Abstract
Background
The optimal sequence of chemotherapeutic agents is not firmly established for the treatment of metastatic colorectal cancer (mCRC). This phase II multi-centre study investigated the efficacy and tolerability of a standard capecitabine plus irinotecan (XELIRI) regimen with bevacizumab in previously untreated patients with mCRC.
Methods
Patients received intravenous irinotecan 175 mg/m2 on day 1 and oral capecitabine 1000 mg/m2 (800 mg/m2 for patients >65 years of age) twice daily on days 2–8, followed by a 1-week rest, and bevacizumab 5 mg/kg as an intravenous infusion on day 1 every 2 weeks.
Results
Seventy-seven patients were included in the intention-to-treat and safety populations. Progression-free survival at 9 months was 61%. The overall response and disease control rates were 51% and 84%, respectively. Median progression-free and overall survival times were 11.9 and 24.8 months, respectively. 48 patients (62%) had at least one grade 3/4 adverse event, the most common being asthenia, diarrhoea and neutropenia. Quality of life varied little over the study period with mean visual analogue scale general health scores ranging from 71 to 76 over cycles 1–11.
Conclusion
Our study found irinotecan and capecitabine administered fortnightly with bevacizumab in patients with mCRC to be an effective and tolerable regimen.
Trial registration
clinicaltrials.gov identifier NCT00875771. Trial registration date: 04/02/2009.
Similar content being viewed by others
Background
According to the World Health Organisation’s most current statistics there are just over 12.6 million cases of cancer diagnosed each year. Colorectal cancer is the 5th most common and accounts for 9.7% of all cancers [1]. Standard treatments for patients with metastatic colorectal cancer (mCRC) usually consist of combination chemotherapy based on fluorouracil or capecitabine plus either oxaliplatin or irinotecan, and a targeted agent such as bevacizumab, cetuximab or panitumumab [2,3]. The most commonly used chemotherapy regimens are fluorouracil with folinic acid plus oxaliplatin (FOLFOX), fluorouracil with folinic acid plus irinotecan (FOLFIRI), capecitabine plus oxaliplatin (XELOX), and capecitabine plus irinotecan (XELIRI). However, the optimal sequence of chemotherapeutic agents is not firmly established, and most patients will receive a fluoropyrimidine, irinotecan and oxaliplatin over the course of their treatment.
Randomised phase III studies have shown that the addition of bevacizumab to first- or second-line chemotherapy regimens extends overall survival and/or progression-free survival in patients with mCRC compared with chemotherapy alone [4-7]. Consequently, bevacizumab is indicated for the first- and second-line treatment of patients with mCRC [2,3].
Some uncertainty surrounds the most effective and tolerable schedule for administering irinotecan-based regimens such as XELIRI. The BICC-C and EORTC 40015 studies suggested that 3-weekly administration of irinotecan plus capecitabine can be associated with unacceptable gastrointestinal side effects [8,9], although both studies were confounded by the concomitant use of celecoxib, which is known to be associated with gastrointestinal toxicity. Consequently, different drug doses and administration regimens have been investigated with the aim of improving the tolerability of the combination of irinotecan and capecitabine [10-12].
We have previously shown that 2-weekly irinotecan plus capecitabine (irinotecan on day 1 every 2 weeks; plus capecitabine on days 1–7, followed by a week of rest) was effective and well tolerated in patients with mCRC [13]. Preclinical studies had shown this 2-weekly schedule, which is similar to the FOLFIRI schedule, to be more effective than the standard 3-weekly regimen and to allow the administration of higher capecitabine doses [14]. In our study, the adverse-event profile of XELIRI was acceptable, with asthenia, nausea, vomiting and diarrhoea being the most commonly observed grade 3/4 adverse events (occurring in 7–9% of patients). Dose delays and reductions occurred in <12% of patients for irinotecan and <5% of patients for capecitabine [13]. We subsequently incorporated bevacizumab into this regimen and demonstrated that this combination of a targeted agent with chemotherapy was effective and well tolerated in patients with mCRC [15].
