Abstract
Background
Cytokine levels have been extensively described in pregnant subjects under normal and pathological conditions, including mood-related disorders. Concerning chemokines, very few studies have reported their association with psychiatric disorders during pregnancy. Therefore, we explored the chemokine profile in women exhibiting anxiety and depression during late pregnancy in the present study.
Methods
One hundred twenty-six pregnant women in the 3rd trimester of pregnancy, displaying moderate to severe anxiety (ANX) alone and women exhibiting moderate to severe anxiety with comorbid depression (ANX + DEP), and 40 control pregnant women without affective disorders (CTRL) were evaluated through the Hamilton Anxiety Rating Scale (HARS) and the Hamilton Depression Rating Scale (HDRS). Serum chemokine levels of MCP-1 (CCL2), RANTES (CCL5), IP-10 (CXCL10), Eotaxin (CCL11), TARC (CCL17), MIP-1α (CCL3), MIP-1β (CCL4), MIG (CXCL9), MIP-3α (CCL20), ENA-78 (CXCL5), GROα (CXCL1), I-TAC (CXCL11) and IL-8 (CXCL8)] were measured by immunoassay. Clinical, biochemical, and sociodemographic parameters were correlated with HARS and HDRS score values.
Results
Serum levels of most chemokines were significantly higher in the ANX and in the ANX + DEP groups, when compared to the CTRL group. Positive correlations were observed between MIP-1α/CCL3, MIP-1β/CCL4, MCP-1/CCL2, MIP-3α/CCL20, RANTES/CCL5, Eotaxin/CCL11, and I-TAC/CXCL11 with high scores for anxiety (HARS) (p < 0.05) and for depression (HDRS) (p < 0.004). After controlling clinical measures for age + gwk + BMI, chemokines such as IL-8/CXCL8, MCP-1/CCL2 and MIP-1β/CCL4 were found associated with high scores for anxiety (p < 0.05) in the ANX group. TARC/CCL17 and Eotaxin/CCL11 showed significant associations with high scores for depression (p < 0.04) whereas, MCP-1/CCL2 and MIP-1α/CCL3 were significantly associated with high scores for anxiety (p < 0.05) in the ANX + DEP group. Using a multivariate linear model, high serum levels of MIP-1β/CCL4 and Eotaxin/CCL11 remained associated with depression (p < 0.01), while, IL-8/CXCL8, MIP-1β/CCL4, MCP-1/CCL2, and MIP-1α/CCL3 were associated with anxiety (p < 0.05) in the symptomatic groups.
Conclusions
Our data show that serum levels of distinct chemokines are increased in women exhibiting high levels of affective symptoms during late pregnancy. Our results suggest that increased levels of anxiety, depressive symptoms, and mood-related disorders may promote changes in specific functional chemokines associated with a chronic inflammatory process. If not controlled, it may lead to adverse obstetric and negative neonate outcomes, child development and neuropsychiatric alterations in the postnatal life.
Highlights
Chemokine levels increase in affective disorders during pregnancy.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a debilitating condition with a high prevalence and multisymptomatic nature, representing the third cause of disability worldwide [1, 2]. MDD has a complex and multifactorial etiology that arises from complex interactions among genetic, developmental, and environmental factors, reflecting the heterogeneity of the disorder [3]. Such heterogeneity is reflected in the estimates of MDD individuals that receive antidepressant treatment, showing that only a third of patients receive adequate treatment, and up to half of them relapse despite the increasing number of antidepressant drugs currently available [2, 3]. Psychosocial stress and systemic disease can both affect the onset of depression, as shown for the comorbidity of depression in patients with diabetes, cancer, or cardiac disease, which is 17–29%, a percentage range much higher than MDD itself in the general population (10.3%) [3].
Current evidence suggests that the prevalence of depression in women during pregnancy is higher than the prevalence observed in similarly aged non-pregnant women [4]. Different studies reported that the prevalence of perinatal depression ranges between 2 and 21% [5, 6], increasing to 31% upon the self-report scales used to screen the healthy pregnant population [6]. A systematic review reported that prevalence rates for depression during pregnancy were 7.4, 12.8, and 12.0% for the first, second, and third trimesters, respectively [5, 6], whereas the prevalence of prenatal anxiety was reported between 15.8 and 25% [7, 8] and from 13 to 31.7% in the postpartum period [9]. Worth note is that the co-morbidity of both affective symptoms was 9.5% during pregnancy and 7.6% in the postpartum [10], and the impact of affective symptoms during the perinatal period included obstetric and neonatal adverse outcomes, family dynamic consequences, child morbidity, and mortality, medical complications among other issues in the postnatal life [11].
Immune and endocrine systems have been reported to be altered in pregnant women with affective disorders [12, 13]. Pregnant women with severe anxiety displayed higher cortisol levels than women without anxiety; conversely, dehydroepiandrosterone-sulphate (DHEA-S) levels were found significantly lower in women with high levels of anxiety when compared to healthy controls [14]. In a similar vein, previous studies showed that women exhibiting anxiety and comorbid depression in the third trimester of pregnancy exhibited significant increases of Th1 and Th17-related cytokines and higher ratios of Th1: Th2 immune balance, which correlated with high scores for anxiety and depressive symptoms [15]. These data suggest that variations in cytokine concentrations are likely influenced by the intensity of depressive and anxiety symptoms. Such inflammatory process could start escalating in vulnerable subjects with high anxiety levels, becoming conspicuous as severe depression emerges in pregnant women [16].
Other crucial immune mediators are the chemotactic cytokines or chemokines. These immune mediators participate in controlling cell migration and cell positioning during development, homeostasis, and inflammation. The common function of chemokines is characterized by the direct movement of leukocytes, the increased immune activity, and leukocyte recruitment [17].
The chemokine family consists of ≅ 50 endogenous ligands that bind to distinct rhodopsin-like seven-transmembrane-spanning receptors identified in humans and mice [18]. Chemokines are small, 8- to 12 kDa protein ligands that promote increased motility and directional migration after binding their cell-surface receptors. Chemokines and their gradients are detected upon signaling pertussis toxin-sensitive Gi-type G protein-receptors on target cells [18, 19].
Based on their function, chemokines are classified as follows: a) Th1, CD8 and NK trafficking-related chemokines: IP-10/CXCL10, I-TAC/CXCL11, and MIG/CXCL9; b) Neutrophil trafficking-related chemokines: IL-8/ CXCL8, ENA-78/CXCL5, and GROα/CXCL1; c) Macrophage-NK migration/ lymphoid tissue T cell/DC interaction-related chemokines: RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4; d) Monocyte/macrophage trafficking-related chemokine: MCP-1/CCL2; e) Th17 response/B cell/DC homing to gut-associated lymphoid tissue: MIP-3α/CCL20; f) Th2 response/Th2 cell migration/Treg, lung and skin-homing: TARC/CCL17; and g) Eosinophil and basophil migration: Eotaxin/CCL11 [18].
Chemokines have been implicated in neurobiological processes relevant to psychiatric disorders, such as synaptic transmission and plasticity, neurogenesis, and neuron-glia communication [20,21,22]. Disruption of these functions by activating the inflammatory response has been implicated in the pathogenesis and development of MDD [23, 24]. Chemokines promote innate and adaptive immune systems interactions, thus shaping and providing the necessary context for developing optimal adaptive immune responses [17].
