Abstract
Background
Even undergoing mechanical thrombectomy (MT), patients with acute vertebrobasilar artery occlusion (AVBAO) still have a high rate of mortality. Tirofiban is a novel antiplatelet agent which is now widely empirically used in acute ischemic stroke (AIS). In this study, we aimed to evaluate the safety and efficacy of tirofiban as adjunctive therapy for MT in AVBAO.
Methods
From October 2016 to July 2021, consecutive AVBAO patients receiving MT were included in the prospective stroke registry. The short-term outcomes were (1) symptomatic intracerebral hemorrhage (sICH); (2) in-hospital death; (3) National Institute of Health Stroke Scale (NIHSS) at discharge. The Long-term outcomes were: (1) modified Rankin Scale (mRS) at 3 months; (2) death at 3 months.
Results
A total of 130 eligible patients were included in the study, 64 (49.2%) patients received tirofiban. In multivariate regression analysis, no significant differences were observed in all outcomes between the tirofiban and non-tirofiban group [sICH (adjusted OR 0.96; 95% CI, 0.12–7.82, p = 0.97), in-hospital death (adjusted OR 0.57; 95% CI, 0.17–1.89, p = 0.36), NIHSS at discharge (95% CI, -2.14–8.63, p = 0.24), mRS (adjusted OR 1.20; 95% CI, 0.40–3.62, p = 0.75), and death at 3 months (adjusted OR 0.83; 95% CI, 0.24–2.90, p = 0.77)].
Conclusions
In AVBAO, tirofiban adjunctive to MT was not associated with an increased risk of sICH. Short-term (in-hospital death, NIHSS at discharge) and long-term outcomes (mRS and death at 3 months) seem not to be influenced by tirofiban use.
Similar content being viewed by others
Background
Acute vertebrobasilar artery occlusion (AVBAO) leads to a high rate of mortality and disability. It accounts for approximately 1% of all ischemic strokes [1, 2]. Although Mechanical thrombectomy (MT) has been applied to acute anterior circulation stroke (ACS) for more than 7 years, the evidence of MT in AVBAO was still limited. Two previously published randomized controlled trials (RCT), the BEST study and the BASICS study, both failed to prove the superiority of MT over standard medical treatment[3, 4]. However, just recently, two RCTs (BAOCHE study and ATTENTION study) which focused on moderate-to-severe stroke (NIHSS ≥ 10), first confirmed that MT significantly improved the functional outcome of AVBAO[5, 6]. Patients with AVBAO often have typical vascular risk factors, one of the most common stroke subtypes is large artery atherosclerosis (LAA)[7,8,9]. During the MT procedure, endothelial injuries may lead to platelet activation which may cause in situ thrombosis [10]. Thus, the use of antiplatelet agents during the perioperative period may improve MT outcomes. Tirofiban is a non-peptide, selective, and reversible antiplatelet agent. It inhibits the final pathway of platelet aggregation. Previous studies have shown that tirofiban could improve the clinical outcome of anterior circulation occlusion (ACO) after MT. However, for posterior circulation stroke, the efficacy evidence of tirofiban is limited. In this study, we aimed to evaluate the safety and efficacy of tirofiban as adjunctive therapy for MT in AVBAO.
Methods
Study design and patient enrolment
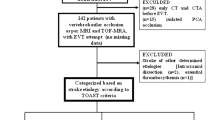
From October 2016 to July 2021, all acute stroke patients with basilar and vertebral artery occlusion receiving MT were enrolled from the Nanjing First Hospital and The First Affiliated Hospital of Chengdu Medical College. The protocol of the Stroke Registry was approved by the hospital ethics committee and informed consent was obtained. The patient’s eligibility for MT was evaluated by neuro-interventionist. All methods were performed in accordance with the relevant guidelines and regulations [11].
Tirofiban was considered for application in the following conditions: (1) severe residual stenosis (≥ 50%) after thrombectomy; (2) rescue treatment with stenting or balloon angioplasty or failed thrombectomy; (3) thrombectomy attempts ≥ 3 times; (4) severe atherosclerosis with a high risk of reocclusion. After 0.25–1 mg intra-arterial bolus, tirofiban was continuously intravenously administered for 16–24 h at a rate of 0.1–0.15 μg/kg/min after MT.
