience, and use social networks to raise awareness and support early symptom presentation.
Abbreviations
- NAEDI:
-
National awareness and early diagnosis initiative
- SPIDER:
-
Sample phenomenon of interest, design, evaluation, research type
- CASP:
-
Critical appraisal skills programme
- OECD:
-
Organisation for economic co-operation and development
References
Ellis L, Coleman M, Rachet B. How many deaths would be avoidable if socioeconomic inequalities in cancer survival in England were eliminated? A national population-based study, 1996-2006. Eur J Cancer. 2012;48(2):270–8.
McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112:S108–15.
Rachet B, Ellis L, Maringe C, Chu T, Nur U, Quaresma M, et al. Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br J Cancer. 2010;103(4):446–53.
Woods LM, Rachet B, Coleman MP. Orogins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19.
Rutherford M, Ironmonger L, Ormiston-Smith N, Abel G, Greenberg D, Lyratzopoulos G, et al. Estimating the potential survival gains by eliminating socioeconomic and sex inequalities in stage at diagnosis of melanoma. Br J Cancer. 2015;112:S116–23.
Macleod U, Mitchell E, Burgess C, Macdonald S, Ramirez A. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. British J Cancer. 2009;101:S92–S101.
Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. 2013;24(3):843–50.
Allgar V, Neal R. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92(11):1959–70.
Ristvedt S, Trinkaus K. Psychological factors related to delay in consultation for cancer symptoms. Psycho-Oncol. 2005;14(5):339–50.
Lyratzopoulos G, Saunders C, Abel G, McPhail S, Neal R, Wardle J, et al. The relative length of the patient and the primary care Interval in patients with 28 common and rarer cancers. Br J Cancer. 2015;112:S35–40.
Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110–8.
Whitaker K, Scott S, Wardle J. Applying symptom appraisal models to understand sociodemographic differences in responses to possible cancer symptoms: a research agenda. Br J Cancer. 2015;112:S27–34.
Low E, Whitaker K, Simon A, Sekhon M, Waller J. Women's interpretation of and responses to potential gynaecological cancer symptoms: a qualitative interview study. BMJ Open. 2015;5(7), e008082.
Sheikh I, Ogden J. The role of knowledge and beliefs in help seeking behaviour for cancer: a quantitative and qualitative approach. Patient Educ Couns. 1998;35(1):35–42.
Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. J Psych Res. 2005;58(4):321–6.
Mitchell E, Macdonald S, Campbell N, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98(1):60–70.
Smith L, Pope C, Botha J. Patients' help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2005;366(9488):825–31.
Hiom S. Diagnosing cancer earlier: reviewing the evidence for improving cancer survival. Br J Cancer. 2015;112:S1–5.
Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, et al. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101:S18–23.
Quaife S, Winstanley K, Robb K, Simon A, Ramirez A, Forbes L, et al. Socioeconomic inequalities in attitudes towards cancer: an international cancer benchmarking partnership study. Eur J Cancer Prev. 2015;24(3):253–60.
Ramirez A, Westcombe A, Burgess C, Sutton S, Littlejohns P, Richards M. Factors predicting delayed presentation of symptomatic breast cancer: a systematic review. Lancet. 1999;353(9159):1127–31.
Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA. Group. Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement. Plos Med. 2009;6(7), e1000097.
McCutchan G, Wood F, Edwards A, Richards, R, Brain K A. Systematic review of cancer awareness, beliefs about cancer and symptomatic presentation in the context of social deprivation. 2014. CRD42014013220 Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013220
Cooke A, Smith D, Booth A. Beyond PICO: The SPIDER Tool for Qualitative Evidence Synthesis. Qual Health Res. 2012;22(10):1435–43.
OECD. http://www.oecd.org/about/membersandpartners/list-oecd-member-countries.htm (2014). Accessed: 6th January 2014.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC Methods Programme. Lancaster: Lancaster University; 2006.
CASP. http://www.casp-uk.net/#!casp-tools-checklists/c18f8 (2014). Accessed 13th May 2014.
Brain KE, Smits S, Simon AE, Forbes LJ, Roberts C, Robbe IJ, et al. Ovarian cancer symptom awareness and anticipated delayed presentation in a population sample. BMC Cancer. 2014;14:171–81. doi:10.1186/1471-2407-14-17.
Forbes L, Atkins L, Thurnham A, Layburn J, Haste F, Ramirez A. Breast cancer awareness and barriers to symptomatic presentation among women from different ethnic groups in East London. Br J Cancer. 2011;105(10):1474–9.
Hunter MS, Grunfield EA, Ramirez AJ. Help-seeking intentions for breast-cancer symptoms: a comparison of the self-regulation model and the theory of planned behaviour. Br J Health Psychology. 2003;8(3):319–33.
