Abstract
Background
So far, the diagnosis of acute artery of percheron (AOP) infarction is uncommon. In this study, patients with acute AOP infarction were studied to explore the relationship of imaging findings, clinical manifestations and prognosis of acute AOP infarction.
Materials
A total of 23 patients with acute AOP infarction in our institution from 2014 to 2019 were reviewed retrospectively. All cases were evaluated by computed tomography (CT) and magnetic resonance imaging (MRI). The modified Rankin scale (MRS), blood examination, electrocardiogram and transthoracic echocardiography were used for detailed clinical and prognostic evaluation. All standard risk factors for these patients were recorded. The MRS scores were performed 90 days after discharge.
Results
Four different types of acute AOP infarction were identified: (a) bilateral paramedian thalamic infarction (BPTI, 52%); (b) bilateral paramedian thalamic with rostral midbrain infarction (BPTRMI, 30%), (c) bilateral paramedian and anterior thalamic infarction (BPATI, 13%), and (d) bilateral paramedian thalamic with red nuclei infarction (BPTRNI, 4%). These patients had consciousness disorder, memory dysfunctions, vertical gaze paresis and mesencephalothalamic syndrome. The 65% of patients with BPTI and BPATI experienced relatively good functional recovery and could carry out daily life activities (MRS score ≤ 2). However, patients with BPTRMI may have an unfavorable outcome.
Conclusions
Although the clinical features are variable, DWI or ADC map can improve the diagnosis of acute AOP infarction patterns. Acute AOP occlusion requires immediate diagnosis and treatment to obtain more favorable outcome and avoid additional unnecessary procedures.
Similar content being viewed by others
Introduction
The arterial supply of thalamus is divided into four regions: the anterior part is supplied by polar artery or thalamic tubercle artery; the paramedian is supplied by the paramedian artery or thalamic perforating artery; the inferolateral supplied by the thalamo-geniculate artery, and the posterior supplied by the posterior choroidal artery [1,2,3]. Perceron G [4] first described the artery of percheron (AOP) in 1973. It is a solitary arterial trunk of the bilateral thalamo-perforating artery originating from P1 segment of the posterior cerebral artery (PCA), which supplies bilaterally the paramedian thalamic territories. The AOP is an uncommon arterial variant. Meanwhile, rostral midbrain has supplied by the superior mesencephalic or rubral arteries, which can branch separately from P1 segment of the PCA or share a common origin with AOP (Fig. 1). Thus, AOP probably supply the rostral midbrain or red nucleus [5,6,7].
Different anatomic regions of the thalamus and its arterial supply. AOP is a solitary arterial trunk of bilateral thalamo-perforating artery originated from P1 segment of one of PCA, which supplies bilaterally the paramedian thalamic territories (A). When the polar artery is absent, AOP not only may supply the paramedian but also the anterior thalamic territories (B). The rostral midbrain has supplied by the superior mesencephalic artery, which share a common origin with AOP (C). The red nucleus has supplied by the rubral artery, which share a common origin with AOP (D). AOP, artery of percheron; PCA, posterior choroidal artery; BA, basilar artery; AN, anterior nucleus; LN, lateral nuclei; MDN, mediodorsal nuclei; PUL, pulvinar; RN, red nucleus
Acute AOP infarction is a rare pattern of ischemia. It can significantly affect patients’ health and may be life-threatening, although the ischemic lesion of paramedian bichulami is relatively small [5, 8]. Therefore, the diagnosis of acute AOP infarction is very important to guide appropriate treatment and prevent additional unnecessary disposal measures [8].
The diameter of AOP is so small that magnetic resonance angiography (MRA) cannot show it, and even digital subtraction angiography (DSA) cannot show its existence, stenosis or occlusion in most cases. Only a few authors found AOP obstruction through DSA [9, 10]. MRI, especially diffusion weighted imaging (DWI), plays an important role in the diagnosis of acute AOP infarction. At present, most neuroimaging literatures only describe the relationship between clinical manifestations and imaging [11, 12]. However, in acute AOP infarction cases, whether imaging findings can suggest prognosis needs to be further confirmed. Whether there is a certain correlation between the clinical characteristics and prognosis of different types of acute AOP infarction information remains to be summarized.