Animal studies showed that an impaired chemokine/receptor -CXCL12/CXCR4- signaling leads to abnormal development, proliferation, and migration of neural progenitor cells [25, 26] and implicated in the reduced neurogenesis in MDD [20, 21]. In the same line, chemokine receptor knockout mice, CCR6, and CCR7 displayed behavioral phenotypes related to psychiatric disorders [27]. Interestingly, clinical studies showed an increase in several chemokines such as CCL2/MCP-1 and CXCL8/IL-8 serum concentrations in MDD subjects [21, 28].
Clinical studies have demonstrated an increase in CCL5 and CCL2 serum levels in subjects displaying generalized anxiety disorder [29], chronic stress [30], and post-traumatic stress disorder (PTSD) [31]. In healthy hospital workers, anxiety scores were found inversely associated with levels of CCL2, CCL5, CCL11, and IL-6 [32]. Recent clinical studies in the perinatal period showed that the expression of several chemokines and their receptors appear to be altered in women exhibiting postpartum depression [33], showing an innate immune activation of pro-inflammatory mediators (IL-6, IL-15, CCL3, GM-CSF) in the peripartum in pregnant women exhibiting affective symptoms [34].
Recent studies showed that plasma LPS level was significantly increased in depressed mothers during their 8–12 weeks gestation and which were associated with higher plasma levels of the pro-inflammatory cytokine (TNF-α) and the chemokine MCP/CCL2) compared to healthy controls [35]. Thus, albeit of the several reports describing the functional activity of chemokines in early pregnancy or pregnant women with depression during the first weeks of gestation, no studies have shown a close relationship between affective disorders and functional chemokines during late pregnancy. Thereby, we described the association found between serum levels of functional chemokines in women exhibiting high levels of depression and anxiety symptoms during late pregnancy.
Methods
Design of the Study
We performed a cross-sectional study from 2014 to 2016, similar to the those reported for cytokine and cortisol studies (Leff-Gelman et al., 2020; Leff Gelman et al., 2019) and performed at the General Hospital of Mexico /OB-Gyn Department (HGM, Hospital General de Mexico, Dr. Eduardo Liceaga of Mexico, Mexico City) and the National Institute of Perinatology/OB-Gyn-Outpatient Control Unit (INPer, Mexico City). Approval from the Institution Ethical Committee was obtained before the beginning of the study (HGM, D1/14/112/04/072, 2014–2016). During the third trimester of gestation pregnant women attended at the OB-Gyn Control Outpatient Units from both institutions were asked to participate in the study. Patients who willingly accepted to participate in it, required a written informed consent before the study. At entry, all participants required third trimester lab tests (blood count, biochemical testing, urinalysis, thyroid function), 2D fetal ultrasound, and Doppler Monitoring (data not shown). All patients were either inhabitants of Mexico City or neighboring states.
Participants
Women between 18 and 30 years old, coursing a healthy pregnancy, during the third trimester (28–40 gwks) were invited to participate in the study. During the initial intervention at the OB-Gyn Control Outpatient Unit, a complete clinical and obstetric assessment was carried out, including sociodemographic parameters (marital status, education level, working status) and anthropometric measures (BMI, weight). Women were interviewed by the psychologist and asked to complete the self-reported questionnaire used to measure anxiety [Hamilton Anxiety Rating Scale (HARS)]. Patients exhibiting a score ≥ 25 in the HARS scale were considered with moderate to high intensity of anxiety symptoms, whereas patients with a cut-off score ≤ 5 were considered as healthy subjects and used as controls. In the same line, women were asked to complete the hetero-reported questionnaire used to measure depression [Hamilton Depression Rating Scale (HDRS)]. Patients exhibiting a score ≥ 24 in the HDRS scale, were considered with high levels of depressive symptoms, whereas patients with a cut-off score ≤ 7 were considered healthy controls. Women recruited into the study showed no smoking, alcohol consumption or drug abuse, including other associated mental (i.e., bipolar disorder, schizophrenia, psychosis, and neuropsychiatric pathologies (i.e., Alzheimer’s, seizures, attention deficit disorder, eating and uncontrolled compulsive disorders).
Exclusion criteria considered the following issues; patients receiving psychotropic medication, illicit substance use, patients having previous psychiatric diagnosis, obstetric pathologies (diabetes, hypertension, preeclampsia), infections, and medical illnesses (neurological, metabolic, cardiovascular, degenerative, endocrine, immune, and rheumatic disorders).
Moreover, participants were excluded from the study upon the demonstration of incomplete questionnaires, absence, abnormal findings in lab tests results and/or incomplete laboratory tests, and inconsistencies in the evaluation of the mood-related disorders.
Upon completing both anxiety and depression scale-related questionnaires, participants exhibiting moderate to high rating scores for anxiety with or without depression were referred to the psychiatric department for mood-disorder management and treatment. After the psychiatry interview and completion of the clinician-rated questionaries, patients were remitted back to the Ob-Gyn outpatient unit to proceed for blood sampling and quantification of serum chemokines.
Criteria and clinician-rated instruments
Criteria considered for recruitment of patients was the presence of high levels of anxiety following the 14 item-Hamilton Anxiety Rating Scale (HARS), which assesses the intensity of anxiety symptoms by a 5-point Likert scale ranging from 0 (no symptom) to 4 (severe anxiety), and higher total scores indicate severe anxiety [36]. Thus, HARS scores are categorized as follows: mild anxiety (score 7–17), moderate anxiety (score 18–24), severe anxiety (score > 25). Patients with a total score > 25 were considered as subjects with moderate to high levels of anxiety [36].
Depressive symptoms were evaluated using the 17-Item Hamilton Depression Rating Scale (HDRS) [37]. The Hamilton Rating Scale for Depression is a multidimensional and clinician-rated scale that has become the standard for clinical trials of depression. For the 17-item version of the Hamilton Rating Scale for Depression, scores can range from 0 to 54. It is generally accepted that scores between 0 and 6 indicating the absence of depression, scores between 7 and 17 indicate mild depression, scores between 18 and 24 indicate moderate depression and scores over 24 indicate severe depression [38, 39]. Therefore, patients with a cutoff > 24 were considered as subjects with moderate to high levels of depression.
Both HDRS and HARS instruments were applied to healthy women to confirm the absence of depressive and anxiety symptoms. Thus, patients showing a cut-off score < 5 in the anxiety-rating scale and a cut-off score < 7 in the depression-rating scale were considered the control group. All patients were evaluated by psychiatric interview following the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2015). Both instruments were validated in the local language [37, 40].
The anxiety scale used in the study has been extensively used to assess the intensity of mood- related items such as, subjective feelings, autonomic and somatic symptoms, cognitive functions, and behavioral responses. In the same line, the depression scale used herein has been widely used to assess the intensity of depressive mood, suicide thought or action, insomnia, irritability, fears, somatic symptoms, libido dysfunction, cognitive function, and insight [41, 42]. Both scales were shown to be reliable, specific, and sensitive clinician-rated instruments [43]. Rating scores obtained from each instrument were checked by the Psychiatric Department, and final evaluations were recorded in a clinical database. Patients exhibiting moderate to higher levels of anxiety and depression symptoms were referred to the psychiatry department for mood-disorder evaluation and treatment.
