Abstract
In the present study, we explored multiple plasma factors to predict the outcomes of patients with AIS after IVT. Fifty AIS patients who received IVT with alteplase were recruited and divided into two groups according to their NIHSS scores. Serum from all subjects was collected to quantitatively analyze the levels of different plasma factors, IL-6, MMP-9, ADAMTS13, TNC, GSN and TRX, using Luminex assays or ELISA measurements. Compared with the levels assessed at the onset of AIS, the levels of MMP-9 (P < 0.001), ADAMTS13 (P < 0.001), and TRX (P < 0.001) significantly decreased after IVT. The level of IL-6 was significantly increased in the NIHSS > 5 group at admission (P < 0.001) compared to the NIHSS ≤ 5 group. AIS patients with a poor prognosis had lower levels of ADAMTS13 at 72 h post-IVT compared with patients with a good prognosis (P = 0.021). IL-6 also was notably higher in the poor outcome group (P = 0.012). After adjusting for confounders, ADAMTS13 at 72 h post-IVT was an independent protective factor for prognosis in AIS patients with an adjusted OR of 0.07 (P = 0.049), whereas IL-6 was an independent predictor of risk for AIS patients with an adjusted OR of 1.152 (P = 0.028). IVT decreased MMP-9, ADAMTS13, and TRX levels in the plasma of AIS patients. Patients with a NIHSS score of less than 5 exhibited lower IL-6 levels, indicating that increased levels of IL-6 correlated with AIS severity after IVT. Therefore, IL-6 and ADAMTS13 might be useful plasma markers to predict the prognosis in AIS patients at 90-days after IVT.
Similar content being viewed by others
Introduction
Stroke is a leading cause of death and the primary cause of disability worldwide, with ischemic stroke accounting for approximately 80% of the cases [1]. Intravenous thrombolytic treatment (IVT) using alteplase within a narrow time window is the leading treatment for acute ischemic stroke (AIS) [2]. However, the benefits of intravenous thrombolysis for recanalization are limited. Ischemia and hypoxia in brain tissue prior to recanalization can result in increased release of pro-inflammatory cytokines, leading to pathological damage, such as blood–brain barrier disruption, brain edema, and cell death. Current studies have suggested that neuroinflammation mediates neuronal damage, increasing neurological deficits during the acute phase of cerebral ischemia [3, 4]. Numerous studies have reported that different inflammatory and cardiogenic biomarkers (e.g., IL-6 and MMP-9) are closely associated with ischemic stroke and its functional prognosis [5,6,7]. Therefore, identifying validated plasma markers to predict the outcome of AIS after IVT is essential to help adjust treatment strategies for patients with AIS.
To explore the potential plasma markers that could be used to predict the AIS outcome after IVT, we prospectively collected peripheral blood from AIS patients before and after IVT to investigate the following plasma biomarkers, interleukin-6 (IL-6), matrix metalloproteinase 9 (MMP-9), a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13 (ADAMTS13), tenascin-C(TNC), gelsolin(GSN), and thioredoxin (TRX). In addition, the relationship between dynamic changes before and after IVT, disease severity, and the predictive value of the plasma markers to assess functional outcomes also were analyzed.
Materials and methods
Patient selection
Patients with AIS admitted to the Department of Neurology at the People's Hospital of Guangxi Zhuang Autonomous Region for IVT between January 2020 and June 2021 were prospectively entered into the study. The study was performed according to the World Medical Association (WMA) Declaration of Helsinki and approved by the Ethics Committee of Guangxi Zhuang Autonomous Region People's Hospital. Given its observational and anonymous nature, the need for patient consent to participate in this study was waived.
The inclusion criteria were as follows: (1) AIS patients who underwent IVT; (2) age was ≥ 18 years; (3) the initial modified Rankin Score (mRS) on admissions ≤ 2.
The exclusion criteria were as follows: (1) unfavorable outcomes occurred before sampling; (2) the patient exhibited significant pre-stroke disability (pre-stroke mRS ≥ 2); (3) evidence of intracranial hemorrhage, subarachnoid hemorrhage, arteriovenous malformation, aneurysm, or intracranial tumor verified by CT/MRI on admission; (4) the presence of pre-existing neurological or psychiatric disease that would confound the neurological evaluation; (5) active or recent hemorrhage and a history of trauma or surgery within two months before stroke onset; (6) concurrent infection at the time of sample collection; (7) the presence of rheumatoid immune diseases, severe liver or kidney disease, hematopathy, or malignant tumors; (8) suspicion of the presence of an infectious embolus or bacterial endocarditis; (9) a baseline platelet count < 50,000/μL; (10) the patient was pregnant or lactating; (11) missing clinical, imaging, or follow-up data or information; and (12) blood samples were of poor quality.