Materials and methods
The study was approved by the institutional review committee. We retrospectively identified 23 patients with acute AOP infarction by searching the medical chart database from 2012 to 2021 (key words: bilateral acute paramedian thalamic infarction).
Inclusion criteria: 1) The clinical manifestations of acute AOP infarction can be found in the medical chart database; 2) All the patients underwent MRI, including axial T2-weighted images (T2WI), axial T1-weighted images (T1WI), axial fluid-attenuated inversion-recovery (FLAIR) and DWI sequence; 3) High signal intensity on DWI or low signal intensity on apparent diffusion coefficients (ADC) map were noted in bilateral paramedian thalami with or without rostral midbrain involvement; 4) Neurological examination was performed during hospitalization; 5) Three months after discharge, modified Rankin Scale (MRS) score was performed; and 6) All the patients were followed up for at least 6 months.
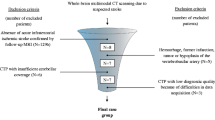
Exclusion criteria: 1) Patients with the top of the basilar artery syndrome, diffuse midline glioma of thalamus, viral encephalitis, arteriovenous fistula (AF) and wernicke encephalopathy; and 2) Patients with incomplete examination or medical records. Steps in consideration of individual data for inclusion were shown in Fig. 2.
Clinical data
Clinical workup included assessment of cardiovascular risk factors, diabetes mellitus history and evaluation of coagulation function. Evaluate the clinical risk factors of each patient, including cardiac embolism, large artery atherosclerosis or small artery occlusion.
Image analysis
Images of all patients with acute AOP infarction were reviewed by 2 senior neuroradiologists. In the Kappa consistency test of the 2 radiologists, Kappa value = 0.750, p <0.001. The acute ischemic territories involved in each patient were recorded under the following categories: only paramedian bithalamus (asymmetric or symmetric), paramedian bithalamic with rostral midbrain, paramedian bithalamic with anterior thalamus (right, left or bilateral), paramedian bithalamus and anterior thalamic with rostral midbrain and paramedian bithalamic with red nucleus (right, left or bilateral). Acute infarction was defined as hyperintense in DWI and hypointense in ADC map.
Results
Clinical syndromes
Table 1 shows the risk factors and neurological symptoms for all cases (age range, 23 to 86 years; mean, 58 ± 14 years; 8 women, 15 men). The most common syndrome was altered mental status in 22 cases (96%), which include 11 cases of hypersomnia (48%), 7 cases of coma (30%) and 4 cases of drowsiness (17%). Other characteristic included: vertical gaze palsy in 8 cases (35%), hypomnesis in 7 cases (30%), oculomotor nerve palsy in 7 cases (30%), disorientation in 5 cases (22%), barylalia and aphasia in 6 cases (26%), movement disorders in 9 cases (39%), ataxia in 5 cases (22%) and hemiplegia in 2 cases (9%).
Imaging findings
Head CT of 22 patients with acute AOP infarctions performed 2 hours after the onset of symptoms showed no acute hemorrhage or ischemia, only one patient had bilateral parathalamic and rostral midbrain hypodensity (Fig. 3). One patient had bilateral paramedian thalamus and left anterior thalamus hypodensity at 24 hours after onset (Fig. 4). MR imaging showed characteristic asymmetricall bilateral paramedian thalamic in 30% (7/23) and symmetrical in 22% (5/23). Acute infarctions of paramedian thalamic and the rostral midbrain was present in 30% of patients (7/23), a distinct pattern of V-shaped hyperintensity on axial DWI along the pial surface of the midbrain in the interpeduncular fossa (Fig. 3) was found in 17% cases (4/23). Bilateral paramedian thalamic ischemia with anterolateral paramedian territory (Fig. 4) in 13% of patients (3/23). Bilateral paramedian thalamic infarction with left red nucleus (Fig. 5) in 4% of patient (1/23). None of the cases had a paramedian bithalamus and anterior thalamic with rostral midbrain. Stenosis or occlusion of the vertebral artery, basilar artery or P1-segments of PCA was observed in 5 patitens, whereas in 15 patients MRA was normal. CT perfusion image demonstrated symmetrically increased mean transit time (MTT) and decreased mean cerebral blood flow (CBF) and mean cerebral blood volume (CBV) in the bilateral paramedian thalami in the in 4 patients (Fig. 6). No bleeding signal was found in SWI of 4 patients.
