Abstract
Background
Community-based exercise is a continuation and complement to inpatient rehabilitation for Parkinson's disease and does not require a professional physical therapist or equipment. The effects, parameters, and forms of each exercise are diverse, and the effect is affected by many factors. A meta-analysis was conducted to determine the effect and the best parameters for improving motor symptoms and to explore the possible factors affecting the effect of community-based exercise.
Methods
We conducted a comprehensive search of six databases: PEDro, PubMed/Medline, CENTRAL, Scopus, Embase, and WOS. Studies that compared community-based exercise with usual care were included. The intervention mainly included dance, Chinese martial arts, Nordic walking, and home-based exercise. The primary outcome measure was the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) score. The mean difference (95% CI) was used to calculate the treatment outcomes of continuous outcome variables, and the I2 statistic was used to estimate the heterogeneity of the statistical analysis. We conducted subgroup analysis and meta-regression analysis to determine the optimal parameters and the most important influencing factors of the exercise effect.
Results
Twenty-two studies that enrolled a total of 809 subjects were included in the analysis. Exercise had a positive effect on the UPDRS-III (MD = -5.83; 95% CI, -8.29 to -3.37), Timed Up and Go test (MD = -2.22; 95% CI -3.02 to -1.42), UPDRS ((MD = -7.80; 95% CI -10.98 to -6.42), 6-Minute Walk Test (MD = 68.81; 95% CI, 32.14 to 105.48), and Berg Balance Scale (MD = 4.52; 95% CI, 2.72 to 5.78) scores. However, the heterogeneity of each included study was obvious. Weekly frequency, age, and duration of treatment were all factors that potentially influenced the effect.
Conclusions
This meta-analysis suggests that community-based exercise may benefit motor function in patients with PD. The most commonly used modalities of exercise were tango and tai chi, and the most common prescription was 60 min twice a week. Future studies should consider the influence of age, duration of treatment, and weekly frequency on the effect of exercise.
PROSPERO trial registration number
CRD42022327162.
Similar content being viewed by others
Background
Parkinson's disease (PD) is a progressive neurodegenerative disorder with both motor and nonmotor symptoms, defined by the accumulation of misfolded alpha-synuclein and the loss of dopaminergic neurons [1,2,3,4,5]. The number of individuals with PD increased rapidly from approximately 6.1 million in 2016 and has grown by 2.5 over the past three decades [6, 7]. By 2040, with aging and industrialization, the number of patients with PD is expected to reach a staggering 12.9 to 14.2 million [8]. Comprehensive management of PD involves pharmacology, surgical therapies, nursing, and rehabilitation [9,10,11]. The current treatment for PD is mainly drug therapy based on levodopa, but there are certain side effects, such as movement fluctuations, nausea, and psychosis [12, 13]. Physical therapy is the key to improving motor functions such as gait and balance that cannot be improved by drugs, so doctors should recommend it to all patients [14,15,16,17]. Rehabilitation can begin at the time of initial diagnosis and continue throughout the disease course [10, 17]. At present, rehabilitation is mainly performed with equipment or professional therapists, which improves the condition of patients but also increases the economic burden of patients. There is no evidence that PD patients are more likely to contract COVID-19 or have increased mortality [18, 19]. However, motor and nonmotor symptoms worsen in PD [19,20,21,22]. This may be because many patients and others are unable to access physical therapists or visit rehabilitation centers due to the lockdown [23]. How to continue therapy after the therapist's instruction is one of our concerns.
