Abstract
Background
Placenta previa accreta is a life-threatening pregnancy complication, and reducing blood loss during operative treatment remains a major challenge. The aim of our study was to investigate the effect of prophylactic abdominal aortic balloon occlusion (AABO) during caesarean section in women with placenta previa accreta.
Methods
A retrospective study of women with placenta previa accreta was conducted in a tertiary hospital from January 1, 2015, to December 31, 2020. Women were divided into balloon and control groups by whether AABO was performed. Baseline characteristics and pregnancy outcomes were compared in the two groups. A propensity score analysis was applied to minimise the indication bias. The primary outcome was composite, including estimated blood loss (EBL) ≥ 2.0 L, massive transfusion and hysterectomy.
Results
A total of 156 patients participated in this study, with 68 in the balloon group and 88 in the control group. Propensity score analysis showed that women in the balloon group had less EBL (1590.36 ± 1567.57 vs. 2830.36 ± 2285.58 mL, P = 0.02) as well as a lower proportion of EBL ≥ 1.0 L (50.00% vs. 78.57%, P = 0.03), EBL ≥ 2.0 L (21.43% vs. 50.00%, P = 0.03) and EBL ≥ 3.0 L (14.29% vs. 42.86%, P = 0.04). In addition, women in the control group received more red blood cell transfusions (8.43 U ± 9.96 vs. 3.43 U ± 6.27, P = 0.03), and the proportion of massive transfusions was higher (35.71% vs. 7.14%, P = 0.02). The proportions of disseminated intravascular coagulation (0% vs. 28.57%, P < 0.01), haemorrhagic shock (3.57% vs. 32.14%, P = 0.02) and hysterectomy (10.71% vs. 39.29%, P = 0.03) were significantly lower in the balloon group. Sutures were performed more often in the balloon group (64.29% vs. 17.86%, P < 0.01). Multivariate logistic regression analysis showed that AABO was associated with the primary outcome (adjusted odds ratio 0.46, 95% confidence interval 0.23 ~ 0.96, P = 0.04). No serious balloon catheter-related complications occurred in the balloon group.
Conclusion
AABO was an effective and safe approach to improve maternal outcomes for patients with placenta previa accreta.
Similar content being viewed by others
Introduction
The annual incidence of invasive placentation is estimated to be 1 in 300 pregnancies and has continuously increased over the last 50 years due to prior caesarean section, advanced maternal age and assisted reproductive technology [1]. Placenta accreta spectrum (PAS) is frequently associated with placenta previa [2], a life-threatening pregnancy complication. It may cause severe haemorrhaging, adjacent organ damage and infection, increasing maternal and neonatal mortality and morbidities [3]. Scheduled caesarean delivery near term is the management choice for women with placenta accreta in many clinical guidelines [4,5,6]. However, reducing blood loss and preserving fertility remain challenging during the operative treatment of placenta previa accreta.
Prophylactic abdominal aortic balloon occlusion (AABO) is a new technique to block blood perfusion in the uterus, reducing postpartum haemorrhage and the rate of hysterectomy [7,8,9]. However, the efficacy of AABO is uncertain because of the limited amount of literature and cases. Placement of balloon catheters may also cause complications such as thrombosis diseases, haematoma and rarely artery rupture [10,11,12]. Therefore, the objective of our study was to investigate whether AABO during caesarean section was effective and safe for women with placenta previa accreta.
