Abstract
Background
The prognostic value of histone γ-H2AX and 53BP1 proteins to predict the radiotherapy (RT) outcome of patients with rectal carcinoma (RC) was evaluated in a prospective study. High expression of the constitutive histone γ-H2AX is indicative of defective DNA repair pathway and/or genomic instability, whereas 53BP1 (p53-binding protein 1) is a conserved checkpoint protein with properties of a DNA double-strand breaks sensor.
Methods
Using fluorescence microscopy, we assessed spontaneous and radiation-induced foci of γ-H2AX and 53BP1 in peripheral blood mononuclear cells derived from unselected RC patients (n = 53) undergoing neoadjuvant chemo- and RT. Cells from apparently healthy donors (n = 12) served as references.
Results
The γ-H2AX assay of in vitro irradiated lymphocytes revealed significantly higher degree of DNA damage in the group of unselected RC patients with respect to the background, initial (0.5 Gy, 30 min) and residual (0.5 Gy and 2 Gy, 24 h post-radiation) damage compared to the control group. Likewise, the numbers of 53BP1 foci analyzed in the samples from 46 RC patients were significantly higher than in controls except for the background DNA damage. However, both markers were not able to predict tumor stage, gastrointestinal toxicity or tumor regression after curative RT. Interestingly, the mean baseline and induced DNA damage was found to be lower in the group of RC patients with tumor stage IV (n = 7) as compared with the stage III (n = 35). The difference, however, did not reach statistical significance, apparently, because of the limited number of patients.
Conclusions
The study shows higher expression of γ-H2AX and 53BP1 foci in rectal cancer patients compared with healthy individuals. Yet the data in vitro were not predictive in regard to the radiotherapy outcome.
Similar content being viewed by others
Background
Each year in Germany, about 65 000 people are diagnosed with the colorectal cancer (CRC) and more than 25 000 people die of the disease [1]. Of those CRC, approximately one third will be distal to the rectosigmoid junction and designated as rectal cancer (RC). Patients with locally advanced RC receive preoperative chemo- and radiation therapy (RT) in order to reduce the possibility of recurrence and to improve survival [2]. However, this depends on the tumor regression grade (TRG) which strongly varies between individual patients [3]. A variety of potential indicators of the success of preoperative chemo- and RT and among others, p53, EGFR, Ki-67, p21, tumor oxygenation, immune reaction, and DNA damage response etc., are currently studied (for review, see [3, 4]). However, no reliable marker that can predict patients’ response to curative RT is currently available [3].
DNA damage repair mechanisms serve as a guard system that protects cells against genetic instability. Both genetic instability and impaired DNA damage repair have been suggested as factors underlying increased susceptibility to tumorigenesis (for reviews, see [5, 6]). The significance of genetic instability and impaired DNA repair in tumor development is particularly well proven by the Ataxia telangiectasia, Fanconi anemia and Nijmegen breakage syndrome, the diseases also known as chromosomal breakage disorders. Indeed, these chromosome instability syndromes are characterized by defects in DNA repair, predisposition to different forms of cancer and increased chemo- and radiation sensitivity (for review, see [7]). Besides these rare diseases, nearly all solid tumors are genetically unstable [5].
Genomic instability in cancer and DNA repair mechanisms have been analyzed in various population-based studies using a variety of assays that assess DNA fragmentation by means of the Comet assay, micronucleus test, chromosomal aberrations, sister chromatid exchanges, etc. Several of these studies have revealed impaired DNA repair capacity in peripheral blood mononuclear cells (PBMCs), exposed in vitro to ionizing radiation (IR) or UV from breast cancer patients, as evaluated by the chromosome aberration assay [8–10] as well as by the micronucleus test [11–13]. In addition, phosphorylation of histone H2AX can serve as a further valuable marker of DNA integrity and repair [14]. Constitutive expression of histone γ-H2AX was suggested to indicate disruption of the DNA damage repair pathway and/or genetic instability in breast cancer [15]. Moreover, altered expression of many H2A variants was found to be associated with cancer [16].
In addition, the kinetics of induction and disappearance of γ-H2AX foci might be related to the efficiency of “repair” of higher order chromatin organization [17]. An impaired DNA repair was found by counting γ-H2AX foci in blood cells from children with tumors [18]. However, the initial numbers of γ-H2AX foci after in vitro irradiation were found very similar among the groups studied [18]. At the same time, Brzozowska et al. (2012) found by a flow cytometer, an increased expression of histone γ-H2AX in irradiated blood lymphocytes from normal donors, as compared to tumor patients with prostate cancer [19]. But the difference was not confirmed when γ-H2AX foci were counted by fluorescence microscopy [19]. Several studies [10, 19–25] evaluated histone γ-H2AX as a marker to predict the toxicity in normal tissue during RT of tumor patients, however, with contradictory conclusions. Some of the quoted studies [19, 21–23] revealed no correlation between either acute or late side effects of RT and expression of histone γ-H2AX. However, other studies [18, 20, 25] found that the loss of histone γ-H2AX correlated with high-grade toxicity from RT treatment. Henríquez-Hernández et al. (2011) suggest that lower levels of initial DNA damage may be associated with a lower risk of suffering from severe late subcutaneous RT-induced toxicity [24].