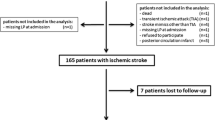
Ninety-five consecutive patients were included in the study. Of the 95 patients, 15 patients were excluded due to the presence of infection on admission, 2 patients had malignant tumors, 1 patient had a rheumatic immune disease,2 patients had incomplete clinical data and missing follow-up data, and 1 patients’ blood samples were of low quality. The remaining eligible patients (n = 74) were divided into two groups based on their NIHSS scores. One group consisted of patients with a NIHSS score greater than 5 (NIHSS > 5) (n = 36), and the other group of patients had a NIHSS score equal to or less than 5 (NIHSS ≤ 5) (n = 38).
Clinical information
IVT was carried out according to international guidelines. 0.9 mg alteplase/kg body weight was administered with a maximum dose of 90 mg. The drug was administered as an infusion of a 10% bolus dose within 1 min, followed by an infusion of a 90% dose within 60 min. The NIHSS score was assessed at admission and at 24 h and one week after stroke onset to determine neurological function. Baseline demographic information and vascular risk factors were collected from the patients, including baseline stroke severity (NIHSS score at admission), pre-stroke mRS, cerebrovascular risk factors (age, sex, hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary artery disease, atherosclerosis, as well as smoking and alcohol consumption status). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times within 24 h of admission, and the mean systolic and mean diastolic blood pressures were calculated. Biochemical parameters, including liver function, coagulation, uric acid, creatinine, and other laboratory tests, including blood cell count, were obtained before or 24 h after thrombolytic therapy. Computed tomography, magnetic resonance and digital subtraction angiography, electrocardiography, and carotid ultrasound were used to determine the stroke etiology. The clinical outcome at 90 days after AIS was assessed using the mRS. An mRS ≥ 2 or death was defined as a poor prognosis [8, 9]. The data were obtained using a telephone follow-up interview by a physician who was unaware of the patients’ clinical information and factor levels.
Biomarker assay assessment
Blood samples were collected before stroke onset (t0), 24 h after thrombolytic therapy (t1), and 72 h after thrombolytic therapy (t2). The samples were immediately centrifuged at 3,000 g for 15 min (Thermo Scientific Haraeus Multifuge 3SR plus centrifuge, US) and stored at -80 °C until analyzed.
The levels of IL-6, MMP-9, TNC, and ADAMTS13 were determined using a Luminex R&D System kit (Labex Bio, Shanghai, China), following the manufacturer’s instructions. This method utilized different antibody factors covalently cross-linked to specifically coded microspheres. Each coded microsphere was dyed with a different fluorochrome in different ratios, resulting in a corresponding fluorescent code and assay. The coefficients of variation (CVs) were 0.56% for IL-6, 0.58% for MMP-9, 0.44% for TNC, and 0.3% for ADAMTS13.
The TRX and GSN antibodies in serum were determined using a quantitative sandwich enzyme immunoassay (ELISA), following the manufacturer's instructions (Service Bio, Wuhan, China). The CVs for the TRX inter-assay and intra-assay ranged from 8 to 10%, respectively. The lower detection limit was 1.172 ng/mL, and the line range was 4.688 ng/ml to 300 ng/ml. The CVs for the GSN inter-assay and intra-assay ranged from 8 to 10%, respectively, and exhibited a range of 3.12 ng/ml to 200 ng/ml with a lower detection limit of 0.78 ng/ml.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 24.0 and Graphpad prism 8.0.2. Continuous variables that conformed to a normal distribution were expressed as means ± standard deviation. Other continuous variables that did not exhibit a normal distribution were expressed as medians and interquartile ranges (25% to 75%). Categorical variables were expressed as composition ratios. The plasma markers were labeled as continuous variables, log-transformed, and tested using repeated-measures ANOVA. The Pearson's chi-square test was used for categorical variables to test for differences in baseline characteristics. We also used binary logistic regression analysis to detect risk factors affecting the severity of illness and adverse prognosis. After correcting for potential confounders, including age and gender, independent influences associated with the prognosis of patients who underwent intravenous thrombolysis for AIS were screened using univariate and multivariate logistic regression analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each endpoint. Subject (receiver) operating characteristic (ROC) curves were used to assess the plasma markers (IL-6, MMP-9, ADAMTS13, TNC, GSN, and TRX) and determine the optimal cut-off point, sensitivity, and calculated specificity.