Methods
This retrospective study was conducted using medical records of women with placenta previa accreta in a single tertiary hospital from January 1, 2015, to December 31, 2020. Placenta previa was diagnosed through routine prenatal ultrasound in the third trimester by experienced ultrasonographers. Ultrasonographic diagnosis of placenta accreta was based on previously reported criteria [13,14,15] when at least one of the following abnormal ultrasound findings were present: loss of retroplacental clear zone, myometrial thinning, multiple placental lacunae, subplacental hypervascularity, turbulent flow in lacunae and bladder wall interruption. Magnetic resonance imaging (MRI) was performed according to the managing obstetrician’s discretion. Characteristics of MRI considered suggestive of placenta accreta included the following [16,17,18]: uterine bulging, intraplacental dark bands on T2-weighted imaging, heterogeneous placental signal, bladder tenting, placental protrusion into the cervix, or more than one of these characteristics. Placenta accreta was confirmed during surgery when there was a lack of spontaneous complete separation of the placenta from its basal plate, requiring manual removal or after direct visualization of placental tissue protruding through the uterine serosa. The degree of placenta accreta was diagnosed by clinical criteria and histologic criteria according to the International Federation of Gynecology and Obstetrics (FIGO) classification from 2018 [19]. The inclusion criteria were women diagnosed with placenta previa and suspicious accreta by ultrasound or MRI before caesarean delivery who were confirmed to have accreta after caesarean delivery. The exclusion criteria were multiple pregnancies and women with a delivery week < 28 weeks.
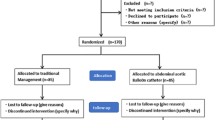
A total of 156 women participated in this study (Fig. 1). They were divided into a balloon group (n = 68) and a control group (n = 88) based on whether they received AABO. Prophylactic placement of balloon catheters was performed at the surgeon’s discretion. All women who received balloon catheters provided written informed consent. For all women in the control group, written informed consent was signed for caesarean delivery without prophylactic placement of balloon catheters. For women in the balloon group, the balloon catheter was performed before surgery, and the procedure was as follows. The catheter was inserted via the right femoral artery and placed at the abdominal aorta (above the division of the common iliac artery and under the renal ostia from the aorta) under fluoroscopic guidance with the balloon deflated. Then, the patient was transferred to the operating room for caesarean delivery. The balloon was inflated immediately after cord clamping. The balloon was inflated for no longer than 10 min and was then deflated for 1 min if the bleeding was active. If the bleeding became inactive, the balloon was kept deflated until the surgery was completed. After delivering the neonate, gentle controlled cord traction was performed in hopes of detaching the placenta if no placenta percreta was suspicious and no heavy bleeding happened. If removal of the placenta failed and the surgeons considered conservative management reasonable, surgical procedures including manual removal of the placenta, clamping and resecting the residual adherent tissue and the affected uterine wall, focal suturing on the placental detachment surface, and the uterine wall reconstruction were performed. Intraoperative haemostatic approaches such as uterine compression sutures, intrauterine gauze packing, intrauterine balloon tamponade and uterine artery ligation could be performed to control bleeding at the surgeon’s discretion. When all conservative therapies failed, the decision to perform a hysterectomy was made by at least two experienced surgeons. The catheter was removed after the surgery, and uterine artery embolisation was adopted if the patient was haemodynamically stable but exhibited persistent slow bleeding. For women in the control group, caesarean delivery was directly performed, and the perioperative management of the two groups was similar.
Maternal characteristics and pregnancy outcomes were collected. Maternal outcomes included operation time, estimated blood loss (EBL), autologous blood transfusion, homologous transfusion including packed red blood cells (PRBCs) transfusion, fresh frozen plasma (FFP) transfusion and other blood transfusions, intraoperative uterine compression suture, intrauterine gauze packing, intrauterine balloon tamponade, intraoperative bladder injury, hysterectomy, disseminated intravascular coagulation (DIC), haemorrhagic shock, uterine artery embolisation after surgery, relaparotomy, maternal length of antibiotic use, maternal length of hospital stay after surgery, complications of thromboembolism diseases, haematoma and artery rupture. The amount of intraoperative EBL was determined by adding blood volume in the surgical suction bottle (excluding amniotic fluid), that on gauze, and the visually estimated volume on the operating table. The primary outcomes included EBL ≥ 2.0 L, massive transfusion (transfusion of 10 or more units of packed red blood cells) and hysterectomy. Neonatal outcomes included gestational weeks at delivery, birthweight, Apgar score ≤ 7 at 1 min, Apgar score ≤ 7 at 5 min, neonatal intensive care unit (ICU) admission, assisted ventilation, pulmonary surfactant use, the complication of neonatal respiratory distress syndrome (NRDS), wet lung, neonatal asphyxia, neonatal infection, hyperbilirubinemia, antibiotic administration and neonatal length of hospital stay.