Despite numerous studies quoted above into the relationship between cellular in vitro assays, tumor risk and clinical RT outcomes, a common opinion has not yet been made. The controversies cited above prompted us to evaluate whether the histone γ-H2AX test is able to predict the clinical RT outcome of RC patients and to discriminate them from healthy subjects. We examined both intrinsic and radiation-induced histone γ-H2AX foci expression in PBMCs from a group of unselected RC patients (n = 53) and a group of healthy controls (n = 12). PBMCs from a group (n = 27) of RC patients with an adverse (grade 2–3) clinical gastro-intestinal (GI) reaction to RT have also been retrospectively analyzed. In addition to γ-H2AX, we analyzed the foci of 53BP1 (p53-binding protein 1), a well-known sensor protein of DNA damage [26]. DNA double-strand breaks (DSB) attract the 53BP1 protein to the surrounding chromatin, where the 53BP1 is recruited by methylated H3 Lys 79 and signals chromatin/DNA damage [26] in a γ-H2AX-dependent manner.
Methods
Study population and blood selection
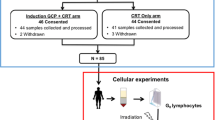
The study was performed on PBMCs isolated from two groups of individuals: (i) a group (n = 53) of unselected patients with locally advanced RC who were prospectively included in the study and their blood samples were collected before and after the first 5 clinical radiation fractions; and (ii) a group of apparently healthy donors (n = 12), mainly hospital personal. None of the healthy controls was previously exposed to clinical radiation. All participants were asked to complete a questionnaire on their medical histories and lifestyles, including genetic diseases, alcohol consumption and smoking habit (Additional file 1: Tables S1 and S2). The study was approved by the Ethics Committee of University of Würzburg and all patients and donors gave written informed consent.
All recruited RC patients underwent preoperative radio-chemotherapy treatment at the Department of Radiation Oncology, University Hospital of Würzburg. Locoregional tumor stage was evaluated according to the standard UICC criteria (endoscopy, endorectal ultrasound and MRI) which resulted in 11, 35, and 7 cases scored as stage II, III, and IV, respectively (Additional file 1: Tables S1 and S2). All patients received 3D conformal pelvic irradiation of the primary tumor and the regional lymphatics by means of a 6 MV linear accelerator (Siemens Concord, CA, USA) at a dose rate of 2 Gy/min. The regimen comprised 28 fractions of 1.8 Gy five times a week giving a total dose of 50.4 Gy. In addition, almost all (98 %) patients received 2 cycles of 5-FU (1000 mg/m2, c.i. 5 days a week) during the 1st and 5th weeks.
Side effects of RT
Rectal (e.g. proctitis with rectal discomfort, diarrhea or bleeding) and hematological (e.g. leukocyte counts, platelets and hemoglobin) toxicities due to radio-chemotherapy were determined during and at the end of the RT according to the RTOG [27] and NCI CTCAE v. 4.03 score. Tumor regression grade (TRG) after chemo- and RT was determined according to Dworak et al. (1997) and identified “good” (TRG 3, TRG 4) and “bad” (TRG 0, TRG 1 and TRG 2) responders [28].
Blood sampling and isolation of cells
PBMCs were separated from the heparinized blood samples by density-gradient centrifugation using Ficoll-Histopaque 1077 (Sigma 1077–1, Deisenhofen, Germany) according to the manufacturer's instructions. PBMCs were washed twice with Ca2+- and Mg2+-free physiological phosphate-buffered saline (PBS, Sigma D-8537) and finally resuspended in the RPMI 1640 (Sigma R-8758) supplemented with 10 % FBS, glutamine (1 mM), and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively), hereafter denoted as complete growth medium (CGM), and incubated at 37 °C in a humidified atmosphere enriched with 5 % CO2 until irradiation.
In vitro X-ray irradiation
The final cell density of isolated G0 unstimulated PBMCs was adjusted to 1 × 106 cells/ml and the samples were placed at 37 °C in a 5 % CO2 incubator. X-irradiation (0.5 and 2 Gy) was performed using a 6 MV Siemens linear accelerator (Siemens Concord, CA, USA) at a dose rate of 2 Gy/min. Non-irradiated cells were treated in similar way, but at a zero radiation dose.