Data collection and outcome measures
Baseline characteristics were collected on admission. Computerized tomography (CT) or magnetic resonance imaging (MRI) was performed before and 24 h after MT to exclude intracranial bleeding. Telephone follow-up was performed to evaluate long-term outcomes at 3 months after stroke.
Outcome measures
Short-term outcomes were: (1) symptomatic intracerebral hemorrhage (sICH), according to the ECASS II classification [12]; (2) in-hospital death; (3) National Institute of Health Stroke Scale (NIHSS) at discharge.
Long-term outcomes were: (1) modified Rankin Scale (mRS) at 3 months (good outcome = mRS score 0–2); (2) death at 3 months.
Statistical analysis
Baseline characteristics were presented as mean (Standard Deviation); median (interquartile range) or n (%). Mann–Whitney U-test, independent sample t test or Fisher exact test were used to compare the outcomes of the two groups. The influence of tirofiban on each outcome was evaluated by the multivariate logistic regression model. Confounding factors were identified as clinically relevant and with p < 0.1 on univariate analysis. Statistical analyses were performed by SPSS 22.0 (IBM Inc., Armonk, NY, USA). A statistically significant difference was defined as p < 0.05.
Results
From October 2016 to July 2021, a total of 130 eligible patients were included in the study. Of them, 64 (49.2%) patients received tirofiban. Tirofiban group had a higher rate of large artery atherosclerosis (73% in tirofiban group vs. 53% in non-tirofiban group) and the non-tirofiban group had a higher rate of cardioembolism (38% in non-tirofiban group vs. 17% in tirofiban group). This discrepancy may be the origin of the differences in baseline characteristics that the tirofiban group had a higher rate of hypertension (p = 0.012), a higher level of LDL (p = 0.011), and non-tirofiban group had a higher rate of atrial fibrillation (p = 0.018), and a higher level of PT/INR (p < 0.001) (maybe caused by warfarin use to treat atrial fibrillation). The baseline characteristics of included patients were presented in Table 1.
Short-term outcomes
During hospitalization, tirofiban group compared with the control, sICH (4.7% vs. 12.1%; p = 0.128), NIHSS score at discharge (10 vs.13; p = 0.352) was not significantly different between the two groups. However, in-hospital death (14.1% vs. 28.8%; p = 0.041) was significantly lower in the tirofiban group. The results of short-term outcomes are summarized in Table 2.
Long-term outcomes
AT 3 months, 29 (46.0%) patients in tirofiban group and 25 (38.5%) in the control group had favorable outcomes, which were not significantly different (p = 0.386). 16 (25.4%) patients in tirofiban group were dead at 3 months, which was lower than control group (46.2%) (p = 0.014) (see Table 2).
Multivariate regression analysis
We used multivariable logistic regression analysis to adjust the potential confounders. The results showed that the use of tirofiban was not associated with sICH (adjusted OR 0.96; 95% CI, 0.12–7.82, p = 0.97), in-hospital death (adjusted OR 0.57; 95% CI, 0.17–1.89, p = 0.36), NIHSS at discharge (95% CI, -2.14–8.63, p = 0.24). At 3 months, favorable outcome (adjusted OR 1.20; 95% CI, 0.40–3.62, p = 0.75) and death rate (adjusted OR 0.83; 95% CI, 0.24–2.90, p = 0.77) were not influenced by tirofiban either (see Table 3).
Sensitivity analysis
LAA patients were further analyzed. The definition of LAA was in accordance with the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) criteria [13]. Multivariable logistic regression analysis was performed in two models. Model 1 was adjusted by the same confounders of overall AVBAO patients. Model 2 was adjusted by confounders that showed p < 0.1 on univariate analysis in LAA patients. The results revealed that in-hospital death, death at 3 months, and the favorable outcome were still unaffected by tirofiban (see Table 4).
Discussion
Acute vertebrobasilar artery occlusion (AVBAO) leads to high mortality and disability. In the MT procedure, a high recanalization rate (81–92%) was achieved in AVBAO patients [14, 15]. However, only about 30% of them obtained good functional outcomes [3, 9], which is lower than patients with anterior circulation occlusion (ACO) (approximately 50%) [16, 17]. The association between successful recanalization and good functional outcome was still controversial [18, 19].