Results
Clinical characteristics
Ninety-five patients were recruited, including 43 men and 52 women. Only 74 patients (43 males and 31 females) with adequate admission data were included in the study. The patients had a median NIHSS score on admission of 5.5(IQR 4 to 13). Patients were categorized into two subgroups according to their NIHSS scores. One group included patients with a NIHSS score high than 5 (NIHSS > 5; IQR 7.5 to 17.5, median: 13; n = 36). The other group included patients with a NIHSS score equal to or less than 5 (NIHSS ≤ 5; IQR 3 to 5, median: 4; n = 38) (Table 1).
The mean age of the low NIHSS score group was significantly higher than that of the high NIHSS score group (P = 0.022). The median NIHSS score 24 h after admission was 4 (IQR 2 to 8.5). Patients in the high NIHSS score group (IQR 3 to 17, median: 5) had a significantly higher NIHSS score 24 h after admission than those in the low NIHSS score group (IQR 2 to 4.5, median: 3) (P = 0.006). The median mRS at 90 days was 2.5(IQR 2 to 4), and the mRS at 90 days was significantly higher in the high NIHSS score group (IQR 2 to 4, median: 3) than in the low NIHSS score group (IQR 1 to 3, median: 2) (P = 0.008). There were no significant differences in hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary heart disease, arteriosclerosis, mean systolic and diastolic blood pressure, smoking, and drinking between the two groups.
Changes in plasma factors before and after IVT in patients with AIS
MMP-9, ADAMTS13, and TRX levels were correlated with significant decreased in pre-IVT patients compared to 24 h after IVT (P < 0.05). The level of ADAMTS13 remained significantly decreased at 72 h post-IVT compared to 24 h after IVT (P = 0.008). The levels of IL-6, TNC, and GSN were not significantly different among the patients when assessed pre-IVT or at 24 h and 72 h after IVT (P > 0.05) (Fig. 1 and Table 2).
Relationship between plasma factors and NIHSS scores at admission
A NIHSS score greater than 5 at admission is thought to indicate the presence of severe neurological deficits. Therefore, the relationship between stroke severity and plasma factors was analyzed further. Univariate analysis demonstrated that the IL-6 level was significantly increased at admission in the NIHSS > 5 group compared to the NIHSS ≤ 5 group (P < 0.001), indicating a positive correlation between the level of IL-6 and the severity of the illness at admission. However, there were no significant differences in the levels of MMP-9, ADAMTS13, TRX, TNC, and GSN between the NIHSS > 5 and NIHSS ≤ 5 groups (Table 2).
Independent risk predictors of disease severity
After adjusting for age and gender, the multifactorial logistic analysis revealed that the level of IL-6 before IVT still was significantly associated with the NIHSS score at admission (OR 219.963, 95% CI 8.039–6018.562, P = 0.001) (Table 3), suggesting that the pre-IVT IL-6 level was an independent predictor of disease severity in patients with AIS. In addition, the pre-IVT MMP-9 level (OR 0.046, 95% CI 0.005–0.958, P = 0.046) also correlated with the NIHSS score on admission after adjustment.
The predictive significance of plasma markers for neurological function at 90 days after IVT
At the 90-day follow-up examination, 21(28%) patients with AIS and IVT exhibited a poor prognosis. We found that serum ADAMTS13 levels at 72 h after IVT were significantly lower in AIS patients with poor outcomes compared with those with a good prognosis 551,181.00(IQR 440,539.00 to 788,283.50) pg/ml vs. 769,107.00(IQR 565,397.50 to 1,366,444.50) pg/ml, P = 0.021). The level of serum IL-6 at 72 h post-IVT was significantly higher in the poor outcome group [8.72 (IQR 3.04–26.89) pg/ml vs. 3.72 (IQR, 1.88–8.58) pg/ml, P = 0.012] (Fig. 2). However, the levels of MMP-9, TNC, GSN, and TRX at 72 h post-IVT were not significantly different between patients with a good prognosis and those with a poor outcome.