Immunofluorescence staining for γ-H2AX and 53BP1foci
A cell aliquot (2–3 × 105) of control or irradiated cells was cytocentrifuged at various time points after IR on a glass slide and fixed for 15 min in ice-cold methanol, and then for 1 min in 100 % acetone at −20 °C. Slides were washed three times for 5 min in PBS and blocked with 4 % FBS-PBS for 1 h at room temperature [29]. Blindly coded slides were incubated overnight at 4 °C with either anti-phospho-histone H2AX (Millipore, Schwalbach, Germany, # 05–636), or anti-53BP1 (Novus Biologicals, Cambridge, UK, # NB 100–304) antibodies followed by incubation with respective secondary antibodies conjugated with Alexa Fluor 488 or 594 nm. Slides were counterstained with 0.2 μg/ml of DAPI (4’,6’-diamidino-2-phenylindole) in antifade solution (1.5 % N-propyl-gallate, 60 % glycerol in PBS) and examined using a Leica DMLB epifluorescence microscope (at a 1000x magnification) coupled to a cooled CCD camera (ColorView 12, Olympus Biosystems, Hamburg, Germany). Camera control and image acquisition were done with image analysis software (Olympus Biosystems, Hamburg, Germany). The foci were counted by eye in 500 cells per each treatment condition, no threshold for γ-H2AX or 53BP1 was set. The cells with apoptotic morphologies or cells with bright nuclei (intense, complete coverage of the nuclei with foci staining) were excluded from the analyses. Because the wide-field microscopic setup used here does not allow three-dimensional microscopy with Z-planning, two-dimensional images were captured from the focal plane. However, in order to detect all foci in the 3D-room we used the possibility to focus manually through the whole nucleus. All experiments were counted by one and the same, trained person.
Statistics
Data are presented as mean (± SE). Mean values were compared by the Student's t-test or one way ANOVA. The threshold of statistical significance was set at p < 0.05. Statistics was performed with the program Origin 8.5 (Microcal, Northampton, MS, USA).
Results
DNA damage and its repair were evaluated up to 24 h after exposure to 0.5 Gy or 2 Gy of X-rays in vitro or after 5 first clinical radiation fractions. The extent of DNA damage was measured by counting the number of histone γ-H2AX foci, a sensitive marker of DNA DSBs [30]. The mean data from 500 nuclei were determined for the cell samples from each tested individual (Fig. 1). The means for each tested group of individuals are also shown in Fig. 1.
Comparison of histone γ-H2AX foci in PBMCs derived from control donors and unselected RC patients. a DNA damage assessed by means of the histone γ-H2AX assay in non-irradiated and b-d in irradiated PBMCs derived from unselected RC patients (triangles), as compared to the cells from apparently healthy donors (circles). Initial (b), residual (c - 0.5 Gy, 24 h, d - 2 Gy, 24 h) DNA damage were assessed in PBMCs after irradiation with 0.5 Gy (b, c) or 2 Gy (d) in vitro. Filled squares represent the mean values (± SE) for the respective group
The parameters on initial, residual and baseline DNA damage assessed by histone γ-H2AX for each individual, as well as age, sex, and grade of GI toxicity after RT are given Fig. 1 and in Additional file 1: Table S3. Although non-irradiated cells of some RC patients showed remarkably lower intrinsic DNA damage, i.e. in the range of controls, the mean value of background DNA damage (Fig. 1a) was significantly (p < 0.005) higher (0.5 ± 0.1 foci/nucleus) in the group of unselected RC patients, as compared to the group of healthy controls (0.1 ± 0.03). Likewise, irradiated in vitro blood lymphocytes showed higher (p < 0.005) initial (Fig. 1b, 0. 5 Gy, 30 min) and residual (p < 0.005, Fig. 1c and d, 0.5 Gy and 2 Gy, 24 h) expression of the γ-H2AX foci.
In addition, the foci numbers of 53BP1, a sensor of DNA damage [26], were compared between 10 healthy controls and 47 RC patients. As seen in Additional file 1: Figure S1 and Table S4, the mean background expression levels of 53BP1 (Additional file 1: Figure S1A) were very similar in two groups. However, the mean expression of radiation-induced 53BP1 foci (Additional file 1: Figure S1, part B) was not significantly higher (3.6 ± 1.8 foci/nucleus) in the group of RC patients than that in control group (2.4 ± 0.4 foci/nucleus) probably because of the enormous data scattering in the RC group. The numbers of residual 53BP1 foci detected 24 h post-IR (Additional file 1: Figure S1, parts C and D) were found to be significantly (p < 0.05 and p < 0.005 after 0.5 and 2 Gy, respectively) higher in the PBMCs derived from RC patients than that of healthy individuals.
Next, we compared the expression of γ-H2AX and 53BP1 per one and the same nucleus at different time post-IR and radiation doses. Judging from the correlation coefficients given in Additional file 1: Figure S2, there was no (Additional file 1: Figure S2, part A) or weak correlation (Additional file 1: Figure S2, part B) between background (0 Gy) or radiation-induced (30 min after irradiation with 0.5 Gy) expression of both proteins, respectively. At the same time, a strong (R2 = 0.92 and R2 = 0.83) correlation was found between residual amounts of γ-H2AX and 53BP1 foci (Additional file 1: Figure S2, parts C and D).