All participants were screened for depressive and anxiety symptoms, clinical variables, sociodemographic parameters, anthropometric measures.
Screening, recruitment and study groups
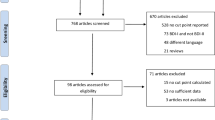
We screened a total of 298 participants at the Ob-Gyn outpatient units at the beginning of the study, and who showed mild to severe anxiety with or without mild to severe depression (n = 131), women with mild to intense levels of anxiety symptoms without depression (n = 113), and healthy pregnant women displaying no affective disorders (n = 54) in the 3rd trimester of pregnancy (see flow diagram, Fig. 1).
Screening and recruitment process of participants into the study. The figure depicts the screening and recruitment process of participants with affective symptoms and healthy pregnant women during the third trimester of pregnancy at the beginning of the study. Both inclusion and exclusion criteria were used to define the study groups formed at the end of the study. (* **) Some participants were excluded due to incomplete or abnormal lab tests, inconsistencies in questionaries, absence to medical/lab appointments, resign or quit from the study (see methods)
Clinical measures, sociodemographic parameters, depressive and anxiety symptoms and other comorbid psychiatry disorders were assessed during patients’ interview. Participants not fulfilling with the items depicted in the inclusion criteria were formaly excuded fom the study (see inclusion and exclusion criteria above). Thus, after the initial screening and elimination process of participants, a total of 166 patients were finally recruited into the study and clustered into three different groups; a) pregnant women exhibiting moderate to severe anxiety without depression (ANX, n = 72); b) pregnant women displaying moderate to severe anxiety and comorbid (moderate to severe) depression (ANX+ DEP, n = 54); and c) healthy pregnant women without affective disorders, used as controls (CTRL, n = 40).
Blood sampling and quantification of serum chemokines
Blood sampling was performed at the main clinical lab facility under aseptic conditions from 7:00–9:00 am, following standardized laboratory procedures for blood extraction and collection. Samples from 176 pregnant women with 8–12 h fasting conditions were collected and processed as described below.
Heparinized tubes (BD, USA) containing blood samples were handled on ice for 30 min and then centrifuged at 1000–2000 x g for 10 min in a Refrigerated Eppendorf Centrifuge 5702R (Eppendorf, USA). Serum fractions were separated and aliquoted into 1.5 mL cryogenic vials and stored at − 70 °C until further use, as previously described [15]. Cryogenic vials were numbered in sequence following the patient’s clinical file and date of sample processing.
Serum chemokines [MCP-1 (CCL2), RANTES (CCL5), IP-10 (CXCL10), Eotaxin (CCL11), TARC (CCL17), MIP-1α (CCL3), MIP-1β (CCL4), MIG (CXCL9), MIP-3α (CCL20), ENA-78 (CXCL5), GROα (CXCL1), I-TAC (CXCL11) and IL-8 (CXCL8)] were quantified by an immunoassay based on multiplex bead array using the LEGENDplex™ Human Proinflammatory Chemokine Panel (Cat. 740,003, BioLegend, USA) following manufacturer’s instructions. Briefly, serum samples (50 μL) were thawed and incubated with specific chemokine-antibody coated beads in a mix buffer at room temperature for 1.5 h in the presence of specific fluorochrome per chemokine. After several washes, samples were analyzed in a FACS Aria III flow cytometer (BD, USA). The chemokine levels were calculated using the LEGENDplexTM Data Analysis Software v 7.0 (Biolegend, CA, USA). The lower limits of detection of each chemokine assayed (manufacturer ‘s data) were: IP-10/CXCL10 (1.4 pg/mL), I-TAC/CXCL11 (7.8 pg/mL), MIG/CXCL9 (9.4 pg/mL), IL-8/CXCL8 (1.4 pg/mL), ENA-78/CXCL5 (1.1 pg/mL), GROα/CXCL1 (3.2 pg/mL), RANTES/CCL5 (4.3 pg/mL), MIP-1α/CCL3 (2.1 pg/mL), MIP-1β/CCL4 (2.3 pg/mL), MCP-1/CCL2 (0.9 pg/mL), MIP-3α/CCL20 (2.5 pg/mL), TARC/CCL17 (0.8 pg/mL) and Eotaxin/CCL11 (0.8 pg/mL). Intra-assay coefficient was < 3.0% and inter-assay covariance was < 5.0%.
Data obtained from flow cytometer analyses by lab observers were uploaded into a lab database. All data collected from patients were handled by personal involved in the protocol following a double-blind study. The number of cryogenic vials, serum chemokine concentration/patient, vacutainer-test tube and patient’s file number, were only known by the present study’s leading researchers.
Statistical analysis
Serum levels of chemokines are shown as mean ± SEM. Clinical variables are depicted in percentage values (%). The parametric t-test with Welch’s correction (two-tailed p-value) was used to compare the means of chemokine serum levels among the study groups. Similarly, Pearson bivariate correlations were performed to assess the associations between biological variables, sociodemographic measures, and questionnaires-related scores among the study groups. Moreover, Pearson correlations were used to detect the associations between serum chemokines and clinical measures after controlling all variables for age, gwk and BMI in the study groups. In addition, a general linear model was used to detect the remaining associations between functional chemokines and affective symptoms in the tested groups. Two-way ANOVA was used to determine significant differences in clinical and biological measures among groups. In detecting significant differences between the parameters assayed in the study, a post hoc Tukey test was performed to establish the differences observed between serum chemokines and clinical variables among the tested groups. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Softwares Inc. USA) and SPSS software v.27.0 (Armonk, NY: IBM Corp). For all the statistical analyses, the p-value ≤ 0.05 was considered significant.
Results
Demographic characteristics
Table 1 shows the sociodemographic characteristics of non-white Latin pregnant women (n = 166) recruited in the study in the 3rd trimester of pregnancy, presenting a mean gwk of 35.1 ± 1.3, and a mean age of 25.7 ± 2.1 years-old participating in the study. Control subjects were older than those corresponding to the anxiety (ANX) and the depressive (ANX+ DEP) groups, respectively. As shown, significant differences were found in the intensity of anxiety symptoms (HARS scores) among the studied groups (t-test, p = 0.000), and between age and gwk (t-test, p < 0.005) among the depressive and the control groups, respectively.
Serum chemokine levels
Regarding the Th1/CD8/NK trafficking-related chemokines, the serum levels of IP-10/CXCL10, I-TAC/CXCL11, and MIG/CXCL9 in the ANX and the ANX + DEP groups were significantly higher than those in the CTRL group. Besides, the serum levels of IP-10/CXCL10 and MIG/CXCL9 in the ANX + DEP groups were significantly higher than those in the ANX group (Fig. 2A). IP-10/CXCL10 and MIG levels were higher compared with those of I-TAC/CXCL11. The serum levels of the Neutrophil trafficking-related chemokine, IL-8/CXCL8, were higher in the ANX and the ANX + DEP groups than those in the CTRL group. Interestingly, ENA-78/CXCL5 and GRO-α/CXCL1 levels were higher in the ANX group compared with the other groups (Fig. 2B).