The present phase II multicentre study was undertaken on behalf of the Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD) to assess the efficacy and tolerability of 2-weekly regimen of irinotecan in combination with capecitabine plus bevacizumab in a larger population of previously untreated patients with mCRC.
Methods
Patients and study design
Patients ≥18 years of age with histologically proven, measurable mCRC that was not initially totally resected were included in this phase II open-label study. To be included, patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and could have had prior surgical treatment of their disease. No prior chemotherapy was allowed, other than adjuvant or neoadjuvant therapy completed at least 6 months before inclusion in the study; patients who received adjuvant therapy must not have progressed during or within 6 months of completing treatment. Additionally each patient was discussed by a multi-disciplinary team within each cancer centre to confirm their suitability for inclusion in the study.
Patients were not eligible for inclusion in the study if they had a history of central nervous system disease, psychiatric disability, clinically significant cardiac disease, lack of integrity of the upper gastrointestinal tract, malabsorption syndrome or inability to take oral medication. Exclusion criteria included any surgical procedures in the 28 days before the start of the study or if any surgery was scheduled to take place during the study. The use of oral anticoagulants or full-dose parenteral thrombolytic agents was not permitted, although low-dose warfarin was allowed. Chronic treatment with high-dose aspirin or antiplatelet agents was not permitted.
Patients with creatinine clearance <30 mL/minute or serum creatinine >1.5 times the upper limit of normal (ULN) were excluded from the study, as were those with: an absolute neutrophil count <1.5 × 109/L; platelet count <100 × 109/L; haemoglobin <9 g/dL; International Normalised Ratio >1.5; total bilirubin >1.5 × ULN; alanine aminotransferase and/or aspartate aminotransferase >2.5 x ULN (or >5 × ULN in case of liver metastases); or alkaline phosphatase >2.5 × ULN (or >5 × ULN in case of liver metastases or >10 × ULN in case of bone metastases).
The study protocol (Study TTD-08-03; EudraCT: 2008-004688-20; clinicaltrials.gov identifier NCT00875771) was approved by the Spanish Medicine Agency as well as the Institutional Review Board and Ethics Committee of each participating site (for details please refer to the Additional file 1). Reference Ethic Committee: “Comité Ético de Investigación Clínica” of the Hospital Universitario de Burgos, Avda. del Cid, 96,09005 Burgos on January 2009. Study procedures were carried out in accordance with the Declaration of Helsinki and its subsequent amendments, and Good Clinical Practice guidelines. Written, informed consent was obtained from all patients before enrolment.
Treatment
Treatment consisted of irinotecan 175 mg/m2 as an intravenous infusion on day 1 every 2 weeks, capecitabine 1000 mg/m2 (800 mg/m2 for patients >65 years of age) twice daily on days 2–8, followed by a 1-week rest, and bevacizumab 5 mg/kg as an intravenous infusion on day 1 every 2 weeks. Treatment was continued until disease progression, unacceptable toxicity or patient withdrawal.
The doses of the chemotherapeutic agents were modified appropriately in each cycle according to the occurrence of toxicities. Once a dose was reduced, doses were not increased in subsequent cycles. If two dose reductions were sanctioned as a result of toxicity, patients experiencing the same complications were withdrawn from the study unless they had achieved an objective response to treatment, in which case the decision to continue treatment was left to the judgment of the investigator. If chemotherapy was delayed, administration of bevacizumab was also delayed. If the administration of chemotherapy was delayed for more than 2 cycles, the patient was withdrawn from the study. If irinotecan was discontinued, capecitabine and bevacizumab were to be continued unless unacceptable toxicity was observed. Similarly, if either capecitabine or bevacizumab were interrupted, treatment with the remaining agents could be continued at the investigator’s discretion.