Out of 53 prospectively recruited RC patients, 27 exhibited an adverse GI reaction to RT, including grade 2 and grade 3 according to RTOG score (see Additional file 1: Table S1). Based on the clinical GI reaction of RT patients we analyzed retrospectively the initial, residual and background DNA damage measured by histone γ-H2AX between the groups of RC patients with normal (RTOG grade 0 and 1, n = 26) and an adverse (RTOG grade 2 and 3, n = 27) clinical reaction to RT (Fig. 2). As seen in Fig. 2, background, induced or residual DNA damage in PBMCs from RC patients with normal or adverse clinical reaction was higher than that from control donors. However, there was no difference between the both groups (grade 0–1 and 2–3) of RC patients in all parameters studied (Fig. 2a-d). Mostly similar data were obtained with the 53BP1 foci except that there was no difference between the background numbers of 53BP1 foci counted in all 3 groups (Additional file 1: Figure S3, parts A-D).
Histone γ-H2AX foci in PBMCs derived from control donors and normally-reacting and radiosensitive (grade 2–3) RC patients. a DNA damage assessed by means of the histone γ-H2AX assay in non-irradiated and b-d irradiated PBMCs derived from normally-reacting RC patients (grade 0 and 1, up triangles) and radiation-sensitive (grade 2 and 3, down triangles) cancer patients compared to cells from apparently healthy donors (circles). Initial (b, 0.5 Gy, 30 min post-IR), residual DNA damage 24 h after in vitro 2 Gy (c) or 72 h after 5 clinical radiation fractions (d) were assessed in PBMCs after irradiation either in vitro (b, c) or in vivo (d). Filled squares represent the mean values (± SE) for the respective group. “n.s.” indicates that the difference was not highly significant (p > 0.05). “n.d.” means not determined. Clinical GI toxicity to RT was controlled at the end of RT (see Additional file 1: Table S2) and used as an indicator for clinical radiosensitivity according to the RTOG score [27]
Further, we split the group of patients (Fig. 3) with an adverse GI reaction to RT (grade 2 and 3) into 2 subgroups showing either grade 2 (n = 19) or grade 3 (n = 8) reaction and compared DNA damage between these groups and a group of normally-reacting (grade 0–1) RC patients. As seen in Fig. 3, we found no differences in the baseline, induced or residual DNA damage assessed by the γ-H2AX foci between the groups.
Histone γ-H2AX foci in PBMCs derived from normally-reacting and radiosensitive (grade 2 and grade 3) RC patients. a DNA damage assessed by means of the histone γ-H2AX assay in non-irradiated and b-d irradiated PBMCs derived from normally-reacting RC patients (grade 0 and 1, up triangles) compared to cells from radiation-sensitive (GI toxicity, Additional file 1: Table S2) RC patients with grade 2 (down triangles, n = 19) and grade 3 (up triangles, n = 8). Peripheral lymphocytes were prepared from the blood samples derived from RC patients. For details, see legend to Fig. 2
In addition to the irradiated in vitro cells, as mentioned in the Methods, blood samples were withdrawn from all recruited RC patients after 5 clinical fractions. As seen in Fig. 4a, the mean number of γ-H2AX foci per patient’s sample after 5 clinical fractions was significantly (p < 0.05) higher (0.90 ± 0.10) than that before RT (0.55 ± 0.07). However, the amounts of γ-H2AX foci (1.0 ± 0.3) after clinical irradiation in a group of RC patients with adverse (grade 3, n = 8) clinical reaction to RT were similar to that of the unselected (n = 53) RC patients.
Effect of clinical radiation on the expression of histone γ-H2AX and 53BP1 foci in blood lymphocytes. a DNA damage was assessed by means of the histone γ-H2AX and b 53BP1 assays before (up triangles) and after 5 clinical fractions in PBMCs derived from unselected (right triangles) RC patients compared with RC patients with an adverse (grade 3, down triangles) clinical GI reaction to RT. Filled squares represent the mean values (± SE) for the respective group. “n.s.” indicates that the difference was not highly significant (p > 0.05)
The quantification of 53BP1 foci after 5 clinical radiation fractions (Fig. 4b) was conducted in a smaller group (n = 46 vs. n = 53 tested for γ-H2AX) RC patients, which however, contained almost all (n = 7) clinically radiation sensitive RC patients with grade 3 GI reaction to RT. Comparison of the mean number of 53BP1 foci per patient’s sample after 5 clinical fractions revealed significantly (p < 0.001) increased foci numbers after clinical irradiation (0.87 ± 0.06 vs. 0.6 ± 0.06 before RT) for the whole group tested. A subset of clinically irradiated RC patients with an adverse clinical reaction to RT showed also an increased but similar number of 53BP1 foci (0.90 ± 0.13) as the group of unselected RC patients.
Next, we asked whether the tumor stage can influence the baseline, induced and residual DNA damage in blood cells of RC patients. We compared the expression of γ-H2AX and 53BP1 foci in the blood lymphocytes of RC patients with different UICC tumor stages (Additional file 1: Table S2). As seen in Fig. 5, no significant difference in the γ-H2AX foci numbers was observed between tumor stage II, III or IV. However, the mean number of the background, induced or residual amount of the γ-H2AX foci in the group with stage IV has the tendency to be always lower than that of the group with the tumor stage III. The same tendency was observed in case of 53BP1 foci (Additional file 1: Figure S4).