Bilateral parathalamic and rostral midbrain hypodensity on axial head CT. Case 2. Axial head CT at 2 hours after onset (A and B) shows hypodensity in the bilateral paramedian thalamus and midbrain involvement (white arrow). Axial DWI and ADC maps (C to F) show acute symmetrical infarctions in the bilateral paramedian thalamus and show a V-shaped acute infarction at the interpeduncular fossa of midbrain
Bilateral paramedian thalamic ischemia with anterolateral paramedian territory. Case 5. Axial head CT at 2 hours after onset (A) shows no hypodensity. Axial DWI and ADC map show acute infarcts (B and C) in the bilateral paramedian thalami (white arrow) and left anterior thalami (black arrow) after 24 hours, meanwhile, head CT show hypodensity (D)
Acute symmetric infarctions in the bilateral paramedian thalamus on MRA and CT perfusion. Case 23. Axial DWI and ADC map (A and B) show acute symmetric infarctions in the bilateral paramedian thalamus (black arrows). MRA demonstrate no occlusion or stenosis in the posterior circulation (C). Hyperacute CT perfusion imaging with white arrows identifying areas of decreased mean blood flow (D)
Treatment
All patients were classified as ischemic cerebrovascular disease after admission treatment. Therapeutic schedule included antiplatelet/anticoagulation, plaque stabilization and collateral circulation improvement. Patient No.4/8/11/14/16/19 were given alteplase in the time window (time window<4.5 h to 6 h). Heparin has also been used post-recanalization after thrombolysis. Low-molecular-weight heparins calcium injection (4000 IU/12 h) by subcutaneous was given to patients No.5/7/9/12/13/17 due to cardioembolism. Patients No.1/2/6/15 were given aspirin 200 mg/d orally. Patients No.3/10/18/20/21/22/23 were treated with aspirin 200 mg/d combined with clopidogrel 7.5 mg/d.
All patients were followed up for 3 months upon discharge. MRS score was shown in Table 1. There were 65% cases of a favorable outcome (MRS score ≤ 2) who experienced a good functional recovery and could carry out daily life activities. Patients partially recovered normal mental status. Patient No.4 was full patient recovery. Patient No.1/2/6/10/13/15/18/19/20 of vertical ophthalmoplegia recovered in various degree. Patient No.3/10/19/23 had memory loss and patient No.5/11/19 had obvious recent memory decline. Patient No.7/8/16/22 had unclear articulation. Patient No.2/6/8/9/10/13/17/18/20/22 had dyskinesia. The patients with rostral midbrain infarction were dependent and unable to walk without assistance. Patient No.5/7/9/10/13/18/20 still had disorientation or mild ataxia symptoms.
Discussion
Etiology and risk factors
Acute ischemia of AOP is uncommon, which accounted for 0.1–0.3% of all cerebral infarction [13, 14]. However, this is likely a conservative estimate due to the insufficient knowledge of it and limitations in the inclusion criteria [15]. AOP arising from P1 segments of the PCA supply predominantly paramedian bithalami with variable contribution to the rostral midbrain or anterolateral thalami [16]. In our studies, hypertension, smoking, diabetes mellitus, and hyperlipemia are risk factors for acute percheron infarction. Small vessel disease and arteriosclerosis are the causes of acute AOP infarcts in 14 patients (61%), which is similar to the previous reports [17]. It is indicated that cardioembolism is the most common cause [18], while only 7 cases (30%) had a potential source of cardiac embolism in our series. In addition, basilar artery dissection is seen in patient No.21 and 22. Atherosclerosis and small vessel disease are the main causes of acute AOP infarction.