At present, rehabilitation research is still mainly focused on new equipment and professional techniques, ignoring economic and practical aspects. A systematic analysis of the short-term efficacy of physical therapy for PD has shown that although there are various physical therapy techniques, there is little difference in efficacy [24, 25]. Community-based exercise is a type of exercise that does not require a professional physical therapist, expensive equipment, or a particular location and is suitable for long-term recovery from illness, with easy access and low cost. Exercise is one of the most effective forms of physical therapy. Studies have also found that exercise reduces the risk of falls among older adults [26]. Animal studies have also found significant benefits in the posttraining PD model. Exercise induces the regulation of different brain regions, reduces cell death pathways, enhances neurotrophic factors and mitochondrial function, increases neurogenesis, and improves synaptic plasticity [27, 28]. People with PD engage in fewer activities of daily living and exercise than their normal peers. They need exercise to continue to consolidate the benefits. Many studies have reported the benefits of exercise at home for people with PD [29, 30]. The effects of exercise can last from 3 to 12 months [31]. In 2014, 19 physiotherapists and Parkinson's patients collaborated to develop the European Physiotherapy Guideline for PD, which described in detail the forms of exercise [32]. The main exercises included dancing, Chinese martial arts, Nordic walking, and home-based exercises. However, different studies have shown inconsistent results. The parameters vary from study to study. Currently, there has been no meta-analysis on community-based exercise, no discussion on the optimal parameters of exercise for PD, and no investigation into the factors that affect these exercises. We summarized the effects of community-based exercise on Parkinson’s motor symptoms without the help of a physical therapist or device and analyzed the appropriate parameters to provide a reference for Parkinson’s patients. This study also included a subgroup analysis and meta-regression to try to determine the main factors. This meta-analysis was conducted following the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement [33] and was registered on the PROSPERO platform under the number CRD42022327162.
Methods
Search strategies
We performed a systematic search of the literature including studies published from inception to April 2022 in the following databases: PubMed/Medline, PEDro, CENTRAL, Scopus, Embase, and WOS. A combination of subject words and free words was adopted. The following Medical Subject Heading (MeSH) terms were included: “Parkinson’s Disease,” “exercise,” “sports,” “rehabilitation,” and “Physical Therapy Modalities.” The search clauses are listed in Supplementary Appendix S1. The language was limited to English. Additionally, we manually searched the reference list, and Google Scholar was searched to include more articles that met the criteria.
Inclusion and exclusion criteria
The criteria included randomized controlled trials (RCTs) or crossover trials that compared exercise interventions with usual care for PD, regardless of disease stage and severity. The inclusion criteria are shown in Table 1.
The exclusion criteria were as follows: 1) Exercise guided by a physiotherapist; 2) exercise therapy with equipment; 3) the control group received exercise-related therapy, including stretching or walking; and 4) the mean and standard deviation were not reported.
Outcome measures and data extraction
Studies that assessed the effectiveness of the intervention on motor symptoms were included. The primary outcome was the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) score, which was revised to test motor function in individuals with PD in 2008 [34]. The secondary outcomes were as follows: 1) Timed Up and Go test (TUG) for a distance of 3 m; 2) the Unified Parkinson’s Disease Rating Scale (UPDRS), which assesses patients' severity, with higher scores indicating a greater severity; 3) the Berg Balance Scale (BBS); and 4) the 6-Minute Walk Test (6MWT) [32].
Endnote X20 was used to manage the literature. Data were independently extracted from eligible studies by two authors (YCL and HJP). Extracted data were compared, and any discrepancies were resolved through discussion with the third author (TYC). Relevant data, such as study time, sample size, duration of follow-up, duration of exercise, type of exercise, Hoehn and Yahr stage, and country where the studies were conducted, were extracted from all included papers. Data were collected using standard spreadsheets (Excel). If any information was unclear, we contacted the author to provide more detailed data.
Methodological quality and data synthesis
Meta-analysis was performed using the software Review Manager (v.5.4, Nordic Cochrane Center, Copenhagen, Denmark) and Stata 12.0 (StataCorp LLC, College Station, TX, USA). Two reviewers (YCL and HJP) independently assessed the methodological quality. If the two reviewers had different opinions, we called all the participants for discussion, and finally, Professor QY made the decision. The quality of the methods was assessed using the Cochrane Collaboration risk-of-bias method quality assessment tools [35]. The variables we selected were all continuous. We extracted the means and standard deviations of the postintervention results into a predesigned Excel table. The mean difference (MD) was used as the effective value. All studies pooled effects, together with corresponding 95% CIs, to present the results of the meta-analysis. If I2 was < 50%, we used the fixed effects model; otherwise, we used the random effects model. We also performed subgroup analysis for the time of each treatment, frequency of treatment per week, total time of exercise per week, and duration of follow-up to analyze the optimal parameters for exercise to improve Parkinson’s motor symptoms. Finally, we also performed a meta-regression analysis to identify the factors that affected the efficacy. We selected the average age, exercise modality (dance, Chinese martial arts, others), disease stage, number of exercises per week, time of each exercise, total exercise time in the week, length of the intervention, and region of the participants (Asia, Oceania and North America) as covariates.