Correlation between the γ-H2AX foci expression and tumor staging (II, III, IV). Peripheral lymphocytes were prepared from the blood samples derived from RC patients. a Foci counting for γ-H2AX was performed in non-irradiated, b irradiated in vitro with 0.5 and c 2 Gy samples 30 min and 24 h post-IR or d after 5 clinical fractions. Filled squares represent the mean values (± SE) for the respective group. Locoregional tumor stage was evaluated according to the standard UICC criteria (endoscopy, endorectal ultrasound and MRI) which gave 11, 35, and 7 cases (Additional file 1: Tables S1 and S2, pre-RT) scored as stage II, III, and IV, respectively. “n.s.” indicates that the difference was not highly significant (p > 0.05)
In addition, we analyzed if the TRG (Additional file 1: Table S2) after curative RT can be predicted on the basis of both protein markers (Fig. 6). Thus we compared the groups with “bad” (TRG 0–2, n = 34) and “good” (TRG 3–4, n = 19) response to RT. However, we found no differences in the background, induced or residual (in vitro and in vivo) γ-H2AX foci between both groups (Fig. 6). Likewise, no difference between the groups with “bad” (TRG 0–2) and “good” (TRG 3–4) response to RT was observed in the degree of the induction of DNA damage (Additional file 1: Figure S5).
Correlation between the γ-H2AX foci expression and tumor regression grade (TRG). DNA damage assessed by means of the γ-H2AX foci expression in non-irradiated and irradiated peripheral lymphocytes of RC patients with different tumor regression grade (TRG, Additional file 1: Table S2). Up and down triangles show γ-H2AX foci amounts in the cells of RC patients with TRG 0–2 and TRG 3–4, respectively. Filled squares represent the mean values (± SE) for the respective group. “n.s.” indicates that the difference was not highly significant (p > 0.05)
Discussion
This prospective study was performed to unravel if DNA damage in peripheral blood lymphocytes can predict RC patients’ response to combined chemo- und RT or correlated with tumor stage, acute GI toxicity or TRG. Peripheral blood cells isolated from (i) unselected RC patients, and (ii) healthy individuals were analyzed for their DNA damage using the histone γ-H2AX and 53BP1 assays. The analysis of non-irradiated as well as irradiated cell samples revealed significantly higher amounts in the background, induced and residual DNA damage levels in a group of unselected RC patients (Fig. 1) compared with healthy controls. Possible reasons for this can be genetic instability and impaired DNA repair in the cells derived from tumor patients. In addition, one of the reasons can be simultaneous chemotherapy with 4-FU received by the majority of RC patients. Yet our results disagree with several studies [23, 31] who have found no differences in levels of both basal and radiation-induced DNA damage in cells from tumor patients with increased clinical radiosensitivity and healthy controls [23, 31]. The reasons for the discrepancy might reside in the patients’ and controls’ cohorts, cancer stage, treatment prior to blood sampling, arbitrary determined cut-off values, experimental protocols, methods of foci quantification (flow cytometry vs. fluorescence microscopy) as well as in interlaboratory variability. Moreover, in contrast to the present and several other studies [18, 20, 21, 25], which analyzed primary PBMCs or T-cells [19], the paper of Vasireddy et al. (2010) used lymphoblastoid cell lines derived from cells of tumor patients [23]. Besides this, the quantification of histone γ-H2AX foci by fluorescence microscopy seems to differ significantly between laboratories. Thus, the background values of about 0.07-0.08 γ-H2AX foci per lymphocyte in non-irradiated cells reported in [21] are some several times lower than the values presented here in Fig. 1a. However, our foci counts (4.9 ± 0.4) detected in the samples from RC patients 30 min after IR with 0.5 Gy correlated well with the numbers (range 6÷14 with a mean of 9.3) published by van Oorschot et al. (2014) 30 min after irradiation with 1 Gy the lymphocytes derived from prostate cancer patients [32] or with those of Kroeber and colleagues [33] on 136 RC patients.
Next, the unselected RC patients’ group was split into the subgroups according to acute gastro-intestinal toxicities (RTOG, see Additional file 1: Table S2), i.e. showing grade 0–1 and grade 2–3 (Fig. 2). However, retrospective analysis of RC patients with normal (n = 26) and an adverse (n = 27) clinical reaction to RT revealed no differences in the background (Fig. 2a), induction (Fig. 2b) and repair (Fig. 2c) of DNA damage 30 min and 24 h post-IR with 0.5 and 2 Gy in vitro as well as after 5 clinical irradiations (Fig. 2d). Likewise, we found no differences between normally-reacting and sensitive RT patients on the base of 53BP1 marker (Additional file 1: Figure S3). Both tests didn’t allow to identify separately RC patients with grade 2 and grad 3 toxicities (Fig. 3 and Additional file 1: Figure S3).