Concentrations of Th1, CD8, NK trafficking, and Neutrophil trafficking-related chemokines in the ANX, ANX + DEP and CTRL groups. The figures depict the values of the estimated serum concentrations of Th1, CD8, and NK trafficking-related chemokines, IP-10/CXCL10, I-TAC/CXCL11, and MIG/CXCL9 (A), and the Neutrophil trafficking-related chemokines, IL-8/CXCL8, ENA-78/CXCL5, and GROα/CXCL1 (B) in the CTRL, ANX, and ANX + DEP groups. These immune mediators (Th1, CD8, and NK trafficking- and Neutrophil trafficking-associated chemokines) were quantified by flow cytometry (see methods). Values are expressed as the mean ± SEM. The parametric, t-test analysis with Welch’s correction was used to estimate the p values for each functional chemokine assayed in the study among the tested groups. (*) p < 0.05; (**); p < 0.001; (***) p < 0.0001. ANX + DEP, high anxiety plus comorbid depression; ANX, high anxiety; CTRL, control. Data were calculated using GraphPad v.7
The serum levels of the Macrophage-NK migration/lymphoid tissue T cell/DC interaction-related chemokines, MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5 were significantly higher in the ANX and in the ANX + DEP groups when compared to their levels in the CTRL group. The highest levels of these chemokines were observed in the ANX + DEP group (Fig. 3A-B).
Concentrations of the Macrophage-NK migration, lymphoid tissue T cell/DC interaction-related chemokines in the ANX, ANX + DEP, and CTRL groups. The figures depict the values of the serum estimated concentrations of Macrophage-NK migration/ lymphoid tissue T cell/DC interaction-related chemokines, MIP-1α/CCL3, MIP-1β/CCL4 (A) and RANTES/CCL5, (B) in the CTRL, ANX, and ANX + DEP groups. These immune mediators (Macrophage-NK migration, lymphoid tissue T cell, DC interaction-associated chemokines) were quantified by flow cytometry (see methods). The concentration values are expressed as the mean ± SEM. The parametric, t-test analysis with Welch’s correction was used to estimate the p values for each functional chemokine assayed in the study among the tested groups. (*) p < 0.05; (**); p < 0.001; (***) p < 0.0001. ANX + DEP, high anxiety plus comorbid depression; ANX, high anxiety; CTRL, control. Data were calculated using GraphPad v.7
Concerning the monocyte trafficking chemokine, MCP-1/CCL2, its levels were higher in the ANX and the ANX + DEP groups than in the CTRL group. The highest levels were found in the ANX group. Concerning the Th17 response, B cell, and DC homing to gut-associated lymphoid tissue-related chemokine, the serum levels of MIP-3α/CCL20 were higher in the ANX + DEP group with regard to the other groups (Fig. 4A-B).
Concentrations of Monocyte trafficking- and Th17 response, B cell, DC homing to gut-associated lymphoid tissue-related chemokines in the ANX, ANX + DEP and CTRL groups. The figures depict the values of the serum estimated concentrations of Monocyte trafficking-related chemokine, MCP-1/CCL2 (A) and the Th17 response, B cell, DC homing to gut-associated lymphoid tissue-related chemokine, MIP-3α/CCL20 (B) in the CTRL, ANX, and ANX + DEP groups. These immune mediators (Monocyte trafficking- and Th17 response, B cell, DC homing to gut associated lymphoid tissue- chemokines) were quantified by flow cytometry. The concentration values are expressed as the mean ± SEM. The parametric, t-test analysis with Welch’s correction was used to estimate the p values for each functional chemokine assayed in the study among the tested groups. (*) p < 0.05; (**); p < 0.001; (***) p < 0.0001. ANX + DEP, high anxiety plus comorbid depression; ANX, high anxiety; CTRL, control. Data were calculated using GraphPad v.7
Serum levels of the Th2 response, Th2 cell migration, Treg, lung, and skin homing-related chemokine, TARC/CCL17, and the eosinophil and basophil migration-related chemokine, Eotaxin/CCL11, were higher in the ANX and the ANX + DEP groups than in the CTRL group, and the highest levels of TARC/CCL17 and Eotaxin/CCL11 were observed in the ANX and the ANX + DEP groups, respectively (Fig. 5A-B).
Concentrations of Th2 response, Th2 cell migration, Treg, lung and skin homing- and the Eosinophil and basophil migration-related chemokines in the ANX, ANX + DEP, and CTRL groups. The figures depict the values of the serum estimated concentrations of the Th2 response, Th2 cell migration and Treg, lung and skin homing-related chemokine, TARC/CCL17 (A) and the Eosinophil and basophil migration-related chemokine, Eotaxin/CCL11 (B) in the CTRL, ANX, and ANX + DEP groups. These immune mediators (Th2 response, Th2 cell migration and Treg, lung and skin homing- and Eosinophil and basophil migration-related chemokines) were quantified by flow cytometry. The concentration values are expressed as the mean ± SEM. The parametric, t-test analysis with Welch’s correction was used to estimate the p values for each functional chemokine assayed in the study among the tested groups. (*) p < 0.05; (**); p < 0.001; (***) p < 0.0001. ANX + DEP, high anxiety plus comorbid depression; ANX, high anxiety; CTRL, control. Data were calculated using GraphPad v.7
Bivariate correlations between serum chemokines and clinical variables
Table 2 depicts the correlations between serum chemokine levels and clinical variables among the studied groups. As shown, all groups of patients recruited into the study displayed positive correlations with several chemokines, HARS and HDRS scores, and clinical variables.
As shown, only MIP-1α/CCL3, and MIP-1β/CCL4, MIP-3α/CCL20, MCP-1/CCL2, RANTES/CCL5, Eotaxin/CCL11 displayed positive correlations with HARS scores (Pearson, p < 0.04) and with clinical variables used in our study (Pearson; age, p < 0.01; gwk, p < 0.03; BMI, p < 0.05) in the ANX group. Moreover, other functional chemokines (I-TAC/CXCL11, IL-8/CXCL8, ENA-78/CXCL5, GRO-α/CXCL1) also showed related correlations with clinical measures (Pearson, p < 0.05).
In the same vein, MIP-1α/CCL3, MIP-1β/CCL4, MIP-3α/CCL20, and Eotaxin/CCL11 showed positive correlations with high levels of anxiety symptoms (HARS scores) (Pearson, p < 0.01) in the ANX + DEP group; whereas I-TAC/CXCL11, RANTES/CCL5, and MCP-1/CCL2 CCL11 displayed significant correlations with high HDRS scores (Pearson, p < 0.004), in addition of correlating with different clinimetric variables.
Other functional chemokines (IL-8/CXCL8, ENA-78/CXCL5, and Gro-α/CXCL1) (Pearson, p < 0.05) showed similar related associations with clinical variables in the ANX + DEP and CTRL groups (Pearson, p < 0.01), respectively.
Significant differences were found between MIP-1α/CCL3, MIP-1β/CCL4, and MIP-3α/CCL20 and HARS scores in both of the symptomatic groups (Tukey test, p < 0.05); whereas MIP-3α/CCL20 showed significant differences with HARS scores (Tukey test, p < 0.03) in the depressive group (data not shown).