In AVBAO, large artery atherosclerosis is one of the main subtypes of stroke[7,8,9]. A previous observational study showed that MT treatment might be more effective for LAA patients than for cardioembolism (CE) patients [20]. Thrombus histology analysis showed that vertebrobasilar artery thrombus contains a higher amount of red blood cells than anterior circulation [21]. These results indicated that thrombus growth in situ may be a major cause of thrombogenesis in the posterior circulation. Moreover, due to different collateral circulation patterns and lesser infarct volume, the hemorrhagic transformation was less likely to occur in AVBAO than in ACO [22]. For the reasons mentioned above, we presumed that AVBAO patients may benefit from adjunctive antiplatelet therapy in MT.
Tirofiban is a low-molecular-weight nonpeptide agent with a plasma half-life is about 2 h. It has a rapid onset and short duration of action, which makes the platelet function reversible within 4 h of cessation of infusion. Tirofiban is approved by The Food and Drug Administration and indicated for patients undergoing percutaneous coronary interventions (PCI) in the setting of ischemic heart disease. However, currently, it is extensively used off-label as adjunctive antiplatelet agent in selected patients with acute ischemic stroke (AIS) undergoing mechanical thrombectomy (MT). Such off-label use may prevent thrombosis events caused by the MT procedure and increase the recanalization rate. However, it has potential safety, ethical, and legal issues. In AIS, the optimum dosage of tirofiban and the characteristics of potential beneficiaries are not yet well-established. Moreover, although the overall incidence is low, adverse effects such as bleeding and thrombocytopenia may be life-threatening [23].
Several studies have been conducted to evaluate the efficacy and safety of tirofiban in MT for ACO [24,25,26,27,28,29,30]. All of them were observational studies and some of them reported conflicting findings. A subsequent meta-analysis showed that for stroke patients (most of them were ACO), tirofiban combined with MT improved 3 months functional outcome, reduced the risk of 3 months death, and did not increase the risk of sICH [30]. As for AVBAO, the efficacy and safety evidence of tirofiban is limited.
So far, only one study published by Sun et al. [31] aimed to evaluate tirofiban use in posterior circulation infarction. Their results revealed that the risk of sICH, 3 months mortality, and functional outcomes did not differ between the tirofiban and non-tirofiban groups. In our study, we reached the same conclusions. However, there were some differences between Sun et al. and our study. Firstly, Sun et al. only included atherosclerosis patients, but our study included all stroke types. Secondly, we found that NIHSS at discharge also had no significant statistical difference between the two groups. Further subgroup analysis showed that tirofiban was not associated with short-term and long-term outcomes in LAA subgroup patients either.
In conclusion, our study failed to prove the efficacy of tirofiban in AVBAO patients who underwent MT. This finding was inconsistent with the results in ACO patients, which showed that tirofiban improved 3 months functional outcome and reduced the risk of 3 months death [30]. These discrepancies may be attributed to several reasons. Firstly, patients with AVBAO were much more severe than ACO (median NIHSS 32 vs. 17) [3, 17]. The antithrombotic agent has classically been used for mild to moderate stroke [32]. The BASICS study suggested that when the antithrombotic agent was used without another more aggressive therapy (intravenous thrombolysis or mechanical thrombectomy), patients with AVBAO would not have good outcomes [33]. Secondly, the clinical outcomes of MT in AVBAO were mainly correlated with stroke severity and the success of the operation (such as admission NIHSS score, Alberta Stroke Program Early CT score, thrombolysis in cerebral infarction score, and residual stenosis after the procedure) [34,35,36]. The traditional prognostic factors such as gender, glucose, blood pressure, and comorbidities were not crucial in AVBAO [37,38,39]. Thus, the clinical outcomes of AVBAO may not be influenced by adjunctive antiplatelet therapy. Our results indicated that improving interventional techniques to reach more effective recanalization appears to be more critical for AVBAO patients.