Results
Study characteristics
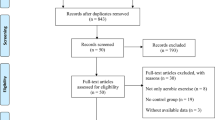
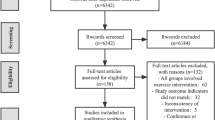
The study selection process is shown in Fig. 1. A total of 151 trials that potentially met the requirements were found by reading titles and abstracts; 130 trials were excluded through intensive reading and comparison of inclusion criteria. Studies were excluded for various reasons, mainly due to the involvement of physiotherapists, incomplete data, or the use of other equipment and techniques. One study [36]reported the presence of a physiotherapist, but given that the study was outdoors and the physiotherapist was a qualified Nordic walking teacher, we included the study as well. A total of 22 full-text studies were included in the analysis [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. The research information of all included studies is shown in Table 2. There were a variety of forms of physical exercise, including dance (N = 5), tango (N = 3), tai chi (N = 5), QiGong (N = 4), yoga (N = 2), Nordic walking (N = 2) and home-based exercise (N = 1). The studies were conducted in Asia (N = 9), North America (N = 9), Oceania (N = 1), and Europe (N = 4). Exercise interventions ranged from 4 weeks to 12 months, with 12 weeks being the dominant treatment length. The frequency of training was 1–5 times per week, but 2–3 times was the most common. Each session lasted 50, 60, or even 90 min.
Methodological quality
The quality assessment of methods using the Cochrane Collaboration risk of bias tool showed that the methodological quality of the included trials varied widely. This was unlikely to be possible with subject blinding, and exercise outside the intervention could not be controlled. Twenty-five studies (92.59%) performed better with assignment blinding, but only 18 studies (66.67%) reported the generation of random sequences. Evaluator blinding was not reported in 9 trials (33.33%), and patients could not be blinded due to the nature of the exercise intervention. Most trials reported data loss and subjects dropping out. The risk of publication bias and other biases was low. The overall risks included in the studies and the risk assessment for each study are shown in Figs. 2 and 3.
Effects of exercise
Outcome of the UPDRS-III
The results of the UPDRS-III were reported in 18 of the 27 included studies. Figure 4a shows that the pooled effect estimates showed that exercise had a positive effect on UPDRS-III scores (MD = -5.83; 95% CI, -8.29 to -3.37; P < 0.00001). However, high heterogeneity was observed in the analysis (P < 0.00001; I2 = 93%). We did not find that eliminating one or more studies significantly changed overall heterogeneity. Two studies showed no improvement in scores, one [57] in which the exercise group scored worse than the control group before intervention and one [38] in which the scores were not statistically significant. However, there was no good reason to exclude these two studies from the analysis. From the funnel plot (Fig. 5a), eight items fell outside the 95% confidence interval. We attempted to further analyze this in a subgroup analysis.
Outcome of the TUG
The results of the TUG were reported in 17 of the 27 included studies. The TUG is a widely used test to assess a patient's ability to transfer. Figure 4b shows that the combined effect estimate suggested that exercise improved the TUG score (MD = -2.22; 95% CI -3.02 to -1.42; P < 0.00001). Figure 4b shows that high heterogeneity was observed in the analysis (P < 0.00001; I2 = 84%). According to the funnel plot (Fig. 5b), many studies fell outside the 95% CI, and no study changed the funnel plot. We attempted to further analyze this in a subgroup analysis.
Outcome of the UPDRS
The UPDRS can reflect the degree of PD. However, the results of the UPDRS were reported in only 6 of the 27 included studies. Figure 4c shows that the combined effect estimates indicated that exercise was beneficial in terms of the UPDRS score (MD = -7.80; 95% CI -10.98 to -6.42; P = 0.02). Figure 4c shows that high heterogeneity was observed in the analysis (P = 0.02; I2 = 60%). From the funnel plot (Fig. 5c), there was some bias among all the studies.