Partial correlations between serum chemokines and clinical variables
As depicted in Table 3, after adjusting clinical data for age, BMI and gwk, altogether in the ANX group, functional chemokines such asIL-8/CXCL8 (Pearson, p = 0.01), MCP-1/CCL2 (Pearson, p = 0.03), and MIP-1β/CCL4 (Pearson, p = 0.05) showed positive correlations with high levels for anxiety symptoms (HARS scores), whereas in the ANX + DEP group, MCP-1/CCL2 (Pearson, p = 0.01), TARC/CCL17 (Pearson, p = 0.02), Eotaxin/CCL11 (Pearson, p = 0.01), and MIP-1α/CCL3 (Pearson, p = 0.003) were found displaying positive correlations with high scores for depression symptoms (HDRS scores) (Pearson, p < 0.04) .
General lineal model
As shown in Table 4, using a multivariate linear model, serum levels of chemokines, such as IL-8/CXCL8 (F = 12.1, p = 0.003), MCP-1/CCL2 (F = 4.7, p = 0.04), MIP-1β/CCL4 (F = 5.7, p = 0.03) and MIP-1α/CCL3 (F = 4.4 p = 0.05) were found associated with high scores for anxiety symptoms (HARS) in the ANX group; whereas high serum levels of IL-8/CXCL8 (F = 4.5, p = 0.05) and MIP-1α/CCL3 (F = 4.7, p = 0.05) remained associated with high scores for anxiety symptoms in the ANX + DEP group, in addition of MIP-1β/CCL4 (F = 17.6, p = 0.004) and Eotaxin/CCL11 (F = 7.9, p = 0.01), which were associated with high scores for depressive symptoms (HDRS).
Discussion
The present study shows that pregnant subjects exhibiting either higher levels of anxiety symptoms and/or comorbid anxiety and depression symptoms, showed higher concentrations of distinct serum chemokines than healthy pregnant women.
Recent clinical studies have focused on the role of functional chemokines in neuropsychiatric diseases [44], including their effects after antidepressant treatment [45]. Chemokines studied in affective disorders [21, 46, 47]; include the monocyte chemoattractant protein (MCP)-1/CCL2 [48,49,50]; the macrophage inflammatory protein (MIP)-1α/CCL3 [51,52,53]; Gro-α/CXCL1 [22, 54, 55]; The IL-8/CXCL8 [48, 56, 57]; TNF-β [58]; IL-16 [58]; CTACK [56]; macrophages migration inhibitory factor (MIF) [56, 59]; Eotaxin/CCL11 [60, 61]; CXCL11/ITAC [62]; MEC/CCL28 [63];TECK/CCL25 [63]; interferon gamma-induced protein (IP)-10/CXCL10 [64, 65]; RANTES/CCL5 [21, 66, 67].
Chemokines play crucial roles during the early stages of pregnancy, not only in the recruitment and functional regulation of decidual immune cells but also in the blastocyst/embryo implantation into the uterus and trophoblast invasion [68]. Several studies showed that chemokine/chemokine receptor interactions control leukocytes and decidual immune cells’ trafficking, recruitment, and maintenance [69]. Our results showed that a large number of chemokines (i.e., IP-10/CXCL10, I-TAC/CXCL11, MIG/CXCL9, RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4, MIP-3α/CCL20, including the IL-8/CXCL8 and Eotaxin/CCL11-related chemokines) were found significantly increased in patients exhibiting high levels of anxiety and depressive symptoms.
Similarly, chemokines such as ENA-78/CCL5, GROα/CXCL1, MCP-1/CCL2, and TARC/CCL17 were increased in women displaying high anxiety levels. Although, at present, it is uncertain how these chemokines are enrolled in affective disorders during pregnancy, recent reports highlight some of the negative effects produced by inflammatory chemokines during pregnancy, as shown for the high levels of inflammatory chemokines (i.e., IL-8, MCP-1, and MIP-1α) measured in the cord blood, and reported to enhance intrauterine inflammation, premature birth, and neonatal complications in perinatal women [70]. Such findings were also associated with other neonatal complications, such as patent ductus arteriosus, respiratory distress syndrome, and chronic lung disease [70].
Interestingly, recent studies showed that mothers of children with autism spectrum disorders (ASD) with intellectual disabilities (DQ < 70) (ASD + ID) had significantly elevated mid-gestational levels of cytokines (GM-CSF, IFN-γ, IL-1α, IL-6) and chemokines (IL-8, MCP-1) when compared to either mother of children with ASD without intellectual disabilities (ASD-noID, DQ ≥70) and mothers of the general population (GP) controls. Conversely, mothers of children with either ASD-noID or developmental delay (DD) had significantly lower levels of the chemokines IL-8 and MCP-1 than mothers of GP controls. Findings that suggested differences in the psychiatric disorders might be related to the plausible expression of early immune biomarkers specific to sub-phenotypes of ASD [71].
Different studies showed that the increased serum concentrations of MCP-1/CCL2, IL-8/CXCL8 in MDD subjects [21] correlated with the onset and progression of MDD (21, 24, 29). In the same line, related studies showed an increase in ENA-78/CCL5 and MCP-1/CCL2 serum levels in subjects displaying generalized anxiety disorder [29], chronic stress [30], and post-traumatic stress disorder [31]. Interestingly, our results are in line with the aforementioned data, showing that chemokines such as MCP-1/CCL2, TARC/CCL17, ENA-78/CXCL5, and GRO-α/CXCL1 were significantly increased in pregnant women exhibiting ANX.
MCP-1/CCL2 appears to be dysregulated in stress-inducing anxiety-like behaviors in humans and rodents [71,72,73]. Animal studies showed that Balb/C mice exposed to chronic ethanol consumption leads to an increase in chemokine levels (MCP-1, MIP-1α, CX3CL1) in the striatum and serum (MCP-1, MIP-1α, CX3CL1) [72]. Alcohol deprivation for 24 h induced IFN-γ levels in the striatum and maintained high levels of some cytokines (IL-1β, IL-17) and chemokines (MIP-1α, CX3CL1) in this brain region [72]. These neuroinflammatory events were associated with an increase in anxiogenic-related behavioral responses [72,73,74]. Interestingly, mice lacking TLR4 or TLR2 receptors protect against ethanol-induced cytokine and chemokine release and associated behavioral effects during alcohol abstinence [72].
Most chemokines assayed in our study, particularly MCP-1/CCL2, MIP-1α/CCL3 and MIP-1β/CCL4, appear to have essential implications in either triggering or modulating mood-related disorders, as demonstrated for functional chemokines such as, MCP-1/CCL2, MIP-1α/CCL3, and IP-10/CXCL10 [75,76,77]. These chemokines have been implicated in the modifications of neurotransmission and alterations in cognitive function [75,76,77], while CXCL8 and CXCL10 were found associated with alterations in neuroendocrine regulation and hypothalamic–pituitary–adrenal axis function [21, 33, 65]. The increased chemokine levels shown herein may reflect the activation of the inflammatory response system in women exhibiting severe anxiety and depression during pregnancy [13, 15, 21].