Our study has several limitations. First, it is a retrospective analysis of a prospective observational study, and the number of patients is limited. Thus, the results should be interpreted with caution. Second, although the patient selection criteria and the dosage range of tirofiban was in accordance with practice guideline, the specific dosage varied for individual use, and it was mainly determined by clinician’s personal assessment. This may bias some effects on safety and efficacy outcomes. Third, some important factors such as prior antithrombotic agents use which may influence the sICH rate, were not recorded in the study. In this study, all patients in the study were enrolled in China, the findings of our study may not be applicable to global populations.
Conclusions
In summary, in AVBAO, tirofiban as adjunctive therapy to MT was not associated with an increased risk of sICH. Short-term and long-term outcomes seemed not to be influenced by tirofiban use.
Availability of data and materials
Data are available on request from the corresponding author.
Change history
28 December 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12883-022-03043-z
Abbreviations
- AVBAO:
-
Acute vertebrobasilar artery occlusion
- MT:
-
Mechanical thrombectomy
- CT:
-
Computerized tomography
- ACO:
-
Anterior circulation occlusion
- MRI:
-
Magnetic resonance imaging
- sICH:
-
Symptomatic intracerebral hemorrhage
- NIHSS:
-
National Institute of Health Stroke Scale
- mRS:
-
Modified Rankin Scale
- LAA:
-
Large artery atherosclerosis
- CE:
-
Cardio embolism
- PCI:
-
Percutaneous coronary interventions
- AIS:
-
Acute ischemic stroke
References
Singer OC, Berkefeld J, Nolte CH, Bohner G, Haring HP, Trenkler J, et al. Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol. 2015;77(3):415–24.
Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar artery occlusion. Lancet Neurol. 2011;10(11):1002–14.
Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:115–22.
Langezaal LCM, van der Hoeven E, Mont’Alverne FJA, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384:1910–20.
Jovin TG, Li C, Wu L, et al. Trial of Thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373–84.
Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361–72.
Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, et al. Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg. 2019;11(12):1174–80.
Bouslama M, Haussen DC, Aghaebrahim A, et al. Predictors of good outcome after endovascular therapy for Vertebrobasilar occlusion stroke. Stroke. 2017;48(12):3252–7.
Writing Group for the BASILAR Group, Zi W, Qiu Z, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. 2020;77(5):561–73.
Rubiera M, Alvarez-Sabin J, Ribo M, Montaner J, Santamarina E, Arenillas JF, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2005;36(7):1452–6.
Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke and vascular neurology. 2020;5(2):159–76.
Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438–41.
Chung JW, Park SH, Kim N, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J Am Heart Assoc. 2014;3(4):e001119. Published 2014 Aug 11. https://doi.org/10.1161/JAHA.114.001119.
Kang DH, Jung C, Yoon W, et al. Endovascular Thrombectomy for Acute Basilar Artery Occlusion: A Multicenter Retrospective Observational Study. J Am Heart Assoc. 2018;7(14):e009419. Published 2018 Jul 7. https://doi.org/10.1161/JAHA.118.009419.
Gory B, Eldesouky I, Sivan-Hoffmann R, Rabilloud M, Ong E, Riva R, et al. Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry. 2016;87(5):520–5.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Kaneko J, Ota T, Unemoto K, Shigeta K, Inoue M, Aoki R, et al. Endovascular treatment of acute basilar artery occlusion: Outcomes, influencing factors and imaging characteristics from the Tama-REgistry of acute thrombectomy (TREAT) study. J Clin Neurosci. 2021;86:184–9.
Pasarikovski CR, Khosravani H, da Costa L, Heyn C, Priola SM, Ku JC, et al. Outcomes of Endovascular Thrombectomy for Basilar Artery Occlusion. Can J Neurol Sci. 2020;47(4):479–85.
Sun B, Shi Z, Pu J, Yang S, Wang H, Yang D, et al. Effects of mechanical thrombectomy for acute stroke patients with etiology of large artery atherosclerosis. J Neurol Sci. 2019;396:178–83.
Berndt M, Poppert H, Steiger K, Pelisek J, Oberdieck P, Maegerlein C, et al. thrombus histology of basilar artery occlusions : are there differences to the anterior circulation? Clin Neuroradiol. 2021;31(3):753–61.
Buchman SL, Merkler AE. Basilar Artery Occlusion: Diagnosis and Acute Treatment. Curr Treat Options Neurol. 2019;21(10):45.