Data were analysed by SPSS statistical software version 22.0. Quantitative variables are presented as the mean ± standard deviation (SD), and qualitative variables are presented as frequencies and percentages. Continuous data were compared using Student’s t test or Wilcoxon rank-sum test for nonnormally distributed continuous variables. Categorical data were compared using the x2 test or Fisher’s exact test. A propensity score analysis with 1:1 matching was applied to minimise the indication bias of the two groups. Univariate analyses were adopted to screen characteristics with P < 0.2, and then a multivariable logistic regression model was adopted to acquire independent predictors for the composite primary outcome. P < 0.05 was considered statistically significant.
Results
A total of 156 eligible women with placenta previa accreta participated in this study, and 68 (43.59%) received prophylactic abdominal aortic balloon before caesarean delivery. The characteristics of the entire cohort and propensity score-matched cohort are shown in Table 1. In the entire cohort, the proportions of gravidity ≥ 4 (36.76% vs. 57.95%, P = 0.01) and prior uterine curettage ≥ 3 (13.24% vs. 26.14%) were significantly different between the two groups. After propensity score matching, 28 women were included in each group, and all characteristics were comparable between the balloon and control groups.
Maternal outcomes are shown in Table 2. In the propensity score-matched cohort, women in the balloon group had significantly less EBL (1590.36 ± 1567.57 vs. 2830.36 ± 2285.58 mL, P = 0.02) and a lower proportion of EBL ≥ 1.0 L (50.00% vs. 78.57%, P = 0.03), EBL ≥ 2.0 L (21.43% vs. 50.00%, P = 0.03) and EBL ≥ 3.0 L (14.29% vs. 42.86%, P = 0.04). The number of RBC transfusions in the control group was significantly higher (8.43 ± 9.96 vs. 3.43 ± 6.27, P = 0.03), and the proportion of massive transfusions was also higher (35.71% vs. 7.14, P = 0.02). The proportions of DIC (0% vs. 28.57%, P < 0.01), haemorrhagic shock (3.57% vs. 32.14%, P = 0.02) and hysterectomy (10.71% vs. 39.29%, P = 0.03) were significantly lower in the balloon group. Sutures were performed more often in women with balloon placement during caesarean Sect. (64.29% vs. 17.86%, P < 0.01). No serious balloon catheter-related complications, including thromboembolic disease, haematoma or artery rupture, occurred in women with balloon occlusion. Neonatal outcomes are shown in Table 3, and there were no significant differences between the two groups in the propensity score-matched cohort (P > 0.05).
Univariate analysis showed that the degree of PAS and AABO were potentially associated with the primary outcome (P < 0.02) (Table 4). Multivariate logistic regression analysis showed that AABO was associated with the primary outcome (adjusted odds ratio 0.46, 95% confidence interval 0.23 ~ 0.96, P = 0.04) (Table 4).
Discussion
In our study, propensity score analysis and multivariate logistic regression analysis were used to investigate over 150 cases of placenta previa and accreta. The results showed that prophylactic AABO effectively reduced blood loss and blood transfusion, decreasing the risk of DIC, haemorrhagic shock and hysterectomy; and did not influence neonatal outcomes. No related complications occurred in any of the patients. However, intraoperative uterine compression sutures were more commonly used in women in the balloon group. Multivariate logistic regression analysis also showed that AABO was associated with women’s primary outcomes.