Outcome of the BBS
The results of the BBS were reported in 10 of the 27 included studies. Several other scales were used to assess balance function. Figure 4d shows that the pooled effect estimates indicated the beneficial effects of exercise on balance. There was a beneficial effect on the BBS scores (MD = 4.52; 95% CI, 2.72 to 5.78; P = 0.002). Figure 4d shows that high heterogeneity was observed in the analysis (P = 0.002; I2 = 66%). From the funnel plot (Fig. 5d), there was some bias among all the studies.
Outcome of the 6MWT
The results of the 6MWT were reported in 6 of the 27 included studies. Figure 4e shows that the estimated pooling effect indicated improvement in the 6MWT scores (MD = 68.81; 95% CI, 32.14 to 105.48; P < 0.0001). Figure 4e shows that severe heterogeneity was observed in the analysis (P < 0.0001, I2 = 83%). From the funnel plot (Fig. 5e), there was some bias among all the studies.
Optimal parameters of exercise
We performed a subgroup analysis and detailed discussion of outcomes involving more than 10 studies. The results of the subgroup analysis of the effects of different exercise intensities on exercise are shown in Table 3.
Training frequency
Subgroup analysis of the frequency of weekly exercise showed a significant difference in the effect of exercise on UPDRS-III scores (P = 0.09, I2 = 57.6%) and TUG scores (P < 0.00001, I2 = 92.4%). An exercise frequency of two and three times a week had significant effects with MDs of -6.65 (95% CI: -10.19 to -3.11) and -3.21 (95% CI: -5.98 to -0.44) on UPDRS-III scores, respectively. An exercise frequency of two and three times a week had significant effects on TUG scores, with MDs of -0.93 (95% CI: -2.53 to 0.66) and -5.70 (95% CI: -6.33 to -4.77), respectively. Twice a week was the most common and effective frequency of exercise in terms of UPDRS-III scores. There was heterogeneity among subgroups in terms of TUG scores, while heterogeneity was significantly reduced when we removed the four times a week subgroup (P = 0.46, I2 = 0%).
Duration of each session
Training duration included 50 min, 60 min, and 90 min. We divided these durations into three subgroups. In the comparison between subgroups, we found no significant difference between subgroups in the effect of the duration of each session on UPDRS-III scores (p = 0.6; I2 = 0%) for all tests between subgroups. However, a 60-min exercise session was the most common choice. A duration of 60 min of exercise per session had a significant effect (MD = -5.82; 95% CI, -8.75, -2.89). There were no differences among the subgroups in terms of TUG scores (P = 0.89; I2 = 0). A duration of 60 min of exercise per session had a significant effect (MD = -1.96; 95% CI, -2.79, -1.13) on TUG scores. The frequency of exercise per week affects motor symptoms and may also be related to the area of evaluation.
Duration of exercise per week
Because of the different training times, the total training time in a week was grouped. The weekly exercise duration included in this study was divided into 60 min, 120 min, 150 min, 180 min, and > 180 min subgroups. The difference between subgroups was not significant for UPDRS-III scores (P = 0.24, I2 = 29.1%) or TUG scores (P = 0.36, I2 = 6.3%). Between 120 and 180 min of total exercise time per week was the most selected parameter. We also analyzed the relationship between the total time in one week and efficacy in the subsequent meta-regression analysis.
Exercise types
For the primary outcome measure UPDRS-III scores, tango, and tai chi were the main forms of exercise. We selected studies with 2–3-month interventions and divided them into tango and tai chi subgroups, including 4 and 6 studies, respectively. Figure 6 shows that there was no significant difference in the subgroup analysis (P = 0.39, I2 = 0%).
Meta-regression analysis
Meta-regression showed that the average age of participants affected the UPDRS-III scores (β = -0.423; 95% CI, -0.742, -0.106; p = 0.012). We also found that the duration of treatment affected the performance of the UPDRS (β = -0.121; 95% CI, -1.987, 0.043; p = 0.0005). When multivariate regression analysis was performed, the heterogeneity of age and treatment duration was reduced (Table 4).