In our study the group (an average age of 45 ± 12 years) of healthy controls was younger than the group of RC patients (mean age of 66 ± 9 years). The data on age dependence of γ-H2AX expression, however, seems quite disputable. Thus, based on the comparison of two donor groups differing markedly in age (31–45 vs. 50–72 years), Firsanov et al. (2011) conclude that the dynamics of γ-H2AX induction is independent of age [34]. In contrast, Sedelnikova et al. (2008) found [35], by comparing two groups with a much larger deviation (21–30 years vs. 60–72 years) in age than in our study, that the fractions of cells containing γ-H2AX foci in older (60–72 years) individuals was higher (about 30 %) than in younger individuals (about 20 %). However, the frequency of γ-H2AX foci in response to IR was found to be age independent [35].
The second indicator of DNA DSB formation studied here was the 53BP1 protein. Given that the γ-H2AX test shows a DSB-induced protein modification and the 53BP1 foci indicate the accumulation of a DSB-modified protein [26, 36], both types of radiation-induced foci should be almost overlapping in fluorescence images [37]. In our hands, however, the 53BP1 assay was less sensitive than the histone γ-H2AX test in case of endogeneous (Additional file 1: Figure S1A, 0 Gy) and induced (Additional file 1: Figure S1B, 0.5 Gy, 30 min) foci. There may be at least two reasons for the observed discrepancy between two assays. Firstly, for the detection of γ-H2AX we used highly specific monoclonal antibodies whereas the 53BP1 protein was detected with less selective polyclonal antibodies. In addition, the 53BP1 foci counting was done for a smaller patient’s group (n = 46), as compared to γ-H2AX assay (n = 53). Nevertheless, residual (24 h post-IR) foci of 53BP1 protein were found to be significantly higher than that from healthy individuals (Additional file 1: Figure S1, parts C and D).
It is known that a minority (about 5 %) of RT patients develop either acute or late radiotoxic responses during or after RT [38]. Among 53 prospectively recruited RC patients in our study we observed 19 and 8 RC patients of patients exhibiting early GI radiotoxicity of grade 2 and 3 during RT, respectively. However, we found no differences in the background, initial and residual DNA damage between irradiated cells from tumor patients with normal (Fig. 3, first data set) and those with an adverse (grade 2 and 3) clinical sensitivity to RT (Fig. 3, second and third data sets). Likewise, we found no difference between normally-reacting (grade 0–1) and radiation-sensitive (grade 3) RC patients after 5 clinical radiation fractions (Fig. 4).
In addition to GI toxicity to curative RT, we analyzed whether the γ-H2AX and 53BP1 foci assays allowed to discriminate between tumor stage (II, III or IV, Fig. 5 and Additional file 1: Figure S4) or TRG after RT of RC patients (Fig. 6). However, both markers were not able to identify either tumor stage or TRG. Interestingly, the mean baseline, induced and residual DNA damage (Fig. 5) was found to be somewhat lower in the group of RC patients with tumor stage IV (n = 7) as compared with the tumor stage III (n = 35). The difference, however, was more like a tendency, apparently because of the limited number of patients, especially with tumor stage IV.
Conclusions
Prospectively recruited RC patients showed on average increased pre-existing, initial and residual DNA damage levels measured by histone γ-H2AX and 53BP1 foci, as compared with the healthy group. However, due to a large interindividual variability, it was not possible to discriminate individually RC patients from healthy controls. Neither it was possible to identify between a minor (n = 8) group of retrospectively identified RC patients with an adverse clinical GI reaction of grade 3 to RT and patients with grade 2 or normally-reacting RC patients. Likewise, the assays were not able to recognize tumor stage or to predict tumor regression grade of RC patients. A larger study would be necessary in order to investigate the complex mechanisms behind the normal tissue radiotoxicity and its correlation with the tumor response to RT.
Abbreviations
- DSB:
-
double-strand break
- GI:
-
gastro-intestinal
- IR:
-
ionizing radiation
- PBMCs:
-
peripheral blood mononuclear cells
- PBS:
-
phosphate buffered saline
- RC:
-
rectal cancer
- RT:
-
radiotherapy
- RTOG:
-
Radiation Therapy Oncology Group
- TRG:
-
tumor regression grade
References
Haug U, Rösch T, Hoffmeister M, Katalinic A, Brenner H, Becker N. [Implementing an Organised Colorectal Cancer Screening Programme in Germany: Opportunities and Challenges]. Gesundheitswesen. Georg Thieme Verlag KG Stuttgart New York 2015;10:775-790. DOI:10.1055/s-0034-1377027
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Shin JS, Tut TG, Ho V, Lee CS. Predictive markers of radiotherapy-induced rectal cancer regression. J Clin Pathol. 2014;67:859–64.
Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–88.
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9.
Thompson LH, Schild D. Recombinational DNA repair and human disease. Mutat Res. 2002;509:49–78.
Carney JP. Chromosomal breakage syndromes. Curr Opin Immunol. 1999;11:443–7.