The relationship between IL-6, ADAMTS13 and AIS outcomes 72 h after IVT. The concentration of ADAMTS13 were decreased in the unfavorable outcome group. However, the levels of IL-6 were significantly increased in the poor outcome group compared with the favorable outcome group. Data are presented as mean ± SEM, *p < 0.05
The prognosis of patients with AIS at 90-days after IVT was used as the dependent variable, and age, smoking, hypertension, diabetes, coronary artery disease, hyperlipidemia, atrial fibrillation, IL-6, MMP-9, ADAMTS13, TRX, TNC, and GSN were considered independent variables. Using univariate logistic regression analysis, we observed that the serum concentrations of IL-6 and ADAMTS13 at 72 h post-IVT were significantly associated with the functional outcome of AIS patients (Table 4). In multivariate logistic regression analysis after adjustment for other indicators, the serum concentration of ADAMTS13 at 72 h post-IVT functioned as a protective factor and was an independent predictor for functional outcome of AIS patients with an adjusted OR of 0.07 (95% CI, 0.005–0.991, P = 0.049), whereas IL-6 at 72 h post-IVT was an independent risk factor in functional outcome with an adjusted OR of 1.152 (95% CI,1.015–1.308, P = 0.028).
To assess the predictive effect of plasma markers on prognosis, we plotted ROC curves. The ROC curves for IL-6 and ADAMTS13 at 72 h post-IVT are shown in Fig. 3. ROC curve analysis determined that the best threshold value of IL-6 at 72 h post-IVT to predict the 90-day prognosis of AIS patients was 5.795 pg/ml, with a sensitivity of 66.7%, a specificity of 72.4%, and a Jorden's index of 0. 391; the area under the curve (AUC) was 0.71 (95% CI, 0.566 to 0.854, P = 0.012). The optimal cut-off value for the ADAMTS13 level at 72 h post-IVT was 634,010.5 pg/ml, with a sensitivity of 72.4%, a specificity of 66.7%, and a Jorden's index of 0.391; the AUC was 0.693 (95% CI, 0.547 to 0.839, P = 0.021), suggesting the level of IL-6 (AUC = 0.71, P = 0.012) exhibited a better predictive effect of a poor prognosis than the level of ADAMTS13 (AUC = 0.693, P = 0.021). Other plasma factors, including MMP-9 (AUC = 0.532, sensitivity 65.5%, specificity 57.1%, P = 0.702), TRX (AUC = 0.573, sensitivity 64.9%, specificity 88.3%, P = 0.382), TNC (AUC = 0.553, sensitivity 75.5%, specificity 67%, P = 0.523), and GSN (AUC = 0.608, sensitivity 78.7%, specificity 64.1%, P = 0.198) showed no significant differences between the mRS < 2 and mRS ≥ 2 groups (P > 0.05) (Fig. 3 and Table 5).
Discussion
In our study, we evaluated the prognostic values of plasma factors in AIS patients who received IVT. We found that the levels of MMP-9, ADAMTS13, and TRX were significantly decreased in AIS patients with IVT. However, the levels of IL-6, TNC, and GSN showed no significant differences after IVT. When the AIS patients were divided into two groups based on their NIHSS scores, the IL-6 levels were significantly higher in the group with high NIHSS scores (NIHSS > 5). Moreover, the ROC curves revealed that IL-6 and ADAMTS13 levels could significantly predict the 90-day prognosis of AIS patients after IVT. These results indicated that IL-6 and ADAMTS13 might be independent factors that are able to predict patient prognosis at 90 days following IVT for AIS.
IL-6 is a critical cytokine involved in the acute phase of the inflammatory response. Numerous studies have shown that the level of IL-6 increases in cerebrospinal fluid (CSF) and peripheral blood after ischemic stroke [10,11,12,13]. In human studies, the serum level of IL-6 is significantly correlated with infarct size and survival in stroke [14, 15]. Francisco et al. [16] demonstrated that IL-6 emerged as the only biomarker independently associated with infarct volume in AIS patients. Similarly, in our study, the levels of IL-6 were increased in AIS patients with NIHSS scores greater than 5. Therefore, the IL-6 levels could be considered an independent factor in predicting the patients’ prognosis 90 days after IVT. Similarly, recent studies have confirmed that a high level of IL-6 after AIS was an independent predictor of poor functional outcomes [17,18,19]. Therefore, high IL-6 levels might partially contribute to poor functional outcomes and death [20].