Discussion
We systematically and comprehensively studied the effect of exercise on motor function and optimal exercise prescriptions among patients with PD. We found that many community-based exercises were an economical and convenient form of physical therapy and a continuation for PD patients after hospital treatment to maintain effects. The key findings of this study were as follows: 1) community-based exercise improved motor symptoms; (2) the most common exercise parameters were 60 min each session, twice a week, and tango and tai chi were the most common movement modalities; 3) the frequency of weekly exercise, age, and the duration of treatment of participants were important factors that affected the results; and 4) no significant heterogeneity was found in subgroup analysis or meta-regression analysis for tango, tai chi, or other exercise methods.
Heterogeneity was so ubiquitous that even if we performed a subgroup analysis or tried to exclude a single study, the heterogeneity could not be significantly changed. The higher heterogeneity is consistent with the results of other exercise interventions [58]. This suggests that the improvement of motor symptoms in PD by exercise is affected by multiple factors. The clinical manifestations of PD vary widely, even in motor symptoms [59]. PD is not just a motor disease; there are many nonmotor symptoms, such as salivary gland issues, visual disturbances, constipation, anxiety, and depression [4, 59]. These nonmotor symptoms can also lead to a reduced willingness to exercise among people with PD. People with PD often fall, making them sedentary and reluctant to exercise [60]. Complications other than Parkinson's motor disease may affect treatment outcomes. The incidence is higher among men than among women at every age [61], but women have a higher mortality rate and progress faster [62, 63]. The optimal parameters for exercise are different for men and women. Sex is also one of the factors that affects exercise among people with PD. The proportion of women is also a factor in the effect. There is still heterogeneity in the same forms of exercise, such as tai chi, dance, and tango groups. However, the heterogeneity between different exercise groups was not obvious. Therefore, our Parkinson's patients do not need to be confused about the optimal form of exercise, and the effects of different exercise forms may be slightly different. This is good news for people who cannot visit a rehabilitation center to see a physical therapist because of financial or transportation reasons. PD is a progressive disease of the nervous system, which may be the reason why age is an important factor. The duration of treatment should be studied for three or six months in the future to increase the homogeneity of the study. If the treatment course is too short, the patient may learn various motor skills, which may exaggerate the curative effect; if it is too long, since PD is a progressive disease [30], it will not be comparable to other treatment cycles. Long-term exercise has not been found to have adverse results among older adults and PD patients [26, 64]. For patients, weekly and lifelong training is recommended. A more standardized, rigorous scientific design is also needed to explore the best exercise prescription.
Our study also has some shortcomings: 1) due to the large heterogeneity, we did not conduct evidence-level classification; 2) we were unable to include studies with the same movement parameters, and more studies on parameters and movement forms are needed in the future; 3) we did not account for changes in other parameters in the subgroup analysis; and 4) some studies did not report the number of men and women, and the sex ratio was not considered as one of the multiple factors in the meta-regression analysis.
It is important to note that in 2020, the WHO recommended 150–300 min of moderate-intensity physical activity per week for all adults, for people over 65 years of age, and for people with disabilities [65]. PD mostly affects people over 60 years of age, and the age at onset of the disease is usually 65 to 70 years [66]. A study in northern Sweden showed that the average age of people with PD was 70.6 years [67]. Moderate-intensity training was beneficial for all older adults over the age of 85, and there was no significant increase in adverse events [68]. Studies have shown no difference in 5-year mortality between moderate-intensity and high-intensity exercise interventions but a downward trend compared with the control group [69]. Studies have shown that exercise is safe and effective in reversing functional decline caused by acute hospitalization among elderly patients (mean age: 87.3; range, 75–101) [70]. Therefore, we believe that the WHO recommendations also apply to PD patients. People with PD have reduced activities of daily living and exercise less per week than healthy elderly people. Studies have shown that intensive exercise therapy can be beneficial for PD patients [71]. Surveys show a global trend of inactivity [72, 73]. People with PD may not have performed exercise that was intense enough in the current study. We should appropriately increase the exercise time of patients in future studies.
Conclusions
This meta-analysis suggests that community-based exercise may benefit motor function in patients with PD. This provides strong evidence for patients to choose more rehabilitation pathways.
The most commonly used modalities of exercise were tango and tai chi, and the most common prescription was 60 min twice a week. However, more direct studies comparing these parameters should be conducted in the future. Age, duration of treatment, and weekly frequency were important factors that influenced the effect.
