Abstract
Background and objectives
To date, programmed intermittent epidural bolus (PIEB) has been widely used in obstetric analgesia, while no optimal PIEB regimen has been proposed. This study aimed to assess effective analgesia in 90% of women (EV90) with different concentrations of ropivacaine (0.075% and 0.1%) combined with 0.5 µg/mL sufentanil, at an interval of 40 min using the biased coin design-up-and-down method (BCD-UDM), and to explore whether there is a difference in EV90 with the increase of ropivacaine concentration.
Methods
In total, 103 primiparous women were assigned to two groups, including group A (n = 52) and group B (n = 51). Parturients in group A were treated with 0.075% ropivacaine and 0.5 µg/mL sufentanil, while those in group B were treated with 0.1% ropivacaine and 0.5 µg/mL sufentanil. Used the biased coin up-and-down sequential allocation method to determine the EV90. The secondary outcomes were sensory block level, motor block, and adverse events (hypotension, urinary retention, and pruritus).
Results
The results revealed that EV90 was 10 mL (95% confidence interval (CI):8.03–11.54) in group A, and EV90 was 9 mL (95% CI:7.49–10.51) in group B by the isotonic regression method. The highest level of the sensory block was T8, and the lowest was T12. No case of hypotension was recorded,and only 4 parturients complained of motor block.
Conclusion
With an interval of 40 min, the optimal PIEB bolus volume of 0.075% ropivacaine and 0.5 µg/mL sufentanil was 10 mL, 0.1% ropivacaine and 0.5 µg/mL sufentanil was 9 mL. Moreover, the PIEB volume decreased along with the higher concentration of ropivacaine.
Trial registration
ChiCTR registration number: ChiCTR2000040917. Registration date: December 15, 2020.
Similar content being viewed by others
Introduction
Neuraxial analgesia is considered the gold standard in labor analgesia, providing the most effective pain relief during childbirth [1]. PIEB was proposed as a more efficacious technique to maintain labor epidural analgesia compared with continuous epidural infusion(CEI). Studies comparing PIEB with CEI have shown that PIEB is associated with the reduced local anesthetic consumption [2,3,4], a lower incidence of breakthrough pain [2, 4, 5], the reduced incidence of cesarean delivery [6], and a greater maternal satisfaction [2, 4, 6,7,8]. There is evidence that PIEB regimens decrease motor block and instrumental deliveries [8, 9]. However, the optimal PIEB regimen has still remained to be determined.
The optimal PIEB regimen has varied significantly among different studies [9,10,11,12,13,14,15]. Bittencourt et al. [16] used a PIEB volume of 10 mL of bupivacaine 0.0625% with fentanyl 2 µg/mL, in which the interval varied between 30 and 60 min, and found an optimal interval of approximately 40 min. Zhou et al. [17] designed a study to identify the optimal interval for PIEB using 10 mL of ropivacaine 0.08% and sufentanil 0.3 µg/mL; the study found that with a fixed 10 mL dose of ropivacaine 0.08% with sufentanil 0.3 µg/mL, the optimal PIEB interval was about 42 min. Another study yielded similar results [15], suggesting that 40 min may be an optimal PIEB interval.
To date, few studies have concentrated on the optimal PIEB volume. Zakus et al. determined the optimal PIEB volume at a 40 min interval to provide effective analgesia in 90% of women, without the use of patient-controlled epidural analgesia (PCEA), and the volume was in the range of 7—12 mL. This study suggested that the optimal PIEB volume of bupivacaine 0.0625% with fentanyl 2 µg/mL administered at a fixed interval of 40 min was approximately 11 mL [18]. Epstein et al. also demonstrated that 10 mL boluses of bupivacaine 0.0625% with fentanyl 2 µg/mL delivered every 40 min produced an effective analgesia without breakthrough pain in 90% of women [15].
Ropivacaine has increasingly been replaced with bupivacaine in obstetric anesthesia because it causes less motor blockade and damage to cardiovascular system and central nervous system toxicity [19, 20]. However, it could not be assumed that the optimal PIEB volume with ropivacaine and sufentanil was the same as that of PIEB with bupivacaine and fentanyl. It is clinically of great importance to determine the optimal PIEB volume for the mixture of ropivacaine and sufentanil.
The present study aimed to evaluate the EV90 with different concentrations of ropivacaine (0.075% and 0.1%) combined with 0.5 µg/mL sufentanil, at an interval of 40 min using the BCD-UDM, and to explore whether there is a difference in EV90 with the increase of local anesthetic concentration.