Parshad R, Price FM, Bohr VA, Cowans KH, Zujewski JA, Sanford KK. Deficient DNA repair capacity, a predisposing factor in breast cancer. Br J Cancer. 1996;74:1–5.
Rigaud O, Guedeney G, Duranton I, Leroy A, Doloy MT, Magdelenat H. Genotoxic effects of radiotherapy and chemotherapy on the circulation lymphocytes of breast cancer patients. Mutat Res. 1990;242:17–23.
Helzlsouer KJ, Harris EL, Parshad R, Fogel S, Bigbee WL, Sanford KK. Familial clustering of breast cancer: possible interaction between DNA repair proficiency and radiation exposure in the development of breast cancer. Int J Cancer. 1995;64:14–7.
Bayens A, Thierens H, Claes K, Poppe B, Messiaen L, De Ridder L, et al. Chromosomal radiosensitivity in breast cancer patients with a known or putative genetic predisposition. Br J Cancer. 2002;87:1379–85.
Scott D, Barber JBP, Levine EL, Burrill W, Roberts SA. Radiation-induced micronucleus induction in lymphocytes identifies a high frequency of radiosensitive cases among breast cancer patients: a test for predisposition? Br J Cancer. 1998;77:614–20.
Scott D, Barber JB, Spreadborough AR, Burrill W, Roberts SA. Increased chromosomal radiosensitivity in breast cancer patients: a comparison of two assays. Int J Radiat Biol. 1999;75:1–10.
Redon CE, Weyemi U, Parekh PR, Huang D, Burrell AS, Bonner WM. γ-H2AX and other histone post-translational modifications in the clinic. Biochim Biophys Acta. 2012;1819:743–56.
Nagelkerke A, van Kuijk SJ, Sweep FC, Nagtegaal ID, Hoogerbrugge N, Martens JW, et al. Constitutive expression of γ-H2AX has prognostic relevance in triple negative breast cancer. Radiother Oncol. 2011;101:39–45.
Monteiro FL, Baptista T, Amado F, Vitorino R, Jerónimo C, Helguero LA. Expression and functionality of histone H2A variants in cancer. Oncotarget. 2014;5:3428–43.
Olive PL, Banáth JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–5.
Rübe CE, Fricke A, Schneider R, Simon K, Kühne M, Fleckenstein J, et al. DNA repair alterations in children with pediatric malignancies: novel opportunities to identify patients at risk for high-grade toxicities. Int J Radiat Oncol Biol Phys. 2010;78:359–69.
Brzozowska K, Pinkawa M, Eble MJ, Müller WU, Wojcik A, Kriehuber R, et al. In vivo versus in vitro individual radiosensitivity analysed in healthy donors and in prostate cancer patients with and without severe side effects after radiotherapy. Int J Radiat Biol. 2012;88:405–13.
Bourton EC, Plowman PN, Smith D, Arlett CF, Parris CN. Prolonged expression of the γ-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer. 2011;129:2928–34.
Fleckenstein J, Kühne M, Seegmüller K, Derschang S, Melchior P, Gräber S, et al. The impact of individual in vivo repair of DNA double-strand breaks on oral mucositis in adjuvant radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:1465–72.
Werbrouck J, De Ruyck K, Beels L, Vral A, Van Eijkeren M, De Neve W, et al. Prediction of late normal tissue complications in RT treated gynaecological cancer patients: potential of the gamma-H2AX foci assay and association with chromosomal radiosensitivity. Oncol Rep. 2010;23:571–8.
Vasireddy RS, Sprung CN, Cempaka NL, Chao M, McKay MJ. H2AX phosphorylation screen of cells from radiosensitive cancer patients reveals a novel DNA double-strand break repair cellular phenotype. Br J Cancer. 2010;102:1511–8.
Henríquez-Hernández LA, Carmona-Vigo R, Pinar B, Bordón E, Lloret M, Núñez MI, et al. Combined low initial DNA damage and high radiation-induced apoptosis confers clinical resistance to long-term toxicity in breast cancer patients treated with high-dose radiotherapy. Radiat Oncol. 2011;6:60.
Djuzenova CS, Elsner I, Katzer A, Worschech E, Distel LV, Flentje M, et al. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol. 2013;8:98.
Huyen Y, Zgheib O, Ditullio RA, Gorgoulis VG, Zacharatos P, Petty TJ, et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature. 2004;432:406–11.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23.
Mahrhofer H, Bürger S, Oppitz U, Flentje M, Djuzenova CS. Radiation induced DNA damage and damage repair in human tumor and fibroblast cell lines assessed by histone H2AX phosphorylation. Int J Radiat Oncol Biol Phys. 2006;64:573–80.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68.
Mumbrekar KD, Fernandes DJ, Goutham HV, Sharan K, Vadhiraja BM, Satyamoorthy K, et al. Influence of double-strand break repair on radiation therapy-induced acute skin reactions in breast cancer patients. Int J Radiat Oncol Biol Phys. 2014;88:671–6.
van Oorschot B, Hovingh SE, Moerland PD, Medema JP, Stalpers LJ, Vrieling H, et al. Reduced activity of double-strand break repair genes in prostate cancer patients with late normal tissue radiation toxicity. Int J Radiat Oncol Biol Phys. 2014;88:664–70.