Availability of data and materials
All data generated or analyzed in this work are included in the published version of the manuscript.
Abbreviations
- PD:
-
Parkinson’s disease
- UPDRS-III:
-
Unified Parkinson’s Disease Rating Scale part III
- MD:
-
Mean difference
- TUG:
-
Timed Up and Go test
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
- BBS:
-
Berg Balance Scale
- 6MWT:
-
6-Minute Walk Test
- H&Y:
-
Hoehn and Yahr
References
Hayes MT. Parkinson’s Disease and Parkinsonism. Am J Med. 2019;132(7):802–7.
Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808.
Simon DK, Tanner CM, Brundin P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin Geriatr Med. 2020;36(1):1–12.
Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–50.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303.
GBD 2016 Neurology Collaborators.Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80.
Dorsey ER, Bloem BR. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018;75(1):9–10.
Nemade D, Subramanian T, Shivkumar V. An Update on Medical and Surgical Treatments of Parkinson’s Disease. Aging Dis. 2021;12(4):1021–35.
Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323(6):548–60.
Feng YS, Yang SD, Tan ZX, Wang MM, Xing Y, Dong F, Zhang F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020;245:117345.
Haas CA. Revisiting brain stimulation in Parkinson’s disease. Science. 2021;374(6564):153–4.
Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–83.
Homayoun H. Parkinson Disease. Ann Intern Med. 2018;169(5):Itc33-itc48.
Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–94.
Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, Shah L, Sackley C, Deane KH, Wheatley K, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004.
Radder DLM. Lígia Silva de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, de Vries NM: Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities. Neurorehabil Neural Repair. 2020;34(10):871–80.
Fearon C, Fasano A. Parkinson’s Disease and the COVID-19 Pandemic. J Parkinsons Dis. 2021;11(2):431–44.
Zhang Q, Schultz JL, Aldridge GM, Simmering JE, Narayanan NS. Coronavirus Disease 2019 Case Fatality and Parkinson’s Disease. Mov Disord. 2020;35(11):1914–5.
Leta V, Rodríguez-Violante M, Abundes A, Rukavina K, Teo JT, Falup-Pecurariu C, Irincu L, Rota S, Bhidayasiri R, Storch A, et al. Parkinson’s Disease and Post-COVID-19 Syndrome: The Parkinson’s Long-COVID Spectrum. Mov Disord. 2021;36(6):1287–9.
Cilia R, Bonvegna S, Straccia G, Andreasi NG, Elia AE, Romito LM, Devigili G, Cereda E, Eleopra R. Effects of COVID-19 on Parkinson’s Disease Clinical Features: A Community-Based Case-Control Study. Mov Disord. 2020;35(8):1287–92.
Song J, Ahn JH, Choi I, Mun JK, Cho JW, Youn J. The changes of exercise pattern and clinical symptoms in patients with Parkinson’s disease in the era of COVID-19 pandemic. Parkinsonism Relat Disord. 2020;80:148–51.
Cartella SM, Terranova C, Rizzo V, Quartarone A, Girlanda P. Covid-19 and Parkinson’s disease: an overview. J Neurol. 2021;268(12):4415–21.
Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, Shah L, Sackley C, Deane KHO, Wheatley K, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004.
Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40.
García-Hermoso A, Ramirez-Vélez R. Sáez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, Valenzuela PL, Lucia A, Izquierdo M: Safety and Effectiveness of Long-Term Exercise Interventions in Older Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2020;50(6):1095–106.
Ferreira AFF, Binda KH, Real CC. The effects of treadmill exercise in animal models of Parkinson’s disease: A systematic review. Neurosci Biobehav Rev. 2021;131:1056–75.
Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lövdén M, Lindenberger U, Bäckman L, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–54.
van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord. 2013;28(11):1587–96.
Amraeva G, Shiderova G, Karimova A, Kaishibayeva G, Izbasarova A. Physical treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2020;79:e65–6.
Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. 2017;13(11):689–703.