It is well known that Th1/Th2 and Th17/Treg immune balances are needed to maintain a successful pregnancy [12] and thereby of the conceptus [78]. It has been shown that pregnant patients with MDD display a preferential Th1-inflammatory response [59]. Recent studies from our group showed that serum levels of Th1, Th2 and Th17-related cytokines in pregnant subjects displaying affective disorders correlated with HARS and HDRS scores [15], and in a similar fashion shown herein, chemokines such as, MIP-1α/CCL3, MIP-1β/CCL4, MCP-1/CCL2, TARC/CCL17, Eotaxin/CCL11, and IL-8/CXCL8-related chemokines showed positive correlations with either HARS or HDRS scores in our pregnant population exhibiting ANX and/or ANX + DEP, respectively. These data posit that chemotactic cytokine appear to impinge at different levels on both neurons and glia cells [24], which ultimately leads to the deregulation of distinct neurotransmission systems shown to be implicated in mood-related disorders) [51, 79], in cognitive functions [21, 22] and in the functional activity of the HPA axis [76, 77].
Moreover, most serum chemokines assayed in the present study were conspicuously increased in women exhibiting mixed anxiety and depression compared with pregnant women only displaying anxiety. These observations support that these disorders during pregnancy represent a highly complex pathological condition associated with several biological responses, such as the increase in cortisol levels [14], glucocorticoid resistance, and inflammatory response [51].
Previous studies showed that BMI significantly correlated with pro-inflammatory cytokines in depressed patients with obesity [52] and pregnant women exhibiting severe anxiety and comorbid depression [15]. However, our results showed that only MCP-1/CCL2 and RANTES/CCL5 were found associated with BMI in pregnant women with ANX, but not in women in women exhibiting ANX + DEP (Table 4). This suggests that such chemokines should be related with inflammatory conditions in patients exhibiting high stress and anxiety symptomology, as previously reported for the increased serum concentrations of Th1/Th17- related cytokines in women with affective disorders [15].
Our results support other clinical studies, that showed that maternal IL-6 and IL-8 correlated with BMI and maternal adiposity in pregnant non-diabetic women during the third trimester of pregnancy [53]. A similar correlation was also observed with high levels of serum and follicular fluid MCP-1 concentrations in obese women, when compared to either overweight and normal-weight women [58], or with MCP-1, and high-molecular-weight (HMW) adiponectin and FFA (free fatty acid) /albumin in women displaying severe pregnancy-induced hypertension [80].
Thus, it appears that chemokines might be linked to women with pathological conditions, in the pregestational period throughout pregnancy, or in the postpartum. For instance, recent studies showed that the tumor necrosis factor ligand superfamily member (TRANCE), the hepatocyte growth factor, IL-18, the Fibroblast Growth Factor (FGF)-23, and CXCL1 were found significantly elevated in women with postpartum depressive symptoms; suggesting that these women show a compromised adaptability of the immune system [81].
Our data suggest that body mass measures (weight < 70 kg, BMI < 30 kg/m2) represent important variables to consider when evaluating depressive symptoms in women from mid to late pregnancy [82], particularly in women exhibiting increased stress responsiveness with high levels of anxiety [62].
Interestingly, different studies revealed that a decrease in chemokine levels (MCP-1, MIP-1α) during the first half of pregnancy is related to the physiological reduction in immune activity and leukocyte migration [83]. Increased levels of serum MIP-1α and decreased levels of MCP-1 in the first trimester were found associated with the development of preeclampsia, premature labor, and neonate-low birth weight [83].
Moreover, our results showed that serum levels of chemokines such as IL-8/CXCL8, MIP-1β/CCL4, MCP-1/CCL2, and Eotaxin/CCL11, remained elevated in both symptomatic groups, after controlling clinical measures by specific confounders; suggesting that such functional chemokines should enhance or interact with several other immune (i.e., Th1-related cytokines) and/or non-immune mediators (i.e., monoamines, prostaglandins, neurosteroids) in limbic structures [5, 12], regulating the intensity of anxiety and depressive symptoms, as demonstrated for Th1/Th17 cytokines in pregnant women with high levels of anxiety and severe depression [15].
Thus, the role of chemokines and their cognate receptors need to be explored deeply in psychiatric disorders in both pregnant and non-pregnant subjects, encompassing their signaling systems on the activated immune (NK, MΦ) cells.
Conclusions and perspectives
Our data show that serum levels of most chemokines are increased in women exhibiting intense affective symptoms during late pregnancy. Our results posit that ANX, affective symptoms, and mood-related disorders may promote changes in immune mediators and their receptors leading to chronic inflammatory processes over time. If not controlled, it may enhance adverse obstetric and negative neonate outcomes, changes in child development and behaviors, and neuropsychiatric disorders.
Limitations
Several limitations found in the present study included the cross-sectional design of the project and not a longitudinal one from early pregnancy to postpartum to detect changes in the chemokine profile at each trimester and in the early postpartum in our recruited population.
Data were collected in women displaying moderate to severe affective symptoms, and the study excluded participants with a history of smoking and alcohol consumption. This group could have shed important information and data in the present study.
Also, the study required the measurements of chemokine concentrations in pregnant women exhibiting moderate to severe depression without anxiety. Moreover, pregestational women displaying affective symptoms could extend the knowledge about the relevance of these disorders before pregnancy and their relation with the serum levels of chemokines, as depicted herein.
Availability of data and materials
All datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- gwk:
-
Gestation weeks
- HARS:
-
Hamilton Anxiety Rating Scale
- HDRS:
-
Hamilton Depression Rating Scale
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders
- CTRL:
-
Control
References
GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6.
Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;35:722–9. https://doi.org/10.1016/j.pnpbp.2010.04.011.
Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006;11:12–21. https://doi.org/10.1017/s1092852900015212.
Accortt EE, Cheadle ACD, Schetter CD. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015;19:1306–37. https://doi.org/10.1007/s10995-014-1637-2.
Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. https://doi.org/10.1097/01.AOG.0000116689.75396.5f.
Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005;119:1–8. https://doi.org/10.1037/e439372005-001.
Fairbrother N, Janssen P, Antony MM, Tucker E, Young AH. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148–55. https://doi.org/10.1016/j.jad.2015.12.082.
Kang Y-T, Yao Y, Dou J, Guo X, Li S-Y, Zhao C-N, et al. Prevalence and risk factors of maternal anxiety in late pregnancy in China. Int J Environ Res Public Health. 2016;13(5):468. https://doi.org/10.3390/ijerph13050468.
Britton JR. Maternal anxiety: course and antecedents during the early postpartum period. Depress Anxiety. 2008;25:793–800. https://doi.org/10.1002/da.20325.
Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–23. https://doi.org/10.1192/bjp.bp.116.187179.
Chinchilla-Ochoa D, Peón PB-C, Farfán-Labonne BE, Garza-Morales S, Leff-Gelman P, Flores-Ramos M. Depressive symptoms in pregnant women with high trait and state anxiety during pregnancy and postpartum. Int J Womens Health. 2019;11:257–65. https://doi.org/10.2147/IJWH.S194021.
Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, Garcia-Cuetara Mdel P, et al. The immune system and the role of inflammation in perinatal depression. Neurosci Bull. 2016;32(4):398–420. https://doi.org/10.1007/s12264-016-0048-3.
Simpson W, Steiner M, Coote M, Frey BN. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Rev Bras Psiquiatr. 2016;38(3):190–6. https://doi.org/10.1590/1516-4446-2015-1899.
Leff-Gelman P, Flores-Ramos M, Carrasco AEÁ, Martínez ML, Takashima MFS, Coronel FMC, et al. Cortisol and DHEA-S levels in pregnant women with severe anxiety. BMC Psychiatry. 2020;20(1):393. https://doi.org/10.1186/s12888-020-02788-6.