Low levels of MMP-9 expression are expected in the normal brain [21]. However, the levels of MMP-9 are elevated after cerebral ischemia, and MMP-9 levels are closely related to the occurrence and development of cerebral infarction [21, 22]. High levels of MMP-9 can be detected in necrotic brain tissue and the ischemic penumbra following a stroke [23]. In the present study, the level of MMP-9 after IVT in AIS patients was lower than before IVT, which might indicate that the necrotic tissue decreased MMP-9 release due to the recanalization of blood vessels after IVT. The NIHSS scores were not correlated with the levels of MMP-9, suggesting that MMP-9 might not reflect disease severity or the amount of necrotic brain tissue present.
Similar to the changes in the levels of MMP-9, the levels of ADAMTS13 and TRX after IVT treatment in AIS patients were lower than before IVT. However, our results contradict the report of Xu et al. that showed that the level of ADAMTS13 was associated with the effects of endovascular treatment or thrombolysis after cerebral infarction in patients with acute stroke. In addition, high levels of ADAMTS13 were associated with arterial recanalization in that study [24]. Decreased levels of ADAMTS13 were associated with poor functional outcomes for AIS patients in this study [24, 25]. Specifically, our results demonstrated that the levels of ADAMTS13 at 72 h post-IVT functioned as a protective factor and was an independent predictor of functional outcome in AIS patients.
TRX is a ubiquitous protein with disulfide reductase activity and plays a critical role in cellular redox control and oxidative stress responses. Increased expression of TRX and TRXR significantly disturbs redox homeostasis [26]. In this study, we investigated for the first time changes in TRX levels before and after IVT and discovered that the level of TRX decreased in AIS patients after IVT. Therefore, we speculated that the reduced TRX levels indicated that the redox homeostasis might not be disturbed following IVT.
There were no significant differences in the levels of TNC and GSN between the NIHSS > 5 and NIHSS ≤ 5 groups before or after IVT. Bharath et al. [27] proved that TNC could induce post-stroke brain damage. The reason why we did not observe significant differences in TNC levels might be due to an insufficient time interval for blood collection. GSN is an actin-severing and capping protein that regulates actin assembly and might be involved in fibroblast activation. We did not observe significant changes in GSN before and after IVT, which might indicate that GSN is not involved in the occurrence and development of cerebral infarction.
Several limitations were associated with this study. The sample size was small, and the patients were recruited from a single center, thus the follow-up prognostic value is very strict. The IL-6, MMP-9, ADAMTS13, TNC, TRX, and GSN levels were detected at only two-time points. The follow-up and chronic-phase data were not available. ADAMTS13 activity and vWF antigen/activity has effects on thrombosis. However, in this study, we did not explore the relationship between ADAMTS13 activity and IVT patients’ outcomes. Finally, the NIHSS scores of patients with mild stroke might be subjectively influenced by physician. Thus, additional studies are necessary to address these issues.
Conclusion
We found that the levels of MMP-9, ADAMTS13, and TRX were significantly decreased in AIS patients who received IVT. Patients with a NIHSS score greater than 5 exhibited higher levels of IL-6. The levels of IL-6 and ADAMTS13 significantly predicted the 90-day prognosis of AIS patients after IVT. Finally, the higher the IL-6 level, the worse the 90-day prognosis of patients who received IVT.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files.
References
GBD 2019 Stroke collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global burden of disease study 2019. Lancet Neurol. 2021;20:795–820.
Turc G, Tsivgoulis G, Audebert HJ, Boogaarts H, Bhogal P, De Marchis GM, et al. European stroke organisation (ESO)-European society for minimally invasive neurological therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J Neurointerv Surg. 2022;14:209.
Jurcau A, Ardelean IA. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J Integr Neurosci. 2021;20:727–44.
Reich DS, Arnold DL, Vermersch P, Bar-Or A, Fox RJ, Matta A, et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20:729–38.
Mechtouff L, Bochaton T, Paccalet A, Da SC, Buisson M, Amaz C, et al. Association of interleukin-6 levels and futile reperfusion after mechanical thrombectomy. Neurol. 2021;96:e752–7.