Keus S, Munneke, M, Graziano, M, European Physiotherapy Guideline for Parkinson’s Disease. KNGF/ParkinsonNet 2014. https://www.parkinsonnet.nl/app/uploads/sites/3/2019/11/eu_guideline_parkinson_guideline_for_pt_s1.pdf. Accessed 8 Aug 2020.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Wroblewska A, Gajos A, Smyczynska U, Bogucki A. The Therapeutic Effect of Nordic Walking on Freezing of Gait in Parkinson’s Disease: A Pilot Study. Parkinsons Dis. 2019;2019:3846279.
Lee HJ, Kim SY, Chae Y, Kim MY, Yin C, Jung WS, Cho KH, Kim SN, Park HJ, Lee H. Turo (Qi Dance) Program for Parkinson’s Disease Patients: Randomized, Assessor Blind, Waiting-List Control. Partial Crossover Study Explore (NY). 2018;14(3):216–23.
Amano S, Nocera JR, Vallabhajosula S, Juncos JL, Gregor RJ, Waddell DE, Wolf SL, Hass CJ. The effect of Tai Chi exercise on gait initiation and gait performance in persons with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):955–60.
Cugusi L, Solla P, Serpe R, Carzedda T, Piras L, Oggianu M, Gabba S, Di Blasio A, Bergamin M, Cannas A, et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation. 2015;37(2):245–54.
Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–43.
Hashimoto H, Takabatake S, Miyaguchi H, Nakanishi H, Naitou Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med. 2015;23(2):210–9.
Michels K, Dubaz O, Hornthal E, Bega D. “Dance Therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement Ther Med. 2018;40:248–52.
Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med. 2015;23(2):175–84.
Choi HJ, Garber CE, Jun TW, Jin YS, Chung SJ, Kang HJ. Therapeutic effects of Tai Chi in patients with Parkinson’s disease. ISRN Neurology. 2013;2013:548240 Epub 2013.
Wan Z, Liu X, Yang H, Li F, Yu L, Li L, Wang Y, Jiang H, Zou J, Du J. Effects of health Qigong exercises on physical function on patients with Parkinson’s disease. J Multidiscip Healthc. 2021;14:941-950 2021.
Liu XL, Chen S, Wang Y. Effects of health Qigong exercises on relieving symptoms of Parkinson’s disease. Evid-Based ComplementAlternat Med. 2016;2016:5935782 Epub 2016.
Li X, Lv C, Liu X, Qin X. Effects of Health Qigong Exercise on Lower Limb Motor Function in Parkinson’s Disease. Front Med. 2022;8:809134.
Cheon S-M, Chae B-K, Sung H-R, Lee GC, Kim JW. The Efficacy of Exercise Programs for Parkinson’s Disease: Tai Chi versus Combined Exercise. J Clin Neurol. 2013;9(4):237–43.
Kalyani HH, Sullivan KA, Moyle GM, Brauer SG, Jeffrey ER, Kerr GK. Dance improves symptoms, functional mobility and fine manual dexterity in people with Parkinson disease: a quasi-experimental controlled efficacy study. Eur J Phys Rehabil Med. 2020;56(5):563–74.
Van Puymbroeck M, Walter AA, Hawkins BL, Sharp JL, Woschkolup K, Urrea-Mendoza E, Revilla F, Adams EV, Schmid AA. Functional Improvements in Parkinson’s Disease Following a Randomized Trial of Yoga. Evid Based Complement Alternat Med. 2018;2018:8516351.
Solla P, Cugusi L, Bertoli M, Cereatti A, Della Croce U, Pani D, Fadda L, Cannas A, Marrosu F, Defazio G, et al. Sardinian Folk Dance for Individuals with Parkinson’s Disease: A Randomized Controlled Pilot Trial. J Altern Complement Med. 2019;25(3):305–16.
Cheung C, Bhimani R, Wyman JF, Konczak J, Zhang L, Mishra U, Terluk M, Kartha RV, Tuite P. Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson’s disease: a pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4:162 Epub 2018.
Gao Q, Leung A, Yang Y, Wei Q, Guan M, Jia C, He C. Effects of Tai Chi on balance and fall prevention in Parkinson’s disease: a randomized controlled trial. Clin Rehabil. 2014;28(8):748–53.
Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41(6):475–81.