Assessments
The response to treatment was assessed using the radiological RECIST criteria [16] at 6-cycle intervals until the disease progressed or the patient died. No independent radiological review committee was established.
Adverse events were assessed at study visits and reported by patients. Adverse events were classified according to the National Cancer Institute Common Toxicity Criteria (CTC) version 3.0 [17]). All adverse events, regardless of their relation to the study treatment, were followed until resolution even if patients had withdrawn from the study.
Quality of life was measured using the EuroQoL 5-Dimensions (3-level) questionnaire (EQ-5D-3 L), a generic instrument used for measuring health status [18]. Quality of life assessments were performed at baseline, before each odd-numbered cycle (3, 5, 7, etc.) and in the 30 days following discontinuation of study therapy. A minimum of three assessments was required for the patient’s data to be included in the quality of life analysis. The EQ-5D-3 L assesses five different aspects of health (mobility, personal care, daily activities, pain/discomfort and anxiety/depression), each with three response categories. In addition, self-assessed general health was recorded using a 20 cm visual analogue scale (VAS) ranging from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”).
Statistical analyses
The primary endpoint of this phase II study was progression-free survival at 9 months. The secondary endpoints were: progression-free survival, overall survival, response rate, safety, resection rate and quality of life. Efficacy analyses were performed on the intention-to-treat population i.e. patients who received at least one dose of study medication. Safety analyses were performed on patients who received at least one dose of study medication (the safety population).
The sample size was based on a single-stage Fleming design, with p0 = 12% at 2 years (equivalent to a median progression-free survival of 8 months), p1 = 25% (equivalent to a median progression-free survival of 12 months), and an alpha error of 0.05 and a beta error of 0.01, resulting in a requirement for seventy-one evaluable patients. Allowing for a 10% dropout rate, seventy-nine patients were planned to be recruited.
Survival analyses were performed using Kaplan–Meier methodology; 95% confidence intervals (CIs) were calculated for the primary and secondary outcomes. Qualitative variables were described using absolute and relative frequencies; quantitative variables were described with means, medians and standard deviation (SD). All analyses were performed using SPSS version 17.0 (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.).
Results
Patients
A total of eighty-one patients were enrolled in the study at twelve Spanish centres between 14 April 2009 and 20 April 2010. Four patients failed the screening process, with three violating entry criteria; one patient was hospitalised prior to initiation of treatment and could not be treated with the study regimen. The remaining seventy-seven patients received treatment and were included in the intention-to-treat and safety populations.
Patient characteristics are summarised in Table 1. A total of sixty-five patients had relevant comorbidities, the most common being hypertension in 32 patients (42%, twenty-seven of whom were taking antihypertensive agents) and 14 patients (18%) with diabetes mellitus. Twenty-one (27%) patients were ≥70 years of age. Fifty patients (65%) had undergone surgical resection of the primary tumour. Of the remaining twenty-seven patients who did not have surgical resection, four presented with intestinal perforation or occlusion.
Tumour KRAS status was determined in seventy-one patients (92%); thirty-six patients (51%) had wild-type KRAS tumours and thirty-five (49%) had mutant KRAS tumours.
Treatment
Patients underwent treatment for a median of 6.2 months (range 0.4–21.6 months) and received a total of 1009 cycles (876 cycles of bevacizumab, 973 cycles of irinotecan and 982 cycles of capecitabine). A median of 12.0 cycles (range 1.0–43.0 cycles) was administered; the median number of cycles of irinotecan, capecitabine and bevacizumab administered were 12.0 (range 1.0–43.0), 12.0 (range 1.0–43.0) and 11.0 (range 1.0–33.0), respectively.
The median relative dose intensities were: bevacizumab 89%, irinotecan 85%, and capecitabine 89%. Absolute median dose intensities were: bevacizumab 2.1 mg/kg/week, irinotecan 77.5 mg/m2/week, capecitabine 1439 mg/day.