Methods
The present study was approved by the Ethics Committee of Ya’an People’s Hospital (Ya’an, China; Approval No. 202015). Parturients who were admitted to the Ya’an People’s Hospital from March 1, 2021, to November 30, 2021, were enrolled. We obtained written informed consent from all parturients prior to enrollment. The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement.
Inclusion criteria were primiparous women with American Society of Anaesthesiologists (ASA) class II-III, gestational age between 37 and 42 weeks, singleton pregnancy, regular contractions, and cervical dilation of 2–3 cm. We excluded women who had a contraindication to epidural analgesia, hypersensitivity to ropivacaine or sufentanil, and those who refused to participate in the study. Withdrawal criteria were failure to perform epidural anesthesia, VAS > 3 after loading dose,cesarean section in the first stage of labor, and loss to follow-up. Parturients were assigned into two groups: group A (ropivacaine 0.075% and sufentanil 0.5 µg/mL) and group B (ropivacaine 0.1% and sufentanil 0.5 µg/mL). The recruitment of women in group A was completed first, followed by group B.
After the parturients arrived in the delivery room, maternal heart rate, blood pressure, oxygen saturation, respiration, and fetal heart rate were continuously monitored. The epidural puncture was performed at the L3–4 vertebral interspace by the midline approach. Using the traditional surface landmarks-based approach. Local infiltration was carried out using 3 mL of 2% lidocaine. Using a loss of resistance to saline technique with a 17G puncture needle, a 19G multiport wire-reinforced epidural catheter (AS-E/SII; TuoRen Medical Co., Ltd., Changyuan, China) was inserted into the epidural space with a depth of about 4 cm. All epidural catheter insertions were performed by a consultant. Then, 3 mL of 1.0% lidocaine (Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) was injected to rule out the possibility of subarachnoid injection or intravenous injection in the next 5 min. Subsequently, a loading dose was administered consisting of two 5 mL boluses of ropivacaine (Hengrui Pharmaceutical Co., Ltd., Nanjing, China) with sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., Wuhan, China), given 5 min apart. To continue with the study, we required that the pain score of the visual analog scale (VAS) ≤ 3 was achieved within 20 min of administering the loading dose.
Subsequently, a solution of ropivacaine (0.075% or 0.1%) with sufentanil 0.5 µg/mL was administered via a PIEB pump (ZZB-IV; Apon Co., Ltd., Nanjing, China), and the infusion rate was 200 mL/h. The first bolus was given at 40 min after the completion of the loading dose, and all subsequent PIEB doses were given at a fixed interval of 40 min. Besides, PCEA was set to 5 mL/time, the lock time was 15 min, and the maximum dose per hour was 32 mL. The range of PIEB volume was between 7 and 12 mL. Each woman was explained how to use PCEA for breakthrough pain and instructed to press the PCEA button if she felt uncomfortable. If the woman pressed the PCEA button or asks for a manual bolus, the bolus was considered inadequate.
The first woman enrolled in the study was administered a bolus of 7 mL. The bolus for the subsequent woman was determined by the response of previous woman and the BCD-UDM. If the bolus volume did not provide adequate analgesia, the bolus for the next woman was increased by 1 mL. In case of adequate analgesia, the next woman’s bolus was coin-randomized with an 11% probability of decreasing by 1 mL and an 89% probability remained the same. In case of adequate analgesia at 7 mL or inadequate analgesia at 12 mL, the bolus for the next woman did not change. The BCD-UDM allocation was carried out using a computer-generated list of random responses. A research assistant used this list to provide the PIEB volume setting for the next woman in a sealed envelope. An anesthetist, who was blinded to the objective of the study, set up the epidural infusion pump. The epidural infusion pump was covered to blind the participant, investigator and nurse.
Baseline data of each woman included physical characteristics, type of labor (spontaneous, instrumental,or cesarean delivery), and use of oxytocin. The sensory block level to ice was detected by applying ice in the midclavicular line, VAS score (0–10, where 0 = no pain and 10 = the highest level of pain), and motor block score (modified Bromage score(MBS): 0 = no motor block; 1 = inability to raise extended leg but able to move knees and feet; 2 = inability to raise extended leg and move knee but able to move feet; 3 = complete motor block of limb). All assessments were completed by a blinded investigator at 20 and 40 min after ending the loading dose, followed by every hour thereafter until the completion of the study.
The primary outcome was adequate analgesia, which was defined as no use of PCEA or request for manual boluses until the woman’s cervix was fully dilated. Secondary outcomes included sensory block level, motor block, and adverse events (hypotension that was defined as a decrease in systolic blood pressure by 20% of baseline, urinary retention, and pruritus).