Kroeber J, Wenger B, Schwegler M, Daniel C, Schmidt M, Djuzenova CS, et al. Distinct increased outliers among 136 rectal cancer patients assessed by γH2AX. Radiat Oncol. 2015;10:36.
Firsanov D, Kropotov A, Tomilin N. Phosphorylation of histone H2AX in human lymphocytes as a possible marker of effective cellular response to ionizing radiation. Cell Tissue Biol. 2011;5:531–5.
Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, et al. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100.
Mochan TA, Venere M, DiTullio RA, Halazonetis TD. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst). 2004;3:945–52.
Lassmann M, Hänscheid H, Gassen D, Biko J, Meineke V, Reiners C, et al. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51:1318–25.
Norman A, Kagan AR, Chan SL. The importance of genetics for the optimization of radiation therapy. A hypothesis. Am J Clin Oncol. 1988;11:84–8.
Acknowledgments
We thank Ines Elsner and Eike Worschech for the expert technical assistance. This work was supported by the grants (#109043; #110274) of the Deutsche Krebshilfe to LVD, CSD and MF.
This publication was funded by the German Research Foundation (DFG) and the University of Würzburg in the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed experiments: CSD, MF and BP. Recruitment of patients, conduction of trial, clinical evaluation: BP and MZ. Performed experiments and summarized primary data: VF, AK, CSD. Analyzed the data: CSD, MF, MG, BP, MZ, LD. Contributed reagents/materials/analysis tools: MG, A-M W-G, MZ, LD. Wrote the paper: MF and CSD. All authors read and approved the final manuscript.
Additional file
Additional file 1: Table S1.
Characteristics of healthy individuals and RC patients undergoing chemo-radiotherapy (Summary). Table S2. Patients’ characteristics in regard to chemo-radiation toxicities and alcohol/tobacco consumption. Table S3. DNA damage measured by the histone γ-H2AX in PBMCs isolated from blood of apparently healthy donors (N) and unselected rectal carcinoma (RC) patients after exposure to 0.5 or 2 Gy of X-irradiation in vitro or after 5 clinical radiation fractions. Table S4. DNA damage measured by the 53BP1 foci in PBMCs isolated from blood of apparently healthy donors (N) and unselected rectal carcinoma (RC) patients after exposure to 0.5 or 2 Gy of X-irradiation in vitro or after 5 clinical radiation fractions. Figure S1. DNA damage assessed by the mean number of 53BP1 foci in non-irradiated (A) and irradiated (B-D) PBMCs derived from unselected RC patients (triangles), as compared to cells from apparently healthy donors (circles). For further details, see legend to Fig. 1. Filled squares represent the mean values (± SE) for the respective group. “n.s.” indicates that the difference was not highly significant (p > 0.05). Figure S2. Correlational analysis of mean γ-H2AX and 53BP1 foci counts from 500 nuclei per sample. Non-irradiated (A) and irradiated with 0.5 (B and C) and 2 Gy (D) lymphocytes were fixed 30 min (B) or 24 h (C, D) post-IR. The expression of both proteins was analyzed simultaneously at each time and IR points for n = 48 blood samples derived from unselected RC patients. Figure S3. DNA damage assessed by means of the 53BP1 assay in non-irradiated (A) and irradiated (B-D) PBMCs derived from normally-reacting RC patients (grade 0 and 1, up triangles) and radiation-sensitive (grade 2 and 3, down triangles) cancer patients compared to cells from apparently healthy donors (circles). Filled squares represent the mean values (± SE) for the respective group. For details, see legend to Fig. 2. Figure S4. Correlation between the 53BP1 foci expression and tumor staging (see Additional file 1: Table S2). Peripheral lymphocytes were prepared from the blood samples derived from RC patients. Foci counting for 53BP1 were performed in non-irradiated (A), irradiated in vitro with 0.5 and 2 Gy samples 30 min and 24 h post-IR (B and C) or 72 h after 5 clinical radiation fractions (D). Filled squares represent the mean values (± SE) for the respective group. Figure S5. Comparison of the γ-H2AX foci expression in peripheral lymphocytes of RC patients differing in tumor regression grade (TRG, Additional file 1: Table S2). Foci counting for γ-H2AX were performed in non-irradiated (up triangles and circles) cells or after 5 clinical radiation fractions (down triangles and diamonds). Filled squares represent the mean values (± SE) for the respective group. (DOC 1915 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Djuzenova, C.S., Zimmermann, M., Katzer, A. et al. A prospective study on histone γ-H2AX and 53BP1 foci expression in rectal carcinoma patients: correlation with radiation therapy-induced outcome. BMC Cancer 15, 856 (2015). https://doi.org/10.1186/s12885-015-1890-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1890-9