Management by a multidisciplinary team for women with placenta previa accreta is imperative because it may cause massive bleeding and be life-threatening [20]. Prenatal diagnosis of PAS is usually accomplished by ultrasound whereas prenatal MRI is commonly performed to diagnosis and describe the depth and topography of placental invasion [2]. Then a pre-planned treatment of PAS could be given to improve maternal outcomes. In recent decades, some intravascular interventional therapies, including bilateral internal iliac artery balloon occlusion (IIABO) and AABO, have been used to reduce intraoperative bleeding and decrease the rate of hysterectomy. However, some studies found that the use of IIABO did not improve maternal outcomes in women with placenta previa accreta [21,22,23], and one study suggested that blocking blood flow in the internal iliac arteries was not enough because blood flow from the external iliac arteries was also observed in placenta previa accreta [24]. On the other hand, the efficacy of AABO is uncertain because of the limited amount of literature and cases. Chen et al. suggested that ABBO was effective in reducing postpartum haemorrhage and blood transfusion and decreasing the risk of hysterectomy, but intrauterine gauze packing was more commonly used in the balloon group [25]. Wang et al. found that ABBO was an effective way to control intraoperative blood loss and blood transfusion and decrease the risk of haemorrhagic shock. However, the rate of hysterectomy was not decreased [26]. Our study showed that AABO effectively reduced blood loss and blood transfusion and decreased the risk of DIC, haemorrhagic shock and hysterectomy, which was partially in accordance with previous studies. Our study also showed that intraoperative uterine compression sutures were more commonly used in women in the balloon group. Solely from a surgical viewpoint, the drier the surgical field is, the better it is. Profuse and brisk bleeding commonly occurs during manual removal of the placenta. It can be prevented by applying AABO, thus making it possible for the operator to complete the oversewing and curettage of the placenta implantation site with acceptable blood loss [27] and eventually decreasing the risk of DIC haemorrhagic shock and hysterectomy.
On the other hand, AABO is an invasive technique. It may cause complications, including initial vessel injury, arterial thrombosis, puncture point haematoma, ischaemic necrosis of the lower limbs, reperfusion injury of tissues and organs, acute renal failure and rarely artery rupture [10,11,12, 28]. It was reported that measurement of the abdominal aortic diameter before the intervention, clearance of the opening of the renal artery and control of the duration of balloon occlusion are essential for decreasing the risk of complications [29]. In our study, the diameter of the abdominal aorta was measured by MRI before surgery to determine the appropriate size of the balloon catheter. Occlusion was maintained for 10 min at a time with an interval of 1 min during surgery. Eventually, no complications occurred in any of the patients. In addition, for short-term neonatal outcomes, the results showed no difference between the two groups. Additionally, minimising foetal radiation exposure was also a favourable aspect for this method. In our study, balloon insertion was performed rapidly by an experienced radiologist. The foetal radiation exposure dose was less than 20 mGy, far less than the standard dose of ≤ 150 mGy recommended by the National Committee on Radiological Protection [30].
Our study had some strengths. First, the number of cases was much larger than that in previous studies (usually no more than 100) to perform propensity score analysis to minimise the indication bias for the decision to use AABO based on the surgeon’s discretion. In addition, the maternal and neonatal outcomes compared in our study were comprehensive. There were also some limitations of our study. This study was retrospectively designed in a single centre and lacked long-term follow-up. In the future, multicentre prospective investigations should be performed with more extended follow-up periods to provide accurate assessment and validation of the clinical efficacy of this method.
Conclusion
AABO was an effective and safe approach to improve maternal outcomes for patients with placenta previa accreta without increasing complications, and it does not cause harm to the newborn.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- PAS:
-
Placenta accreta spectrum
- AABO:
-
Abdominal aortic balloon occlusion
- EBL:
-
Estimated blood loss
- MRI:
-
Magnetic resonance imaging
- FIGO:
-
International Federation of Gynecology and Obstetrics
- PRBCs:
-
Packed red blood cells
- FFP:
-
Fresh frozen plasma
- DIC:
-
Disseminated intravascular coagulation
- NRDS:
-
Neonatal respiratory distress syndrome
- SD:
-
Standard deviation
- IIABO:
-
Iliac artery balloon occlusion
- BMI:
-
Body mass index
- ICU:
-
Intensive care unit
References
Jha P, et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol. 2020;30(5):2604–15. https://doi.org/10.1007/s00330-019-06617-7.
Tinari S, Buca D, Cali G, Timor-Tritsch I, Palacios-Jaraquemada J, Rizzo G, et al. Risk factors, histopathology and diagnostic accuracy in posterior placenta accreta spectrum disorders: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;57(6):903–9. https://doi.org/10.1002/uog.22183.