Trials with a BCD-UDM need a sufficient sample size, which may increase statistical accuracy and make the standard errors smaller [21]. Therefore, to estimate the EV90, We appropriately increased the sample size to include 52 cases in group A and 51 in group B. The EV90 was calculated by isotonic regression, and 95% confidence interval (95% CI) was calculated by bootstrapping. Isotonic regression is a well-described variant of restricted least-squares regression that constrains the point estimates to be either monotonically increasing or monotonically decreasing. Isotonic regression has favorable statistical properties. We used the dose estimator μ3. The isotonic estimator μ3 has a smaller biasand MSE, which was defined as the linearly interpolated estimator of the target dose. It was derived from the two consecutive boluses that success rates enclosed the value of probability of effect ‘Г’ [22]. Statistical analysis was performed using R 3.6.2 software. The descriptive summaries of some indicators of secondary outcomes were performed.
Result
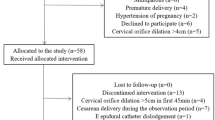
A total of 134 parturients were enrolled in the study. Finally, group A had 52 parturients and group B had 51 parturients included in the data analysis. The study flowchart is shown in Fig. 1. Parturient’s demographic and labor characteristic are summarized in Table 1. The parturients allocation sequences and responses to different PIEB volume are illustrated in Figs. 2 and 3.
The estimated EV90 was 10 mL (95%CI:8.03–11.54) and 9 mL (95%CI:7.49–10.51) with the isotonic regression method in group A and group B, respectively. In 9, 10, and 11 mL groups, effective analgesia was achieved in 25%, 70.83%, and 100% of women in group A, respectively. In 8, 9, and 10 mL groups, effective analgesia was achieved in 50%, 88.46%, and 90.91% of women in group B, respectively. The proportion of women with successful analgesia for each PIEB volume is shown in Table 2. For those patients who did not respond to analgesia, the majority of them received PCEA within 2 h. The details of PCEA timing are summarized in Table 3.
The highest level of sensory block in the included women was T8, and the lowest was T12.No sensory block higher than T6 was observed. No parturient had motor block with a MBS score greater than 1 in both groups. Women who received 10 mL PIEB volume and above exhibited a trend toward higher maximum sensory block levels. The results are summarized in Table 4.
Only one parturient in group B and 2 parturient in group A had pruritus. No maternal hypotension or urinary retention were recorded (Table 1).
Discussion
In the present study, the results demonstrated that 10 mL was the optimal PIEB volume for 0.075% ropivacaine, and 9 mL was the optimal PIEB volume for 0.1% ropivacaine. The corresponding hourly consumption of ropivacaine was 11.25 and 13.5 mg/h in group A and group B, respectively. The incidence of motor block and adverse effects was very low, which was similar to other studies [16, 17, 23]. Although three parturients complained of pruritus, no case of hypotension or urinary retention was reported.
Wu et al. found that 10 mL PIEB volume was better than 8 and 5 mL with 0.1% ropivacaine combined with 0.33 ug/mL sufentanil [24]. Similar results were also observed in other studies [13, 23]. The results suggested an approximately 10 mL PIEB volume, maybe a better setting, regardless of the different concentrations of anesthetics.
Our study found that when the PIEB volume was < 10 mL in group A or < 9 mL in group B, the effectiveness of analgesia was significantly reduced in the two groups, suggesting that it is not possible to reduce the PIEB volume below EV90 with our current PIEB regimen without compromising the quality of analgesia. Similar results were also reported previously [15].
Moreover, in our PIEB regimen at a fixed interval of 40 min, the EV90 showed a decreasing trend with the increase of ropivacaine concentration. But one point of interest was that the hourly consumption of ropivacaine was comparable between the two different anesthetic recipes. Ricardo et al. found that when the concentration of bupivacaine increased from 0.0625% to 0.125% at a fixed bolus volume of 10 mL, the optimal interval was shortened from 40 to 35 min [16]. This suggests that increasing local anesthetic concentration can shorten the optimal interval and reduce the bolus volume, while the increase of local anesthetic concentration may be associated with more complications, such as motor block and the upper sensory block level.
The highest level of sensory block in the present study was T8, which was lower than previously reported levels [16, 18, 25, 26]. In general, the highest level of sensory block is associated with the puncture position, anesthetic and its concentration, pump speed, and other factors. In our study, the puncture position was L3-L4, catheter was inserted by 3 cm, the speed of PIEB pump was 200 mL/h, and the maximum tested PIEB volume was 11 mL, which were all lower or smaller than their corresponding variables used in previous reports. This may explain why the sensory block level was lower in our study.