Relationship imaging diagnosis and clinical features
Acute AOP ischemia has great variability with respect to symmetry, size, and territory, which is mainly due to the thalamic arteries vary between individuals. These differences are related to the parent vessel from which each branch arises, the number and position of the arteries and their tributaries [9, 12, 18]. Paramedian bithalami of the 23 patients showed high signal intensity on FLAIR with restricted diffusion and hypointense in the ADC map (positive in 100%). Valentina Francioni et al. [19] presented mismatch between DWI and FLAIR mismatch of hyperacute paramedian bithalami ischemia, which points out the DWI and ADC map is very important for the differentiation and therapy in acute AOP infarction. Although head CT is not sensitive to acute AOP infarction, it is indispensable to exclude cerebral hemorrhage at admission. It is identified a case describing AOP ischemic on acute CT perfusion imaging for the first time, however, thrombolysis was not performed in that symmetrical perfusion abnormalities were not recognized [20]. Most interestingly, we found that patient No.4/8/11/14 show corresponding bithalamic ischemia changes on acute CT perfusion imaging and was given thrombolysis to avoid deterioration. DSA rarely shows the presence of AOP, more often than not, the AOP is too small to be visualized by angiography. Similarly, although the AOP is rarely visualized with conventional MRA, it is very valuable to observe the stenosis of BA and PCA by MRA.
Obstruction of the AOP oftentimes leads to ischemia of the midbrain (57%) and/or the anterior thalamus (19%) [21], while we found that acute infarction of the rostral midbrain was present in 30% and anterior thalamus in 13%. The polar artery is absent in 30–60% of the population in that PcomA is highly variable (absent or hypoplastic) [22]. Then, paramedian arteries not only may supply the paramedian but also the anterior thalamic territories [11, 23], especially when the polar artery is absent (Fig. 2). Therefore, an infarction of the paramedian bithalamus and the anterior thalamus may be explained by occlusion of a single AOP, rather than synchronous occlusions of an AOP and the polar artery. In addition, patient No.7 showed bilateral paramedian thalamic infarction with only left red nucleus.
Similar to previous studies [23, 24], we also analysis the relationship between AOP infarction region and clinical presentation. Based on previous report,6, 9 No case involving bilateral paramedian and anterior thalamic infarction with midbrain involvement was found. The thalami contain reticular and intralaminar nuclei, associative nuclei, effector nuclei, sensory nuclei and limbic nuclei. Acute infarction of AOP destroys these nuclei in different combinations and result in complex syndromes depending on which nuclei is involved [8]. The typical clinical symptoms are “altered mental status, vertical gaze palsy, and memory impairment”. Other symptoms include: disorientation, hemiplegia, cerebellar ataxia, movement disorders and dysarthria [25]. Disorder of consciousness in 22 cases, except patient No.15, may be due to involvement in the reticular activating system. Excitedly, recent evidences suggest that the paraventricular nuclei supplied the AOP is a key wakefulness-controlling nucleus in the thalamus [26]. The vertical gaze palsy observed in patient No.2/10/13/18/20 with BPTRMI is due to an associated involvement of the midbrain tegmentum, including the interstitial nucleus of Cajal and rostral interstitial nucleus medial longitudinal fasciculus (RINMLF). Meanwhile, vertical gaze palsy in patient No.1/15/19 with BPTI were also reported. The reason may be that the thalami have a vital role in cortical input processing to the RINMLF [17]. Memory dysfunctions are reported on patient No.3/5/9/10/11/19/23 (BPATI in patient No.5/11/19). The dorsomedial nucleus located in paramedian territory is supplied by the AOP, the mammillothalamic tract and anterior nucleus located in anterior territory is supplied by polar artery or AOP, which is usually related to memory function [27]. When the anterior thalamic territory is also involved, memory impairment is typically more severe [8]. In our series, patient No.5/11/19 with BPATI still have memory deficit after 90 days. Oculomotor nerve palsy including ptosis, ocular movement disorders and ocular abnormal reflexes in patient No.2/6/10/13/18/20 with BPTRMI is related to acute ischemia of the oculomotor nucleus located in near midline of mesencephalic. The mesencephalothalamic or thalamopeduncular syndrome included movement disorders, hemiplegia and cerebellar ataxia occurs in patients with BPTRMI is related to midbrain involvement [28]. The superior mesencephalic or rubral artery branch separately from P1 segment of the PCA or share a common origin with AOP, which supply blood to the interpeduncular nucleus, medial part of the red nucleus, nucleus of cranial nerve III and anterior part of the periaqueductal gray matter located in dorsal midbrain [29]. Similar to polar artery, a single AOP may result in BPTRMI, rather than synchronous occlusions of an AOP and the superior mesencephalic or rubral artery.