Li N, Tian Y, Liu C, Qiao C. Feasibility of infrarenal abdominal aorta balloon occlusion in pernicious placenta previa coexisting with placenta accrete. Biomed Res Int. 2018;2018:1–6. https://doi.org/10.1155/2018/4596189.
American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Placenta accreta spectrum. Obstetric care consensus no. 7. American college of obstetricians and gynecologists. Obstet Gynecol. 2018;132(6):e259-75. https://doi.org/10.1097/AOG.0000000000002983.
Jauniaux ERM, et al. Placenta praevia and placenta accreta: diagnosis and management. Green-top guideline no 27a. BJOG. 2018;2018:e1-48. https://doi.org/10.1111/1471-0528.15306.
Allen L, Jauniaux E, Hobson S, Papillon-Smith J, Belfort MA. FIGO consensus guidelines on placenta accreta spectrum disorders: nonconservative surgical management. Int J Gynaecol Obstet. 2018;140:281–90. https://doi.org/10.1002/ijgo.12409.
Chen L, Wang X, Wang H, Li Q, Shan N, Qi H. Clinical evaluation of prophylactic abdominal aortic balloon occlusion in patients with placenta accreta: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):1–8. https://doi.org/10.1186/s12884-019-2175-0.
He Q, Li Y, Zhu M, Peng X, Liu X. Prophylactic abdominal aortic balloon occlusion in patients with pernicious placenta previa during cesarean section: a systematic review and meta-analysis from randomized controlled trials. Arch Gynecol Obstet. 2019;300(5):1131–45. https://doi.org/10.1007/s00404-019-05297-4.
Qiu Z, Hu J, Wu J, Chen L. Prophylactic temporary abdominal aorta balloon occlusion in women with placenta previa accretism during late gestation. Medicine. 2017;96(46):e8681. https://doi.org/10.1097/MD.0000000000008681.
Jerasimos B, et al. Preoperative intravascular balloon catheters and surgical outcomes in pregnancies complicated by placenta accreta: a management paradox. Am J Obstet Gynecol. 2012;207(3):216.e1-216.e5. https://doi.org/10.1016/j.ajog.2012.06.007.
Gagnon J, Boucher L, Kaufman I, Brown R, Moore A. Iliac artery rupture related to balloon insertion for placenta accreta causing maternal hemorrhage and neonatal compromise. Can J Anaesth. 2013;60(12):1212–7. https://doi.org/10.1007/s12630-013-0038-0.
Peng Q, Zhang W. Rupture of multiple pseudoaneurysms as a rare complication of common iliac artery balloon occlusion in a patient with placenta accreta: a case report and review of literature. Medicine. 2018;97(12):e9896. https://doi.org/10.1097/MD.0000000000009896.
Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016;215(6):712–21. https://doi.org/10.1016/j.ajog.2016.07.044.
Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47(3):271–5. https://doi.org/10.1002/uog.14952.
Bhide A, Sebire N, Abuhamad A, Acharya G, Silver R. Morbidly adherent placenta: the need for standardization. Ultrasound Obstet Gynecol. 2017;49(5):559–63. https://doi.org/10.1002/uog.17417.
Ueno Y, Kitajima K, Kawakami F, Maeda T, Suenaga Y, Takahashi S, et al. Novel MRI finding for diagnosis of invasive placenta praevia: evaluation of findings for 65 patients using clinical and histopathological correlations. Eur Radiol. 2014;24(4):881–8. https://doi.org/10.1007/s00330-013-3076-7.
Valentini AL, Gui B, Ninivaggi V, Micco M, Giuliani M, Russo L, et al. The morbidly adherent placenta: when and what association of signs can improve MRI diagnosis? Our experience Diagn Interv Radiol. 2017;23(3):180–6. https://doi.org/10.5152/dir.2017.16275.