Gelman PL, Mancilla-Herrera I, Flores-Ramos M, Takashima MFS, Coronel FMC, Fuentes CC, et al. The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatry. 2019;19(1):104. https://doi.org/10.1186/s12888-019-2087-6.
Stuart-Parrigon K, Stuart S. Perinatal depression: an update and overview. Curr Psychiatry Rep. 2014;16:468. https://doi.org/10.1007/s11920-014-0468-6.
Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7(5):a016303. https://doi.org/10.1101/cshperspect.a016303.
Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16. https://doi.org/10.1016/j.immuni.2012.05.008.
Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1(2):95–104.
de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL. Hippocampal adult neurogenesis: Does the immune system matter? J Neurol Sci. 2017;372:482–95. https://doi.org/10.1016/j.jns.2016.10.052.
Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH. The role of chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci. 2019;20(9):2283. https://doi.org/10.3390/ijms20092283.
Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93–115. https://doi.org/10.1016/j.neubiorev.2014.02.001.
Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26(6):797–810. https://doi.org/10.1038/sj.jcbfm.9600229.
Biber K, Vinet J, Boddeke HW. Neuron-microglia signaling: chemokines as versatile messengers. J Neuroimmunol. 2008;198(1–2):69–74. https://doi.org/10.1016/j.jneuroim.2008.04.012.
Pujol F, Kitabgi P, Boudin H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci. 2005;118(Pt 5):1071–80. https://doi.org/10.1242/jcs.01694.
Peng H, Wu Y, Duan Z, Ciborowski P, Zheng JC. Proteolytic processing of SDF-1 alpha by matrix metalloproteinase-2 impairs CXCR4 signaling and reduces neural progenitor cell migration. Protein Cell. 2012;3(11):875–82. https://doi.org/10.1007/s13238-012-2092-8.
Jaehne EJ, Baune BT. Effects of chemokine receptor signaling on cognition-like, emotion-like and sociability behaviours of CCR6 and CCR7 knockout mice. Behav Brain Res. 2014;261:31–9. https://doi.org/10.1016/j.bbr.2013.12.006.
Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, et al. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;68:1–8. https://doi.org/10.1016/j.pnpbp.2016.02.006.
Ogłodek EA, Szota AM, Just MJ, Moś DM, Araszkiewicz A. The MCP-1, CCL-5 and SDF-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disorders. Pharmacol Rep. 2015;67(1):85–9. https://doi.org/10.1016/j.pharep.2014.08.006.
Castellani ML, De Lutiis MA, Toniato E, Conti F, Felaco P, Fulcheri M, et al. Impact of RANTES, MCP-1 and IL-8 in mast cells. J Biol Regul Homeost Agents. 2010;24(1):1–6.
Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–55. https://doi.org/10.1002/da.20564.
Polacchini A, Girardi D, Falco A, Zanotta N, Comar M, De Carlo NA, et al. Distinct CCL2, CCL5, CCL11, CCL27, IL-17, IL-6, BDNF serum profiles correlate to different job-stress outcomes. Neurobiol Stress. 2018;8:82–91. https://doi.org/10.1016/j.ynstr.2018.02.002.
Petralia MC, Mazzon E, Fagone P, Falzone L, Bramanti P, Nicoletti F, et al. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp Ther Med. 2019;18(1):2055–62. https://doi.org/10.3892/etm.2019.7774.
Osborne LM, Yenokyan G, Fei K, Kraus T, Moran T, Monk C, et al. Innate immune activation and depressive and anxious symptoms across the peripartum: An exploratory study. Psychoneuroendocrinology. 2019;99:80–6. https://doi.org/10.1016/j.psyneuen.2018.08.038.
Zhou Z, Guille C, Ogunrinde E, Liu R, Luo Z, Powell A, et al. Increased systemic microbial translocation is associated with depression during early pregnancy. J Psychiatr Res. 2018;97:54–7. https://doi.org/10.1016/j.jpsychires.2017.11.009.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5.
Ramos-Brieva JA, Cordero-Villafafila A. A new validation of the Hamilton Rating Scale for Depression. J Psychiatr Res. 1988;22(1):21–8.
Cusin C, Yang H, Yeung A, Fava M. Rating scales for depression. In: Baer L, Blais MA, editors. Handbook of clinical rating scales and assessment in psychiatry and mental health. Boston: Humana Press; 2010. p. 7–35. e-ISBN 978-1-59745-387-5. https://doi.org/10.1007/978-1-59745-387-5.
Zimmerman M, Thompson JS, Diehl JM, Balling C, Kiefer R. Is the DSM-5 Anxious Distress Specifier Interview a valid measure of anxiety in patients with generalized anxiety disorder: A comparison to the Hamilton Anxiety Scale. Psychiatry Res. 2020;286:112859. https://doi.org/10.1016/j.psychres.2020.112859.
Lobo A, Chamorro L, Luque A, Dal-Ré R, Badia X, Baró E, et al. Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales. Med Clin (Barc). 2002;118(13):493–936. https://doi.org/10.1016/s0025-7753(02)72429-9.
Maier W, Buller R, Philipp M, Heuser I. The Hamilton anxiety scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–8. https://doi.org/10.1016/0165-0327(88)90072-9.
Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163–77. https://doi.org/10.1176/appi.ajp.161.12.2163.
Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1. https://doi.org/10.1186/1471-244X-14-S1-S1.
Simon SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. 2018;23(1):48–58. https://doi.org/10.1038/mp.2017.205.
Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55(5):4195–206. https://doi.org/10.1007/s12035-017-0632-1.
Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J Neuroinflammation. 2014;11:132. https://doi.org/10.1186/1742-2094-11-132.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res. 2009;29(6):313–26. https://doi.org/10.1089/jir.2008.0027.
Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi-analyte profiling of case–control collections. PLoS One. 2010;5(2):e9166. https://doi.org/10.1371/journal.pone.0009166.
Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, et al. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012;34:71–5. https://doi.org/10.1590/s1516-44462012000100013.
Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36(10):1882–6. https://doi.org/10.1016/j.biocel.2003.10.019.
Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;45:54–63. https://doi.org/10.1016/j.pnpbp.2013.04.009.
Snegovskikh V, Park JS, Norwitz ER. Endocrinology of parturition. Endocrinol Metab Clin N Am. 2006;35(1):173–91 viii. https://doi.org/10.1016/j.ecl.2005.09.012.
Farah N, Hogan AE, O'Connor N, Kennelly MM, O'Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60(1):96–9. https://doi.org/10.1016/j.cyto.2012.05.024.
Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Lee SH, et al. Simultaneous measurement of 23 plasma cytokines in late-life depression. Neurol Sci. 2009;30(5):435–8. https://doi.org/10.1007/s10072-009-0091-1.
Shang Y, Tian L, Chen T, Liu X, Zhang J, Liu D, et al. CXCL1 promotes the proliferation of neural stem cells by stimulating the generation of reactive oxygen species in APP/PS1 mice. Biochem Biophys Res Commun. 2019;515(1):201–6. https://doi.org/10.1016/j.bbrc.2019.05.130.
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. https://doi.org/10.1016/j.jaci.2016.06.033.
Moore BB, Kunkel SL. Attracting attention: discovery of IL-8/CXCL8 and the birth of the chemokine field. J Immunol. 2019;202(1):3–4. https://doi.org/10.4049/jimmunol.1801485.