Treatment and prognosis
In this study, 65% of the patients have a favorable outcome. Complete recovery from bilateral paramedian thalamic infarction has occasionally been reported [8]. At present, the goal of acute AOP occlusion treatment is to promote recanalization [30]. Patient No.4/8/11/14/16/19 were treated with thrombolytic therapy without cerebral hemorrhage or edema on head CT within 6 hours. Patient No.4 recovered completely and the residual cognitive impairment disappeared (MRS score = 0). Patient No.8/11/14/16/19 were fully conscious with partial memory deficit (MRS score = 1). Thrombolytic therapy (time window < 4.5 h–6 h) is considered to be the most effective treatment for acute AOP infarction [31]. Our results are consistent with previous studies. Patients who are not suitable for thrombolysis beyond 6 hours received antiplatelet and heparin therapy. We found a favorable outcome (MRS score = 1 or 2, except patient No.7) in patient No.1/3/5/12/15/17/21/22/23 with BPTI. Meanwhile, an unfavorable outcome (MRS score = 3 or 4) was present in patient No.2/6/9/13/18/20 with BPTRMI, which is consistent with previous studies [10]. All 23 patients we reported took oral anticoagulants to prevent recurrence. According to the literature reports and our cases, we summarized the flow chart of the algorithm for the treatment of acute AOP infarction (Fig. 7).
Differential imaging
There are some other etiologies to consider, such as the “top of the basilar” syndrome, deep venous infarction by thrombosis, wernicke’s encephalopathy and diffuse midline gliomas [32], which should not be confused with AOP infarction (Fig. 8). A single large embolus at the basilar artery tip could mimic acute AOP infarction pattern, but it often accompanies with additional characteristic cerebellar, brainstem and occipital lobe infarcts. Deep venous infarction caused by thrombosis and diffuse midline gliomas do not involve the specific blood-supply territory of the AOP, but multiple arterial regions. Wernicke’s encephalopathy affects not only the thalamus but also the mammillary bodies, tectal plate, and periaqueductal area. Diffuse midline gliomas in bilateral thalamus with space-occupying effect are hypointense on T1WI and hyperintense on T2WI or FLAIR as well as isointensity on DWI.
Differential diagnosis of AOP infarction. Top of the basilar artery syndrome can lead not only to thalamic infarction, but also to additional characteristic cerebellar, brainstem and occipital lobe infarction (A1 and A2). For deep vein infarction caused by thrombosis, high density of bilateral internal cerebral veins (B1, black arrow) can be observed on head CT, and FLAIR shows angiogenic edema involving multiple arterial areas of thalamus (B2). Wernicke’s encephalopathy often implicates pulvinar (C1) with abnormal enhancement of papillary body (C2, white arrow). Diffuse midline glioma in bilateral thalamus with mass effect is hyperintense on FLAIR and isointensity on DWI (D1 and D2)
Limitation
There are some limitations in thist study. Firstly, all the 23 patients were retrospectively enrolled from a single institution. Due to the limited sample size, large scale multicenter research is necessary in the future. Secondly, although many researchers believe that thrombolytic therapy is the first treatment option for acute AOP infarction, there are few cases of AOP occlusion recorded in this study. In our study, only 6 patients received thrombolytic therapy. The development of treatment protocols needs to be further improved through systematic case reports and clinical trials.