Elcioglu OC, Ozkok A, Akpınar TS, Tufan F, Sezer M, Umman S, Besısık SK. Severe thrombocytopenia and alveolar hemorrhage represent two types of bleeding tendency during tirofiban treatment: case report and literature review. Int J Hematol. 2012;96(3):370–5.
Pan X, Zheng D, Zheng Y, Chan PWL, Lin Y, Zou J, et al. Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol. 2019;26(8):1105–10.
Sun C, Li X, Zhao Z, Chen X, Huang C, Li X, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. 2019;10:1100.
Yu T, Lin Y, Jin A, Zhang P, Zhou X, Fang M, et al. Safety and efficiency of low dose intra-arterial tirofiban in mechanical thrombectomy during acute ischemic stroke. Curr Neurovasc Res. 2018;15(2):145–50.
Yi HJ, Sung JH, Lee DH. Safety and efficacy of intra-arterial tirofiban injection during mechanical thrombectomy for large artery occlusion. Curr Neurovasc Res. 2019;16(5):416–24.
Zhang S, Hao Y, Tian X, Zi W, Wang H, Yang D, et al. Safety of intra-arterial tirofiban administration in ischemic stroke patients after unsuccessful mechanical thrombectomy. J Vasc Interv Radiol. 2019;30(2):141-7 e1.
Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, et al. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol. 2020;27(6):1056–61.
Tang L, Tang X, Yang Q. The application of tirofiban in the endovascular treatment of acute ischemic stroke: a meta-analysis. Cerebrovasc Dis. 2021;50(2):121–31.
Sun X, Zhang H, Tong X, Gao F, Ma G, Miao Z. Effects of periprocedural tirofiban vs. oral antiplatelet drug therapy on posterior circulation infarction in patients with acute intracranial atherosclerosis-related vertebrobasilar artery occlusion. Front Neurol. 2020;11:254.
Dornak T, Herzig R, Sanak D, Skoloudik D. Management of acute basilar artery occlusion: should any treatment strategy prevail? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(4):528–34.
Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8(8):724–30.
Alexandre AM, Valente I, Consoli A, Piano M, Renieri L, Gabrieli JD, et al. Posterior circulation endovascular thrombectomy for large-vessel occlusion: predictors of favorable clinical outcome and analysis of first-pass effect. AJNR Am J Neuroradiol. 2021;42(5):896–903.
Yao J, Xu M, Zhou R, Zhu Y, Yan B, Lu J, et al. The efficacy and risk factors of mechanical thrombectomy for the treatment of vertebrobasilar artery occlusion: a single center study. Ann Palliat Med. 2021;10(4):4697–704.
Wu L, Zhang D, Chen J, Sun C, Ji K, Li W, et al. Long-term outcome of endovascular therapy for acute basilar artery occlusion. J Cereb Blood Flow Metab. 2021;41(6):1210–8.
Harvey RL. Predictors of Functional Outcome Following Stroke. Phys Med Rehabil Clin N Am. 2015;26(4):583–98.
van den Berg SA, Uniken Venema SM, Mulder M, Treurniet KM, Samuels N, Lingsma HF, et al. Admission blood pressure in relation to clinical outcomes and successful reperfusion after endovascular stroke treatment. Stroke. 2020;51(11):3205–14.
Sung SF, Chen YW, Hung LC, Lin HJ. Revised iScore to predict outcomes after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(6):1634–9.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from Jiangsu Pharmaceutical Association (A202109); National Natural Science Foundation of China (81870940, 82171295); Health Commission of Sichuan Province (18ZD008).
Author information
Authors and Affiliations
Contributions
XP—Patient recruitment, data analysis, writing of manuscript. MX—Patient recruitment, data analysis. YF, SL and YL—Patient recruitment, data collection. JZ—Study design, statistical method advises. JY—Study design, revise the manuscript and supervise the study. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol of the Stroke Registry was approved by the ethics committee of Nanjing First Hospital (document number: KY20130424-01). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Affiliation 5 was removed for author Jie Yang.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, X., Xu, M., Fei, Y. et al. Influence of tirofiban on stroke outcome after mechanical thrombectomy in acute vertebrobasilar artery occlusion. BMC Neurol 22, 460 (2022). https://doi.org/10.1186/s12883-022-02996-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02996-5