There are some limitations in the present study. First, the study was conducted under a fixed PIEB interval and PIEB pump speed, hindering the generalization of the results. These limitations made the conclusions only suitable for these strictly set conditions. Second, as we only followed up women during the first stage of labor, we do not know how well our regimen works during the second stage of labor. Detailed study for different labor stages may find better PIEB regimen for labor analgesia.
In conclusion, the EV90 of 0.075% ropivacaine and 0.5% sufentanil with a fixed interval of 40 min is 10 mL, and 9 mL for 0.1% ropivacaine and 0.5% sufentanil. The PIEB volume decreased along with the higher concentration of ropivacaine. However, there was no significant difference in the local anesthetic hourly consumption between the two groups. The incidence of motor block and adverse effects was very low in the two groups. Our results suggested that approximately 10 mL PIEB volume may be the optimal PIEB regimen, regardless of the different concentrations of anesthetics. Further attention should be paid to the ropivacaine concentration, different local anesthetics, and the optimal bolus interval to optimize the PIEB regimen. Moreover, more research is needed in the future to optimize the PIEB program and to address the analgesic needs of parturients in all stages of labor.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- PIEB:
-
Programmed intermittent epidural bolus
- BCD-UDM:
-
Biased coin design up-and-down sequence method
- EV90:
-
Optimal effective bolus volume required for effective analgesia in 90% of women
- 95% CI:
-
95% Confidence Interval
- CEI:
-
Continuous epidural infusions
- VAS:
-
Visual analogue scale
- MBS:
-
Modified Bromage score
- PCEA:
-
Patient-controlled epidural analgesia
References
Sng BL, Sia ATH. Maintenance of epidural labour analgesia: the old, the new and the future. Best Pract Res Clin Anaesthesiol. 2017;31:15–22.
Xu J, Zhou J, Xiao H, et al. A systematic review and metaanalysis comparing programmed intermittent bolus and continuous infusion as the background infusion for parturient controlled epidural analgesia. Sci Rep. 2019;9:2583.
George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia:a systematic review and meta-analysis. Anesth Analg. 2013;116:133–44.
Sng BL, Zeng Y, de Souza NNA, et al. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database Syst Rev. 2018;5:CD011344.
Fidkowski CW, Shah S, Alsaden MR. Programmed intermittent epidural bolus as compared to continuous epidural infusion for the maintenance of labor analgesia: a prospective randomized single-blinded controlled trial. Korean J Anesthesiol. 2019;72(5):472–8.
Nunes J, Nunes S, Veiga M, et al. A prospective, randomized, blinded-endpoint, controlled study – continuous epidural infusion versus programmed intermittent epidural bolus in labor analgesia. Braz J Anesthesiol. 2016;66(5):439–44.
Rodríguez-Campoó MB, et al. Patient intermittent epidural boluses (PIEB) plus very low continuous epidural infusion (CEI) versus patient-controlled epidural analgesia (PCEA) plus continuous epidural infusion (CEI) in primiparous labour: a randomized trial. J Clin Monit Comput. 2019;33(5):879–85.
González IPR, Domínguez EE, García CQ, et al. Comparison between different epidural analgesia modalities for labor. Rev Esp Anestesiol Reanim (Engl Ed). 2019;66(8):417–24.
Capogna G, Camorcia M, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113(4):826–31.
Carvalho B, George RB, Cobb B, McKenzie C, Riley ET. Implementation of programmed intermittent epidural bolus for the maintenance of labor analgesia. Anesth Analg. 2016;123:965–71.
Huang R, Zhu J, Zhao Z, et al. The effect of programmed intermittent epidural bolus compared with continuous epidural infusion in labor analgesia with ropivacaine: a meta-analysis of randomized controlled trials. Ann Palliat Med. 2021;10(3):2408–20.
Shatalin D, Arzola C, Downey K, et al. Programmed intermittent epidural bolus for labour analgesia during first stage of labour: a sequential allocation trial to determine the effective interval time between boluses of a fixed volume of 2.5 mL of bupivacaine 0.25% plus fentanyl 8 g·mL1. Can J Anesth. 2021;68(5):653–60.
Yuru F, Hou W, et al. Programmed intermittent epidural bolus decreases the incidence of intra-partum fever for labor analgesia in primiparous women: a randomized controlled study. Arch Gynecol Obstet. 2019;300(6):1551–7.
Liu J, Lin Y, Qiang L, et al. Comparison of continuous epidural infusion and programmed intermittent epidural bolus in labor analgesia. Ther Clin Risk Manag. 2016;12(1):1107–12.