Treatment was delayed in fifty-seven patients (74%) resulting in delays in 160 of the 1009 cycles (16%). The most common reasons for delayed doses were: neutropenia (25 cycles; 16%), administrative reasons (23 cycles; 14%); diarrhoea (19 cycles; 12%) and patient decision (15 cycles; 9%).
Bevacizumab was delayed in 119 cycles (14%) in twenty-four patients (31%), most commonly as a result of thromboembolism (67 cycles; 56%), fistula (9 cycles; 8%), wound-healing complications (8 cycles; 7%), and surgery (9 cycles; 8%). The irinotecan dose was reduced or delayed in 78 cycles (8%) in thirty-seven patients (48%) most commonly as a result of asthenia (12 cycles; 15%), diarrhoea (13 cycles; 17%) and at the discretion of the investigator (22 cycles; 28%). The capecitabine dose was reduced or delayed in 85 cycles (9%) in forty-six patients (60%) primarily as a result of diarrhoea (18 cycles; 21%).
Most patients received more than one line of treatment with sixty-two patients (81%) receiving second-line therapy, twenty-eight of whom had second-line bevacizumab-containing regimens. Thirty-seven patients received third-line and later lines of therapy.
Efficacy
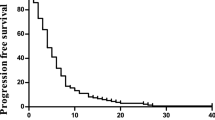
Patients were followed for a median of 23.3 months (range 0.4–39.6 months). Efficacy outcomes are shown in Table 2 and Figure 1. Progression-free survival at 9 months (the primary endpoint) was 61% (95% CI: 48–73%). The median progression-free survival was 11.9 months (95% CI: 10.8–13.1 months) and median overall survival was 24.8 months (95% CI: 19.9–29.7 months). The overall response rate was 51% (95% CI: 39–62%) and the disease control rate was 84% (95% CI: 74–91%).
Median progression-free survival was 12.0 months (95% CI: 6.6–17.5 months) in patients with wild-type KRAS tumours and 11.8 months (95% CI: 10.7–13.0 months) in those with mutant KRAS tumours (P= 0.985) (Figure 2). Overall survival was also similar in patients with wild-type and mutant KRAS tumours: 28.5 months (95% CI: 21.4–35.6 months) versus 27.9 months (95% CI: 21.4–34.3 months), respectively (P= 0.659; Figure 2). Confirmed response rates were 44.4% in patients with wild-type KRAS tumours and 37.1% in those with mutant KRAS tumours (P >0.05).
Seventeen patients (22%) had surgical resection of metastases during the study (65% liver metastases, 18% lung metastases, 12% peritoneal metastases and other sites). The median time to surgery after treatment initiation was 6.7 months. Twelve patients (71%) underwent R0 resection, three (18%) had an R1 resection and two (12%) were not evaluable. Thirteen of the seventeen patients who underwent surgical resection had further treatment (chemotherapy or immunotherapy). With respect to second-line chemotherapy six patients received post-surgical treatment with bevacizumab plus capecitabine/irinotecan and three patients received other bevacizumab-containing regimens. The remaining patients received a variety of other regimens that included oxaliplatin, cetuximab and panitumumab.
Safety
To date, forty-five patients (58%) have died and thirty-two (42%) are still alive. The causes of death were: progressive disease (N = 35), adverse events (N = 7) and unknown (N = 3). Adverse events leading to death included: multi-organ failure (N = 2), gastrointestinal perforation (N = 2), respiratory and cardiac insufficiency due to chronic obstructive pulmonary disease (N = 1), myocardial infarction (N = 1) and respiratory insufficiency (N = 1). One of the gastrointestinal perforation events was considered to be related to treatment with bevacizumab; the two multi-organ failures were considered following discussion and scrutiny by the investigators to be related to capecitabine/irinotecan, rather than to disease progression as these events were reported in the context of toxicities.