Buyuk E, Asemota OA, Merhi Z, Charron MJ, Berger DS, Zapantis A, et al. Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: a pilot study. Fertil Steril. 2017;107(3):632–640.e3. https://doi.org/10.1016/j.fertnstert.2016.12.023.
Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–10. https://doi.org/10.1111/j.1600-0897.2010.00852.x.
Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, et al. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48:13–5. https://doi.org/10.1016/j.jpsychires.2013.10.007.
Teixeira AL, Gama CS, Rocha NP, Teixeira MM. Revisiting the role of Eotaxin-1/CCL11 in psychiatric disorders. Front Psychiatry. 2018;9:241. https://doi.org/10.3389/fpsyt.2018.00241.
Karlsson L, Nousiainen N, Scheinin NM, Maksimow M, Salmi M, Lehto SM, et al. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy—the FinnBrain birth cohort study. Arch Womens Ment Health. 2017;20(1):39–48. https://doi.org/10.1007/s00737-016-0672-y.
Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry. 2013;54(7):953–61. https://doi.org/10.1016/j.comppsych.2013.03.026.
Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–204. https://doi.org/10.4049/jimmunol.168.7.3195.
Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18:230–3. https://doi.org/10.1016/j.euroneuro.2007.06.004.
Suffee N, Richard B, Hlawaty H, Oudar O, Charnaux N, Sutton A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem Soc Trans. 2011;39(6):1649–53. https://doi.org/10.1042/BST20110651.
Blank T, Detje Claudia N, Spieß A, Hagemeyer N, Brendecke Stefanie M, Wolfart J, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44:901–12. https://doi.org/10.1016/j.immuni.2016.04.005.
Imakawa K, Imai M, Sakai A, Suzuki M, Nagaoka K, Sakai S, et al. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol Reprod Dev. 2006;73(7):850–8. https://doi.org/10.1002/mrd.20496.
Du M-R, Wang S-C, Li D-J. The integrative roles of chemokines at the maternal–fetal interface in early pregnancy. Cell Mol Immunol. 2014;11(5):438–48. https://doi.org/10.1038/cmi.2014.68.
Otsubo Y, Hashimoto K, Kanbe T, Sumi M, Moriuchi H. Association of cord blood chemokines and other biomarkers with neonatal complications following intrauterine inflammation. PLoS One. 2017;12(5):e0175082. https://doi.org/10.1371/journal.pone.0175082.
Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2017;22(2):273–9. https://doi.org/10.1038/mp.2016.77.
Harper KM, Knapp DJ, Park MA, Breese GR. Age-related differences in anxiety-like behavior and amygdala CCL2 responsiveness to stress following alcohol withdrawal in male Wistar rats. Psychopharmacology. 2017;234(1):79–88. https://doi.org/10.1007/s00213-016-4439-y.
Chen HJ, Antonson AM, Rajasekera TA, Patterson JM, Bailey MT, Gur TL. Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl Psychiatry. 2020;10(1):19. https://doi.org/10.1038/s41398-020-00876-5.
Pascual M, Montesinos J, Marcos M, Torres J-L, Costa-Alba P, García-García F, et al. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol. 2017;22(6):1829–41. https://doi.org/10.1111/adb.12461.
Dalgard C, Eidelman O, Jozwik C, Olsen CH, Srivastava M, Biswas R, et al. The MCP-4/MCP-1 ratio in plasma is a candidate circadian biomarker for chronic post-traumatic stress disorder. Transl Psychiatry. 2017;7(2):e1025. https://doi.org/10.1038/tp.2016.285.
Merendino RA, Di Pasquale G, De Luca F, Di Pasquale L, Ferlazzo E, Martino G, et al. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate–severe depression. Mediat Inflamm. 2004;13:205–7. https://doi.org/10.1080/09511920410001713484.
Marciniak E, Faivre E, Dutar P, Pires CA, Demeyer D, Caillierez R, et al. The Chemokine MIP-1α/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. https://doi.org/10.1038/srep15862.
Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85(1):71–5. https://doi.org/10.1016/j.jri.2010.01.006.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90. https://doi.org/10.1016/j.neuron.2019.03.013.
Naruse K, Noguchi T, Sado T, Tsunemi T, Shigetomi H, Kanayama S, et al. Chemokine and Free Fatty Acid Levels in Insulin-Resistant State of Successful Pregnancy: A Preliminary Observation. Mediat Inflamm. 2012;2012:432575. https://doi.org/10.1155/2012/432575.
Bränn E, Fransson E, White RA, Papadopoulos FC, Edvinsson Å, Kamali-Moghaddam M, et al. Inflammatory markers in women with postpartum depressive symptoms. J Neurosci Res. 2020;98(7):1309–21. https://doi.org/10.1002/jnr.24312.
Mina TH, Denison FC, Forbes S, Stirrat LI, Norman JE, Reynolds RM. Associations of mood symptoms with ante- and postnatal weight change in obese pregnancy are not mediated by cortisol. Psychol Med. 2015;45(15):3133–46. https://doi.org/10.1017/S0033291715001087.
Stokkeland LMT, Giskeødegård GF, Stridsklev S, Ryan L, Steinkjer B, Tangerås LH, et al. Serum cytokine patterns in first half of pregnancy. Cytokine. 2019;119:188–96. https://doi.org/10.1016/j.cyto.2019.03.013.
Acknowledgments
This study was supported by the following grants: FOSSIS/CONACyT Project No. 272458, and INPer Project No. 212250-3000-10916-01-16.
Methods and procedures
All the methods and procedures described in the present study, were performed according to the relevant guidelines and regulations following the declaration of Helsinki.
Author statement
The corresponding author declares that the present study has not been published before in any other journal.
Funding
This study was supported by the following grants: FOSSIS/CONACyT Project No. 272458 and INPer Project No. 212250–3000–10916-01-16.
Author information
Authors and Affiliations
Contributions
PLG and MFR designed and conducted the study. PLG and ICA wrote the body of the manuscript. MFR was major a contributor in reviewing the data and body of the manuscript, and amending several areas of the manuscript. FMCC conducted the clinical intervention in pregnant women to assesses the recruitment of patients into the study. IMH determined the serum levels of chemokines. ICA contributed to the financial support of the study. BFL and PLG contributed to the statistical analysis of data. MFR and GPD conducted the psychiatric intervention and recruitment of participants into the study. GPD and MPMR scored the psychometric instruments applied to the participants. PLG and BFL performed the blood sampling. PLG and JHR performed the lab-processing of fresh blood samples to obtain serum aliquots. PLG and FMCC collected the biochemical and clinical data from participants recruited into the study. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We received the approval of the clinical study from the head of the Ethical and Research Committee of General Hospital of Mexico (Hospital General de Mexico, Dr. Eduardo Liceaga, CD MX) with the project No. DI/14/1 12/04/072. The written Informed Consent was also obtained from all participants recruited in the present study.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing financial interests that could influence the publishing of the final version of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Camacho-Arroyo, I., Flores-Ramos, M., Mancilla-Herrera, I. et al. Chemokine profile in women with moderate to severe anxiety and depression during pregnancy. BMC Pregnancy Childbirth 21, 807 (2021). https://doi.org/10.1186/s12884-021-04225-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-021-04225-2