Kilcoyne A, Shenoy-Bhangle AS, Roberts DJ, Sisodia RC, Gervais DA, Lee SI. MRI of placenta accreta, placenta increta, and placenta percreta: pearls and pitfalls. AJR Am J Roentgenol. 2017;208(1):214–21. https://doi.org/10.2214/AJR.16.16281.
Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146(1):20–4. https://doi.org/10.1002/ijgo.12761.
Morlando M, Collins S. Placenta accreta spectrum disorders: challenges, risks, and management strategies. Int J Womens Health. 2020;12:1033–45. https://doi.org/10.2147/IJWH.S224191.
Chen M, Lv B, He G, Liu X. Internal iliac artery balloon occlusion during cesarean hysterectomy in women with placenta previa accrete. Int J Gynecol Obstet. 2018;145:110–5. https://doi.org/10.1002/ijgo.12763.
Chen M, et al. Internal iliac artery balloon occlusion for placenta Previa and suspected placenta accreta: a randomized controlled trial. Obstet Gynecol. 2020;135(5):1112–9. https://doi.org/10.1097/AOG.0000000000003792.
Schy A. et al. Perioperative prophylactic internal iliac artery balloon occlusion in the prevention of postpartum hemorrhage in placenta previa: a randomized controlled trial. Am J Obstet Gynecol. 2020;223(1). https://doi.org/10.1016/j.ajog.2020.01.024
Iwata A, Murayama Y, Itakura A, Baba K, Seki H, Takeda S. Limitations of internal iliac artery ligation for the reduction of intraoperative hemorrhage during cesarean hysterectomy in cases of placenta previa accrete. J Obstet Gynaecol Res. 2010;36(2):254–9. https://doi.org/10.1111/j.1447-0756.2009.01157.x.
Chen M, Lan X. Clinical evaluation of balloon occlusion of the lower abdominal aorta in patients with placenta previa and previous cesarean section: a retrospective study on 43 cases. Int J Surg. 2016;34:6–9. https://doi.org/10.1016/j.ijsu.2016.08.016.
Wang YL, Su FM, Zhang HY, Wang F, Zhe RL, Shen XY. Aortic balloon occlusion for controlling intraoperative hemorrhage in patients with placenta previa increta/percreta. J Matern Fetal Neonatal Med. 2017;2017:1–5. https://doi.org/10.1080/14767058.2016.1256990.
Panici PB, et al. Intraoperative aorta balloon occlusion: Fertility preservation in patients with placenta previa accreta/increta. J Matern Fetal Neonatal Med. 2012;25(12):2512–6. https://doi.org/10.3109/14767058.2012.712566.
Xie L, Wang Y, Luo FY, Man YC, Zhao XL. Prophylactic use of an infrarenal abdominal aorta balloon catheter in pregnancies complicated by placenta accreta. J Obstet Gynaecol. 2017;37(5):557–61. https://doi.org/10.1080/01443615.2017.1291588.
Peng Z, et al. Prophylactic abdominal aortic balloon occlusion: an effective method of controlling hemorrhage in patients with placenta previa or accreta. Exp Ther Med. 2019;17:1492–6. https://doi.org/10.3892/etm.2018.7066.
Valentin J. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37(2):1–332. https://doi.org/10.1016/j.icrp.2007.10.003.
Acknowledgements
Not applicable.
Funding
This study was funded by National Natural Science Foundation of China (grant number 81571460).
Author information
Authors and Affiliations
Contributions
Huifen Yin: Data collection, Data analysis, Manuscript writing. Rong Hu: Project development, Manuscript revising. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee in the Obstetrics and Gynecology Hospital of Fudan University. All methods were performed in accordance with the relevant guidelines and regulations and we confirm that informed consent was obtained from all subjects. All women who received balloon catheter provided written informed consent and for all women in control group, written informed consent of cesarean delivery without prophylactic placement of balloon catheter was signed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yin, H., Hu, R. Outcomes of prophylactic abdominal aortic balloon occlusion in patients with placenta previa accreta: a propensity score matching analysis. BMC Pregnancy Childbirth 22, 502 (2022). https://doi.org/10.1186/s12884-022-04837-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-04837-2