A total of seventy-six patients (99%) had at least one adverse event related to treatment; forty-eight patients (62%) had at least one grade 3 or 4 adverse event. The most common grade 3 and 4 related adverse events were asthenia, diarrhoea and neutropenia (Table 3). Adverse events of special interest for bevacizumab are summarised in Table 4. Pulmonary embolism occurred in 10 patients, four of whom were >70 years of age; eight of these events were asymptomatic and two were symptomatic.
Quality of life
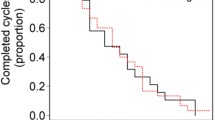
EQ-5D-3 L questionnaires were completed by 70 patients (91%) at cycle 1, 58 of 70 patients (83%) at cycle 3, 49 of 64 patients (77%) at cycle 5, 38 of 55 patients (69%) at cycle 7, 36 of 47 patients (77%) at cycle 9, and 31 of 45 patients (69%) at cycle 11. After this point, the number of patients who completed questionnaires continued to decline, although some patients completed questionnaires until cycle 43.
Patient quality of life did not vary greatly over the study period (Figure 3). Most patients reported having no problems with mobility, patient care or activities of daily living during the first cycles of treatment. More than 50% of patients experienced pain or discomfort in the early cycles, although this proportion decreased as the study progressed. More than half of all patients reported feeling moderately or very anxious, or depressed throughout the study period. Mean VAS general health scores ranged from 71 to 76 over cycles 1–11 (Figure 3).
Discussion
Considerable uncertainty surrounds the most effective use of irinotecan in combination with capecitabine for the treatment of patients with mCRC. Some studies have shown that irinotecan can be associated with significant gastrointestinal toxicities and, as a result, several doses and administration regimens have been investigated in order to maximise efficacy and tolerability.
This study has demonstrated that administering capecitabine–irinotecan plus bevacizumab every 2 weeks is a feasible and tolerable first-line treatment option for patients with mCRC. Cross-study comparisons, which should be made with caution, suggest that median progression-free survival and overall survival in the present study (11.8 months and 24.8 months, respectively) are similar to those reported in other phase II studies of bevacizumab plus XELIRI [19-22] and superior to those in which XELIRI was administered without bevacizumab [8,11,12]. This suggests that the efficacy of treatment was not compromised by the 2-weekly dosing schedule.
The safety profile of capecitabine–irinotecan plus bevacizumab administered every 2 weeks was comparable with reports from other phase II studies. We observed grade 3/4 diarrhoea in 18% of patients, which is similar to the 10–19% reported by others [19-22]; moreover, only one patient had grade 4 diarrhoea. Grade 3/4 neutropenia appeared to be somewhat less common than in other studies, occurring in 10% of patients in our study compared with 12–18% reported in those other studies.
Thromboembolic events have been reported as a complication of treatment with XELIRI–bevacizumab in the French FNCCLC ACCORD 13/0503 study, in which 24% of patients in the XELIRI–bevacizumab arm reported a venous thrombosis or pulmonary embolism [22]. In the present study, thromboembolic events were observed in 17% of patients, most of which were asymptomatic. Four of the eight grade 4 pulmonary emboli occurred in patients who were >70 years of age, amongst whom such events have been reported to be more common [23,24]. Indeed the incidence of thromboembolic events increased with age in bevacizumab-treated patients in the BRiTE registry, although the increase was not statistically significant after adjustment for baseline ECOG performance status, hypertension, the absence of anticoagulant therapy at baseline and prior history of thromboembolic events [25].
Median dose intensities were 89% for bevacizumab, 85% for irinotecan and 89% for capecitabine, suggesting that the regimen was generally well tolerated. These findings were within the confidence intervals of other studies that have reported on tolerability of the combination [21,22,26].
Response to treatment was not dependent on tumour KRAS status, as observed in other studies of bevacizumab plus chemotherapy in patients with mCRC [7,27-29]. As with the prognostic value of KRAS genotype study [29], we found that progression-free survival and overall survival were not extended significantly in KRAS wild-type genotypes over the mutant form. When interpreting these findings, it is important to note that the KRAS analysis was conducted retrospectively in a non-comparative trial.