Kanczuk ME, Barrett NM, Arzola C, et al. Programmed Intermittent Epidural Bolus for Labor Analgesia During First Stage of Labor: A Biased-Coin Up-and-Down Sequential Allocation Trial to Determine the Optimum Interval Time Between Boluses of a Fixed Volume of 10 mL of Bupivacaine 0.0625% With Fentanyl 2 μg/mL. Anesth Analg. 2017;124:537–41.
Bittencourt R, Arzola C, Zakus P, et al. A Biased Coin Up-and-Down Sequential Allocation Trial to Determine the Optimum Programmed Intermittent Epidural Bolus Time Interval Between 5 mL Boluses of Bupivacaine 0.125% With Fentanyl 2 µg/mL. Obstet Anesth Digest. 2020;66(9):1075–81.
Zhou SQ, Wang J, Du WJ, et al. Optimum interval time of programmed intermittent epidural bolus of ropivacaine 0.08% with sufentanyl 0.3 μg/mL for labor analgesia: a biased-coin up-and-down sequential allocation trial. Chin Med J. 2020;133(5):517–22.
Zakus P, Arzola C, Bittencourt R, et al. Determination of the optimal programmed intermittent epidural bolus volume of bupivacaine 0.0625% with fentanyl 2 μg/mL at a fixed interval of forty minutes: a biased coin up-and-down sequential allocation trial. Anaesthesia. 2018;73:459–65.
Riazanova OV, Alexandrovich YS, Guseva YV, Ioscovich AM. A randomized comparison of low dose ropivacaine programmed intermittent epidural bolus with continuous epidural infusion for labour analgesia. Rom J Anaesth Intensive Care. 2019;26:25–30.
Halpern SH, Breen TW, Campbell DC, Muir HA, Kronberg J, Nunn R, et al. A multicenter, randomized, controlled trial comparing bupivacaine with ropivacaine for labor analgesia. Anesthesiology. 2003;98:1431–5.
GoRges M, Zhou G, Brant R, et al. Sequential allocation trial design in anesthesia: an introduction to methods, modeling, and clinical applications[J]. Pediatr Anesth. 2017;27(3):240–7.
Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–52.
Roofthooft E, Barbé A, Schildermans J, et al. Programmed intermittent epidural bolus vs. patient-controlled epidural analgesia for maintenance of labour analgesia: a two-centre, double-blind, randomised study. Anaesthesia. 2020;75(12):1635–42.
Wu Qixing. Effects of different bolus volume on anesthetic result of programmed intermittent epidural bolus in labor analgesia [D]. Wenzhou: Wenzhou Medical University, 2019:1–45.
Huang B, Huang Q, Liao J. Optimization of time interval of programmed intermittent epidural bolus in labor analgesia. J Guangdong Med University. 2018;06:647–9.
Lange EM, Wong CA, Fitzgerald PC, et al. Effect of Epidural Infusion Bolus Delivery Rate on the Duration of Labor Analgesia: A Randomized Clinical Trial. Anesthesiology. 2018;128(4):745–53.
Acknowledgements
We gratefully thank the Yan’an People’s Hospital for their time and willingness to participate in the study. We are grateful to the women who participated in the study.
Funding
Scientific Research Project of Health Commission of Sichuan Province, No.19PJ156.
Author information
Authors and Affiliations
Contributions
Corresponding author: Shuzhi Zhou, Department of Anesthesiology of Ya’an People’s Hospital, E-mail:893,915,648@qq.com. Author Xin Ran(First Author): Conceptualization, Methodology, Investigation, Formal Analysis, Writing-Original Draft;Author Shuzhi Zhou(Corresponding Author): Conceptualization, Funding Acquisition, Resources, Supervision, Writing-Review & Editing;Author Kailan Cao: Visualization, Investigation;Data Curation, Writing-Original Draft;Author Peng He:Resources, Supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was accepted by the ethical review of the Ethics Committee of Ya’an Municipal Personnel Hospital,and ethical approval number: 202015. Participants provided written informed consent prior to being interviewed for the study.All methods were performed in accordance with the relevant guidelines and regulations.All methods were performed in accordance with the guidelines and regulations in the Declaration of Helsinki to promote ethical standards and respect for the participants that ensured their safety and protected their health and rights.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ran, X., Zhou, S., Cao, K. et al. Optimization of programmed intermittent epidural bolus volume for different concentrations of ropivacaine in labor analgesia: a biased coin up-and-down sequential allocation trial. BMC Pregnancy Childbirth 22, 590 (2022). https://doi.org/10.1186/s12884-022-04912-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-04912-8