Low E, Waller J, Menon U, Jones A, Reid F, Simon A. Ovarian cancer symptom awareness and anticipated time to help-seeking for symptoms among UK women. J Fam Plan R Health. 2013;39(3):163–71.
Quaife S, Forbes L, Ramirez A, Brain K, Donnelly C, Simon A, et al. Recognition of cancer warning signs and anticipated delay in help-seeking in a population sample of adults in the UK. Br J Cancer. 2014;110(1):12–8.
Ristvedt SL, Pruitt SL, Trinkhaus KM. Appraisal of emerging symptoms of colorectal cancer: associations with dispositional, demographic, and tumour characteristics. J Behav Med. 2014;37(4):698–708. doi:10.1007/s10865-013-9519-4.
Kakagia D, Trypsiannis G, Karanikas M, Mitrakas A, Lyratzopoulos N, Polychronidis A. Patient-Related Delay in Presentation for Cutaneous Squamous Cell Carcinoma. A Cross-Sectional Clinical Study. Onkologie. 2013;36(12):738–44.
Siminoff L, Thomson M, Dumenci L. Factors associated with delayed patient appraisal of colorectal cancer symptoms. Psycho-Oncol. 2014;23(9):981–8.
Chonjnacka-Szwalowska G, Koscielak R, Karasiewicz K, Majkowicz M, Kozaka J. Delays in seeking cancer diagnosis in relation to beliefs about the curability of cancer in patients with different disease locations. Psychol Health. 2013;28(2):154–70.
Oliveria SA, Christos PJ, Halpern AC, Fine JC, Barnhill RL, Berwick M. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol. 1999;52(11):1111–6.
Freidman LC, Kalidas M, Elledge R, Dulay MF, Romero C, Chang J, et al. Medical and psychological predictors of delay in seeking medical consultation for breast symptoms in women in a public sector setting. J Behav Med. 2006;29(4):327–34.
Gould J, Fitzgerald B, Fergus K, Clemons M, Baig F. Why women delay seeking assistance for locally advanced breast cancer. Can Oncol Nurs J. 2010;20(1):23–9.
Schmid-Wendtner M, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389–94.
Walter F, Birt L, Cavers D, Scott S, Emery J, Burrows N, Cavanagh G, MacKie R, Weller D, Campbell C. 'This isn't what mine looked like': a qualitative study of symptom appraisal and help seeking in people recently diagnosed with melanoma. BMJ Open. 2014;4(7). doi:10.1136/bmjopen-2014-005566
Coates R, Bransfield D, Wesley M, Hankey B, Eley J, Greenberg R, et al. Differences between black and white women with breast cancer in time from symptom recognition to medical consulation. J Nat Cancer Inst. 1992;84(12):938–50.
Burgess C, Hunter M, Ramirez A. A qualitative study of delay among women reporting symptoms of breast cancer. Br J Gen Pract. 2001;51(473):967–71.
Caplan L. Patient delay in seeking help for potential breast cancer. Public Health Rev. 1995;23(3):263–74.
Burgess C, Ramirez A, Richards M, Love S. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77(8):1343–8.
Meechan G, Collins J, Petrie KJ. The relationship of symptoms and psychological factors to delay in seeking medical care for breast symptoms. Prev Med. 2003;36(3):374–8.
O'Mahony M, Hegarty J. Factors influencing women in seeking help from a health care professional on self discovery of a breast symptom, in an Irish context. J Clin Nurs. 2009;18(14):2020–9.
van Osch L, Lechner L, Reubsaet A, de Nooijer J, de Vries H. Passive cancer detection and medical help seeking for cancer symptoms: (in)adequate behavior and psychosocial determinants. Eur J Cancer Prev. 2007;16(3):266–74.
Simon A, Waller J, Robb K, Wardle J. Patient Delay in Presentation of Possible Cancer Symptoms: The Contribution of Knowledge and Attitudes in a Population Sample from the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2272–7.
McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. 2003;36(5):525–35.
Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56(11):2727–32.
Trivers KF, Rodriguez JL, Hawkins NA, Cooper CP, Polonec L, Gelb CA. Intention to seek care for symptoms associated with gynaecologic cancers, HealthStyles survey, 2008. Prev Chronic Dis. 2011;8(6):1–9.
Waller J, Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, et al. Awareness of cancer symptoms and anticipated help seeking among ethnic minority groups in England. Br J Cancer. 2009;101:S24–30.
Cockburn J, Paul C, Tzelepis F, McElduff P, Byles J. Delay in seeking advice for symptoms that potentially indicate bowel cancer. Am J Health Behav. 2003;27(4):401–7.
Whitaker K, Scott S, Winstanley K, Macleod U, Wardle J. Attributions of Cancer 'Alarm' Symptoms in a Community Sample. Plos One. 2014;9(12):e114028.