Quality of life, as measured using the EQ-5D-3 L questionnaire, was maintained throughout the study, suggesting that treatment did not have a substantial negative impact on patients’ everyday activities. The evidence supports the validity of the EQ-5D-3 L tool in measuring quality of life in cancer patients [30], although the 5-level classification system, EQ-5D-5 L, has less ceiling effect and greater discriminative power [31] and we would consider using this tool in future studies. It is tricky to compare the VAS scores in Figure 3 with the EQ-5D-3 L scores. The subjective nature of the VAS scores gives some insight into the psychological tolerance of the effect of treatment on patients than the more objective EQ-5D-3 L. This self-perception of wellbeing improves over the course of the study. It would be interesting to investigate this (perhaps ‘placebo-like’) phenomenon more deeply in a separate study.
Conclusions
In conclusion, this multicentre phase II study supports the use of irinotecan and capecitabine administered every 2 weeks with bevacizumab in patients with mCRC. The study included patients with multiple comorbidities, and elderly patients, and therefore indicates that this is an effective and tolerable regimen.
Abbreviations
- ECOG:
-
Eastern Cooperative Oncology Group
- FOLFIRI:
-
Fluorouracil with folinic acid plus irinotecán
- FOLFOX:
-
Folinic acid plus oxaliplatino
- mCRC:
-
Metastatic colorectal cancer
- TTD:
-
Spanish cooperative group for the treatment of digestive tumors
- XELIRI:
-
Capecitabine plus irinotecán
- XELIRI:
-
Capecitabine plus irinotecán
- XELOX:
-
Capecitabine plus oxaliplatin
References
Ferlay J, Shin H, Bray F, Forman D, C Mathers, Parkin D. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. 2010. Lyon, France: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr. Accessed on 10 November 2013.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2013) Colon cancer Version 3. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed on 6 March 2013.
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:2479–516.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:2013–9.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Eastern Cooperative Oncology Group Study E3200: Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:1539–44.
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. ML18147 Study investigators: continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:4779–86.
Köhne C-H, De Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2008;19:920–6.
Tewes M, Schleucher N, Achterrath W, Wilke HJ, Frings S, Seeber S, et al. Capecitabine and irinotecan as first-line chemotherapy in patients with metastatic colorectal cancer: results of an extended phase I study. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2003;14:1442–8.
Borner MM, Bernhard J, Dietrich D, Popescu R, Wernli M, Saletti P, et al. A randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: efficacy, quality-of-life and toxicity. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2005;16:282–8.
Bajetta E, Di Bartolomeo M, Mariani L, Cassata A, Artale S, Frustaci S, et al. Randomized multicenter Phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer. 2004;100:279–87.
Garcia-Alfonso P, Muñoz-Martin A, Mendez-Ureña M, Quiben-Pereira R, Gonzalez-Flores E, Perez-Manga G. Capecitabine in combination with irinotecan (XELIRI), administered as a 2-weekly schedule, as first-line chemotherapy for patients with metastatic colorectal cancer: a phase II study of the Spanish GOTI group. Br J Cancer. 2009;101:1039–43.
Kolinsky K, Zhang Y-E, Dugan U, Heimbrook D, Packman K, Higgins B. Novel regimens of capecitabine alone and combined with irinotecan and bevacizumab in colorectal cancer xenografts. Anticancer Res. 2009;29:91–8.
García-Alfonso P, Muñoz-Martin AJ, Alvarez-Suarez S, Jerez-Gilarranz Y, Riesco-Martinez M, Khosravi P, et al. Bevacizumab in combination with biweekly capecitabine and irinotecan, as first-line treatment for patients with metastatic colorectal cancer. Br J Cancer. 2010;103:1524–8.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v3.0. 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 10 November 2013.