Kunkel D, Fitton C, Roberts L, Pickering RM, Roberts HC, Wiles R, Hulbert S, Robison J, Ashburn A. A randomized controlled feasibility trial exploring partnered ballroom dancing for people with Parkinson’s disease. Clin Rehabil. 2017;31(10):1340–50.
Wu PL, Lee M, Wu SL, Ho HH, Chang MH, Lin HS, Huang TT. Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson’s disease patients: a randomized controlled trial. Jpn J Nurs Sci. 2021;18(3):e12418 2021.
Vergara-Diaz G, Osypiuk K, Hausdorff JM, Bonato P, Gow BJ, Miranda JGV, Sudarsky LR, Tarsy D, Fox MD, Gardiner P, et al. Tai Chi for reducing dual-task gait variability, a potential mediator of fall risk in Parkinson’s disease: a pilot randomized controlled trial. Glob Adv Health Med. 2018;7:2164956118775385 Epub 2018.
Wang LC, Ye MZ, Xiong J, Wang XQ, Wu JW, Zheng GH. Optimal exercise parameters of tai chi for balance performance in older adults: A meta-analysis. J Am Geriatr Soc. 2021;69(7):2000–10.
Blesa J, Foffani G, Dehay B, Bezard E, Obeso JA. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci. 2022;23(2):115–28.
Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson’s disease: A complex and evolving picture. Mov Disord. 2017;32(11):1524–36.
Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55(9):1358–63.
Cerri S, Mus L, Blandini F. Parkinson’s Disease in Women and Men: What’s the Difference? J Parkinsons Dis. 2019;9(3):501–15.
Dahodwala N, Shah K, He Y, Wu SS, Schmidt P, Cubillos F, Willis AW. Sex disparities in access to caregiving in Parkinson disease. Neurology. 2018;90(1):e48–54.
Mak MKY, Wong-Yu ISK. Exercise for Parkinson’s disease. Int Rev Neurobiol. 2019;147:1–44.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna). 2017;124(8):901–5.
Linder J, Stenlund H, Forsgren L. Incidence of Parkinson’s disease and parkinsonism in northern Sweden: a population-based study. Mov Disord. 2010;25(3):341–8.
Izquierdo M, Morley JE, Lucia A. Exercise in people over 85. BMJ. 2020;368:m402.
Stensvold D, Viken H, Steinshamn SL, Dalen H, Støylen A, Loennechen JP, Reitlo LS, Zisko N, Bækkerud FH, Tari AR, et al. Effect of exercise training for five years on all cause mortality in older adults-the Generation 100 study: randomised controlled trial. BMJ. 2020;371:m3485.
Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, Sáez de Asteasu ML, Lucia A, Galbete A, García-Baztán A, Alonso-Renedo J, González-Glaría B, Gonzalo-Lázaro M, et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern Med. 2019;179(1):28–36.
Gamborg M, Hvid LG, Dalgas U, Langeskov-Christensen M. Parkinson’s disease and intensive exercise therapy - An updated systematic review and meta-analysis. Acta Neurol Scand. 2022;145(5):504–28.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):e1077–86.
Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2020;4(1):23–35.
Acknowledgements
We thank all the people who conducted the studies that contributed to this meta-analysis and systematic review.
Funding
This work was supported by the Ministry of Science and Technology of the People's Republic of China, (grant numbers National Key R&D Plan/2017YFC1308504), the Science and Technology Department of Sichuan Province (grant numbers Project of Science and Technology Department of Sichuan Province/2021YJ0184), and the Science and Technology Bureau of Enshi Prefecture: Guiding Project of Science and Technology Bureau of Enshi Tujia and Miao Autonomous Prefecture/JCY2019000031.
Author information
Authors and Affiliations
Contributions
YCL and QY designed and implemented the search strategy. TYC, HJP, and WTT selected the studies. YCL, TYC, and HJP performed data extraction and risk of bias assessment. YCL and TYC performed the data analysis. CY, QCH, CY, and ZZQ contributed to the writing of the paper. All authors were involved in the interpretation of the review. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, CL., Huang, JP., Wang, TT. et al. Effects and parameters of community-based exercise on motor symptoms in Parkinson’s disease: a meta-analysis. BMC Neurol 22, 505 (2022). https://doi.org/10.1186/s12883-022-03027-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-03027-z