Facione N, Miaskowski C, Dodd M, Paul S. The self-reported likelihood of patient delay in breast cancer: New thoughts for early detection. Prev Med. 2002;34(4):397–407.
Fitzpatrick P, Corcoran N, Fitzpatrick J. Prostate cancer: how aware is the public? Br J Urology. 1998;82(1):43–8.
Cameron A, Hinton J. Delay in seeking treatment for mammary tumours. Cancer. 1968;21(6):1121–6.
Facione NC, Facione PA. The cognitive structuring of patient delay in breast cancer. Soc Sci Med. 2006;63(12):3137–49.
Tomlinson C, Wong C, Au HJ, Schiller D. Factors associated with delays to medical assessment and diagnosis for patients with colorectal cancer. Can Fam Physician. 2012;58:495–501.
Goldsen RK, Gerhardt PR, Handy VH. Some factors related to patient delay in seeking diagnosis for cancer symptoms. Cancer. 1957;10(1):1–7.
Samet JM, Hunt W, Lerchen ML, Goodwin JS. Delay in seeking care for cancer symptoms: a population-based study of elderly New Mexicans. J Natl Cancer Inst. 1988;80(6):432–8.
Lam WW, Fielding R, Chan R, Or A. Factors influencing delayed presentation with symptomatic breast cancer in Hong Kong Chinese women. Hong Kong Med J. 2009;15(3):4–7.
Forbes LJ, Warburton F, Richards MA, Ramirez AJ. Risk factors for delay in symptomatic presentation: a survey of cancer patients. Br J Cancer. 2014;111(3):581–8. doi:10.1038/bjc.2014.304.
Li WW, Lam WW, Wong JH, Chui A, Chan M, Or A, et al. Waiting to see the doctor: understanding appraisal and utilization components of consultation delay for new breast symptoms in Chinese women. Pyshcooncology. 2012;21(12):1316–23. doi:10.1002/pon.2038.
Rauscher GH, Ferrans CE, Kaiser K, Campbell RT, Calhoun EE, Warnecke RB. Misconceptions about breast lumps and delayed medical presentation in urban breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19(3):640–7. doi:10.1158/1055-9965.EPI-09-0997.
Burgess CC, Ramirez AJ, Smith P, Richards MA. Do adverse life events and mood disorders influence delayed presentation of breast cancer? J Psychosom Res. 2000;48(2):171–5.
Greer S. Psychological aspects: delay in treatment of breast cancer. Proc R Soc Med. 1974;67(6):470–3.
Carter-Harris L, Hermann CP, Draucker CB. Pathways to lung cancer diagnosis. J Am Assoc Nurse Pract. 2015. doi:10.1002/2327-6924.12242.
Esteva M, Leiva A, Ramos M, Pita-Fernandez S, Gonzalez-Lujan L, Casamitjana M, et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer. 2013;13(87). doi:10.1186/1471-2407-13-87.
Loehrer PJ, Greger HA, Weinberger M, Musick B, Miller M, Nichols C, et al. Knowledge and beliefs about cancer in a socioeconomically disadvantaged population. Cancer. 1991;68(7):1665–7.
Magarey CJ, Todd PB, Blizard PJ. Psycho-social factors influencing delay and breast self-examination in women with symptoms of breast cancer. Soc Sci Med. 1977;11(4):229–32.
Rozniatowski O, Reich M, Mallet Y, Penel N, Fournier C, Lefebvre JL. Psychosocial factors involved in delayed consultations by patients with head and neck cancer. Head Neck. 2005;27(4):274–80. doi:10.1002/hed.20157.
Mor V, Masterson-Allen S, Goldberg R, Guadagnoli E, Wool MS. Pre-diagnostic symptom recognition and help seeking among cancer patients. J Community Health. 1990;15(4):253–66.
Temoshok L, DiClemente RJ, Sweet DM, Blois MS, Sagebiel RW. Factors related to patient delay in seeking medical attention for cutaneous malignant melanoma. Cancer. 1983;54(12):3048–53.
Brouha XD, Tromp DM, Hordijk G, Winnubst JA, de Leeuw R. Oral and pharyngeal cancer: analysis of patient delay at different tumour stages. Head Neck. 2005;27(11):939–45.
Richard MA, Grob JJ, Avril MF, Delaunay M, Gouvernet J, Wolkenstein P, et al. Delays in diagnosis and melanoma prognosis (I): the role of patients. Int J Cancer. 2000;89(3):271–9.
Marlow LA, McGregor LM, Nazaroo JY, Wardle J. Facilitators and barriers to help-seeking for breast and cervical cancer symptoms: a qualitative study with an ethnically diverse sample in London. Psychooncology. 2014;23(7):749–57. doi:10.1002/pon.3464.
O’Mahony M, Hegarty J, McCarthy G. Women’s help seeking behaviour for self-discovered breast cancer symptoms. Eur J Oncol Nurs. 2011;15(5):410–8. doi:10.1016/j.ejon.2010.10.011.
Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: a qualitative study. J Adv Nurs. 2008;61(3):336–43. doi:10.1111/j.1365-2648.2007.04542.x.
Whitaker KL, Macleod U, Winstanley K, Scott SE, Wardle J. Help seeking for cancer ‘alarm’ symptoms: a qualitative interview study of primary care patients in the UK. Br J Gen Pract. 2015;65(631):e96–e105.
Grant E, Silver K, Bauld R, Day R, Warnakulasuria S. The experiences of young oral cancer patients in Scotland: symptom recognition and delays in seeking professional help. Br Dent J. 2010;208(10):465–71. doi:10.1038/sj.bdj.2010.450.
Facione NC, Dodd MJ. Women's narratives of help seeking for breast cancer. Cancer Pract. 1995;3(4):219–25.
Facione NC, Dodd MJ, Holzemer WM, Meleis AI. Help-seeking for self-discovered breast symptoms. Implications for early detection. Cancer Pract. 1997;5(4):220–7.
Pedersen AF, Olesen F, Hansen R, Hansen RP, Zachariae R, Vedsted P. Social support, gender and patient delay. Br J Cancer. 2011;104(8):1249–55.
Brandner S, Muller-Nordhorn J, Stritter W, Fotopoulou C, Sehouli J, Holmberg C. Symptomization and triggering processes: Ovarian cancer patients' narratives on pre-diagnostic sensation experiences and the initiation of healthcare seeking. Soc Sci Med. 2014;119:123–30.
Scott S, McGurk M, Grunfield E. Patient delay for potentially malignant oralsymptoms. Eur J Oral Sci. 2008;116(2):141–7.
Scott S, Grunfield E, Auyeung V, McGurk M. Barriers and triggers to seeking help for potentially malignant oral symptoms: implications for interventions. J Public Health Dent. 2009;69(1):34–40.
Cooper C, Polonec L, Stewart S, Gelb C. Gynaecologic cancer symptom awareness, concern and care seeking among US women: a multi-site qualitative study. Fam Pract. 2013;30(1):96–104.
Lauver D, Coyle M, Panchmatia B. Women's reasons for and barriers to seeking care for breast-cancer symptoms. Womens Health Issues. 1995;5(1):27–35.
Cochran S, Hacker N, Berek J. Correlates of delay in seeking treatment for endometrial cancer. J Psychosom Obstet Gynaecol. 1986;5(4):245–52.
Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax. 2005;60(4):314–9.
Gollwitzer P. Goal achievement: The role of intentions. Eur Rev Soc Psychol. 1993;4:441–85.
Whitaker KL, Winstanley K, Macleod U, Scott SE, Wardle J. Low cancer suspicion following experience of cancer ‘warning sign’. Eur J Cancer. 2015. http://dx.doi.org/10.1016/j.ejca.2015.07.014
Robb KA, Simon AE, Miles A, Wardle J. Public perceptions of cancer: a qualitative study of the balance of positive and negative beliefs. BMJ Open. 2014;4(7), e005343.
Kahneman D. Thinking fast and slow. London: Penguin; 2011.
Edwards M, Wood F, Davies M, Edwards A. 'Distributed health literacy': a longitudinal qualitative analysis of the roles of health literacy mediators and social networks of people living with a long term condition. Health Expect. 2013. doi:10.1111/hex.12093
Pescosolido B, Boyer C. How do people come to use mental health services? Current knowledge and changing perspectives. In: Scheid T, Brown T, editors. A Handbook for the study of mental health: Social contexts, theories and systems. New York: Cambridge University Press; 1999. p. 392–411.
Dobson CM, Russel AJ, Rubin GP. Patient delay in cancer diagnosis: what do we really mean and can we be more specific? BMC Health Serv Res. 2014;14:(387). doi:10.1186/1472-6963-14-387.
Acknowledgements
The authors would like to thank Dr Stephanie Smits for her contribution to interpretation of data from quantitative studies. This study was funded by Tenovus Cancer Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GMM, KEB, AGE and FW were responsible for the concept and design and conduct of the study. AGE gave additional advice on methodology. GMM was responsible for collection of data and manuscript preparation. RR was responsible for double checking at all stages of the search. KEB, AGE and FW extensively reviewed and edited the manuscript drafts. All authors were involved in interpretation of results and approved the final version of the manuscript.
Additional file
Additional file 1: Appendix 1.
Search terms. (DOCX 13 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
McCutchan, G.M., Wood, F., Edwards, A. et al. Influences of cancer symptom knowledge, beliefs and barriers on cancer symptom presentation in relation to socioeconomic deprivation: a systematic review. BMC Cancer 15, 1000 (2015). https://doi.org/10.1186/s12885-015-1972-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1972-8