Oemar M, Oppe M. EQ-5D-3L User Guide: Basic information on how to use the EQ-5D-3L instrument. Version 5.0. 2013. Available at: http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-3L_UserGuide_2013_v5.0_October_2013.pdf. Accessed on 11 November 2013.
Pectasides D, Papaxoinis G, Kalogeras KT, Eleftheraki AG, Xanthakis I, Makatsoris T, et al. XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis. BMC Cancer. 2012;12:271.
Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, et al. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br J Cancer. 2012;106:453–9.
Renouf DJ, Welch S, Moore MJ, Krzyzanowska MK, Knox J, Feld R, et al. A phase II study of capecitabine, irinotecan, and bevacizumab in patients with previously untreated metastatic colorectal cancer. Cancer Chemother Pharmacol. 2012;69:1339–44.
Ducreux M, Adenis A, Pignon J-P, François E, Chauffert B, Ichanté JL, et al. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer Oxf Engl 1990. 2013;49:1236–45.
White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I–4–I–8.
Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med. 2004;164:2260–5.
Kozloff M, Yood MU, Berlin J, Flynn PJ, Kabbinavar FF, Purdie DM, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14:862–70.
Ardavanis A, Kountourakis P, Mantzaris I, Malliou S, Doufexis D, Sykoutri D, et al. Bevacizumab added to the irinotecan and capecitabine combination for advanced colorectal cancer: a well-tolerated, active and convenient regimen. Anticancer Res. 2008;28:3087–92.
Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–8.
Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:2675–82.
Bruera G, Cannita K, Di Giacomo D, Lamy A, Troncone G, Dal Mas A, et al. Prognostic value of KRAS genotype in metastatic colorectal cancer (MCRC) patients treated with intensive triplet chemotherapy plus bevacizumab (FIr-B/FOx) according to extension of metastatic disease. BMC Med. 2012;10:135.
Pickard AS, De Leon MC, Kohlmann T, Cella D, Rosenbloom S. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care. 2007;45:259–63.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2013;22:1717–27.
Acknowledgements
The authors wish to thank the patients, and the medical and nursing staff of all the participating institutions; Inma Ruiz de Mena at the TTD Data Center; Dynamic Solution for monitoring, statistics and data management. Support for third-party medical writing assistance was provided by Roche Farma, Spain.
Financial support for this research trial was provided by Roche Farma, S.A.
The findings have been presented in part at:
● American Society of Clinical Oncology (ASCO) Annual Meeting Proceedings. J Clin Oncol29: 2011 (suppl; abstr e13018).
● 14th World Congress on Gastrointestinal Cancer (2012) Ann Oncol23 (suppl 4): iv103-iv104.
● American Society of Clinical Oncology (ASCO) (2013) Gastrointestinal Cancers Symposium. J Clin Oncol Vol31, No 4_suppl (February 1 Supplement): 501.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
P Garcia-Alfonso: Consultant /Advisory Role: Amgen, Bayer, Merck, Roche and Sanofi. E. Aranda: Consultant /Advisory Role: Amgen, Bayer, Celgene, Merck, Roche and Sanofi. All remaining authors have declared no conflict of interests.
Authors’ contributions
PGA and EA were responsible for conception and design, and data analysis and interpretation. PGA, MC, AM, AS, MGG, CG, BM, EGF, BQ, ALL, FL, MJG, AO and EA were responsible for collection and assembly of data, provision of study materials or patients, manuscript writing and final approval of manuscript.
Authors’ information
Supported by the TTD, Madrid, Spain.
Additional file
Additional file 1:
Institutional Review Board and Ethics Committee.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Garcia-Alfonso, P., Chaves, M., Muñoz, A. et al. Capecitabine and irinotecan with bevacizumab 2-weekly for metastatic colorectal cancer: the phase II AVAXIRI study. BMC Cancer 15, 327 (2015). https://doi.org/10.1186/s12885-015-1293-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1293-y