Douglas AS, Shearer JA, Okolo A, Pandit A, Gilvarry M, Doyle KM. The relationship between cerebral reperfusion and regional expression of matrix metalloproteinase-9 in rat brain following focal cerebral ischemia. Neurosci. 2021;453:256–65.
Montaner J, Ramiro L, Simats A, Hernández-Guillamon M, Delgado P, Bustamante A, et al. Matrix metalloproteinases and ADAMs in stroke. Cell Mol Life Sci. 2019;76:3117–40.
Tsivgoulis G, Goyal N, Katsanos AH, Malhotra K, Ishfaq MF, Pandhi A, et al. Intravenous thrombolysis for large vessel or distal occlusions presenting with mild stroke severity. Eur J Neurol. 2020;27:1039–47.
Tang H, Yan S, Wu C, Zhang Y. Characteristics and outcomes of intravenous thrombolysis in mild ischemic stroke patients. Front Neurol. 2021;12:744909.
Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–98.
Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLOS MED. 2009;6:e1000145.
Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40.
McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45.
Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2.
Beridze M, Sanikidze T, Shakarishvili R, Intskirveli N, Bornstein NM. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11:41.
Purroy F, Farré-Rodriguez J, Mauri-Capdevila G, Vicente-Pascual M, Farré J. Basal IL-6 and S100b levels are associated with infarct volume. Acta Neurol Scand. 2021;144:517–23.
Reiche E, Gelinksi JR, Alfieri DF, Flauzino T, Lehmann MF, de Araújo M, et al. Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab Brain Dis. 2019;34:789–804.
Li X, Lin S, Chen X, Huang W, Li Q, Zhang H, et al. The prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis. 2019;10:544–56.
Mengel A, Ulm L, Hotter B, Harms H, Piper SK, Grittner U, et al. Biomarkers of immune capacity, infection and inflammation are associated with poor outcome and mortality after stroke - the PREDICT study. BMC Neurol. 2019;19:148.
Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–51.
Abdelnaseer MM, Elfauomy NM, Esmail EH, Kamal MM, Elsawy EH. Matrix metalloproteinase-9 and recovery of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:733–40.
Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54.
Taylor A, Vendramin C, Singh D, Brown MM, Scully M. von Willebrand factor/ADAMTS13 ratio at presentation of acute ischemic brain injury is predictive of outcome. Blood Adv. 2020;4:398–407.
Bustamante A, Ning M, García-Berrocoso T, Penalba A, Boada C, Simats A, et al. Usefulness of ADAMTS13 to predict response to recanalization therapies in acute ischemic stroke. Neurol. 2018;90:e995-1004.
Prochazka V, Jonszta T, Czerny D, Krajca J, Roubec M, Macak J, et al. The role of von Willebrand factor, ADAMTS13, and cerebral artery thrombus composition in patient outcome following mechanical thrombectomy for acute ischemic stroke. Med Sci Monit. 2018;24:3929–45.
Mahmood DF, Abderrazak A, El HK, Simmet T, Rouis M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal. 2013;19:1266–303.
Chelluboina B, Chokkalla AK, Mehta SL, Morris-Blanco KC, Bathula S, Sankar S, et al. Tenascin-C induction exacerbates post-stroke brain damage. J Cereb Blood Flow Metab. 2022;42:253–63.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No.82160239), Guangxi Natural Science Foundation (No. 2020GXNSFAA297002), Guangxi Zhuang Autonomous Region Health Department (No. S2019089).
Author information
Authors and Affiliations
Contributions
Siyi Li, Huanhuan Lu, Xin Zhong, Shuxuan Huang prepared the manuscript and performed experiments. Xue Jiao, Guoyong He, Bingjian Jiang, Yuping Liu, Zhili Gao, Jinhong Wei, Yushen Lin conducted many of the experiments. Zhi Chen, Yanhua Li conceived of the idea for the project, contributed to the experimental design and assisted in the preparation of the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study involving human participants were reviewed and approved by the Ethics Committee of Guangxi Zhuang Autonomous Region people's Hospital. The ethics committee waived the requirement of written informed consent for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, H., Li, S., Zhong, X. et al. Immediate outcome prognostic value of plasma factors in patients with acute ischemic stroke after intravenous thrombolytic treatment. BMC Neurol 22, 359 (2022). https://doi.org/10.1186/s12883-022-02898-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02898-6