Conclusion
AOP is a rare anatomical variation of arteries. Due to the small lumen of AOP, angiography usually cannot show occlusion of AOP. Therefore, angiography should not be used in the diagnosis of acute AOP infarction. DWI and ADC are the best methods to diagnose acute AOP infarction. Clarifing the relationship between imaging and clinical manifestations is helpful to improve the disease management of acute AOP infarction.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AF:
-
Arteriovenous fistula
- CTP:
-
Computed tomography perfusion
- AOP:
-
Artery of percheron
- BPATI:
-
Bilateral paramedian and anterior thalamic infarction
- BPTI:
-
Bilateral paramedian thalamic infarction
- BPTRMI:
-
Bilateral paramedian thalamic with rostral midbrain infarction
- BPTRNI:
-
Bilateral paramedian thalamic with red nuclei infarction
- CT:
-
Computed tomography
- DSA:
-
Digital subtraction angiography
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Modified Rankin scale
- PCA:
-
Posterior cerebral artery
- SWI:
-
Susceptibility weighted imaging
References
Raina S, Thakur A, Sharma R, Sharma A. Hyperactive Delirium and Bilateral Ptosis: Bilateral Thalamic Infarcts Due to Artery of Percheron Occlusion. J Assoc Physicians India. 2019;67(12):77–8.
Gökçal E, Şengül Y, Uslu F. Investigation of risk factors, topographic location and stroke mechanisms of unilateral isolated and posterior cerebral ARTERY thalamic infarcts. Ideggyogy Sz. 2019;72(7–8):251–6.
Semenova U, Medvednik R, Popov V, Jinnah HA, Shaikh AG, Sedov A. Neuronal Activity of Pallidal Versus Cerebellar Receiving Thalamus in Patients with Cervical Dystonia. Cerebellum. 2021;20(2):151–9.
Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973;205(1):1–13.
Naldi A, Mirandola L, Di Liberto A, Cavallo R, Boghi A. Asynchronous bithalamic infarction in presence of artery of Percheron. Neurol Sci. 2021;42(10):4353–6.
Moon Y, Eah KS, Lee EJ, Kang DW, Kwon SU, Kim JS, et al. Neuro-Ophthalmologic Features and Outcomes of Thalamic Infarction: A Single-Institutional 10-Year Experience. J Neuroophthalmol. 2021;41(1):29–36.
Elsayed S, Al Balushi A, Schupper A, Shoirah H. Artery of Percheron occlusion with first-pass recanalisation of the first segment of posterior cerebral artery. BMJ Case Rep. 2021;14(4):e237968.
Kheiralla O, Alghamdi S, Aljondi R, Tajaldeen A, Bakheet A. Artery of Percheron Infarction: A Characteristic Pattern of Ischemia and Variable Clinical Presentation: A Literature Review. Curr Med Imaging. 2021;17(5):669–74.
Kheiralla O. Artery of Percheron infarction a rare anatomical variant and a diagnostic challenge: Case report. Radiol Case Rep. 2021;16(1):22–9.
Musa J, Rahman M, Guy A, Kola E, Guy A, Hyseni F, et al. Artery of Percheron infarction: A case report and literature review. Radiol Case Rep. 2021;16(6):1271–5.
Shams A, Hussaini SA, Ata F, Abdelhady M, Danjuma M. Bilateral Thalamic Infarction Secondary to Thrombosis of Artery of Percheron. Cureus. 2021;13(3):e13707.
Snyder HE, Ali S, Sue J, Unsal A, Fong C, Deng Z. Artery of Percheron infarction with persistent amnesia: a case report of bilateral paramedian thalamic syndrome. BMC Neurol. 2020;20(1):370.
Rissardo JP, Caprara AF. Isolated acute pseudobulbar palsy with infarction of artery of percheron: case report and literature review. Afr Health Sci. 2021;21(1):166–71.
Gonçalves DB, Barreira RP, Torres T, Correa BM, Rossette VM, Marques T, et al. Vertical one-and-a-half syndrome in a patient with pecheron artery ischemia: A case report. Radiol Case Rep. 2021;16(12):3908–10.
Ribeiro B, Marchiori E. Evaluation of neuroimaging findings in thalamic lesions: what can we think. Radiol Bras. 2021;54(5):341–7.
Yamaguchi T, Yakushiji Y. Bilateral Paramedian Thalamic Infarct. Am J Med. 2022;135(1):e24–5.
Macedo M, Reis D, Cerullo G, Florêncio A, Frias C, Aleluia L, et al. Stroke due to Percheron Artery Occlusion: Description of a Consecutive Case Series from Southern Portugal. J Neurosci Rural Pract. 2022;13(1):151–4.
Vinod KV, Kaaviya R, Arpita B. Artery of Percheron Infarction. Ann Neurosci. 2016;23(2):124–6.
Francioni V, Pirro F, D'Anna G, Cairo M, Appollonio I, Ferrarese C, et al. DWI/FLAIR mismatch during hyperacute infarction of the percheron artery: time is thalamus. Case Rep Neurol. 2020;12(1):127–30.
Isikbay M, Narsinh KH, Arroyo S, Smith WS, Cooke DL, Higashida RT, et al. Computed tomography perfusion abnormalities after carotid endarterectomy help in the diagnosis of reversible cerebral vasoconstriction syndrome. J Vasc Surg Cases Innov Tech. 2021;7(1):171–5.
Lazzaro NA, Wright B, Castillo M, Fischbein NJ, Glastonbury CM, Hildenbrand PG, et al. Artery of percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010;31(7):1283–9.
Li S, Kumar Y, Gupta N, Abdelbaki A, Sahwney H, Kumar A, et al. Clinical and neuroimaging findings in thalamic territory infarctions: a review. J Neuroimaging. 2018;28(4):343–9.
Garcia-Grimshaw MA, Peschard-Franco M, Gutierrez-Manjarrez FA. Bilateral thalamic ischemic stroke secondary to occlusion of the artery of percheron. Cureus. 2018;10(5):e2676.
Shah M, Nadeem MD, Saleem A, Khan MT, Asghar N. Bilateral thalamic infarction due to artery of Percheron occlusion: A case report. J Pak Med Assoc. 2021;71(12):2817–9.
Puri PR, Sijapati A. Bilateral internuclear and internal ophthalmoplegia due to artery of Percheron infarction. Clin Case Rep. 2017;5(3):280–4.
Ren S, Wang Y, Yue F, Cheng X, Dang R, Qiao Q, et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362(6413):429–34.
MacLellan A, Boyle K. Computed Tomography Perfusion Abnormalities in Bilateral Thalamic Infarction Due to Artery of Percheron Occlusion. J Stroke Cerebrovasc Dis. 2017;26(8):e170–1.
Galvez-Ruiz A. Post-traumatic Vertical Gaze Paresis in Nine Patients: Special Vulnerability of the Artery of Percheron in Trauma. Middle East Afr J Ophthalmol. 2015;22(3):370–6.
Ramalho C, Almeida M, Gomes F, Silva M, Peixoto J, Rodrigues S. Artery of Percheron Occlusion: A Diagnostic Challenge. Eur J Case Rep Intern Med. 2021;8(11):003020.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Santos M, Rodrigues A, Albuquerque A, Santos F, Bandeira A, Magalhães M, et al. Artery of Percheron Ischaemic Stroke: A Classic Presentation of a Rare Case. Eur J Case Rep Intern Med. 2021;8(12):003045.
Tuttle C, Boto J, Martin S, Barnaure I, Korchi AM, Scheffler M, et al. Neuroimaging of acute and chronic unilateral and bilateral thalamic lesions. Insights Imaging. 2019;10(1):24.
Acknowledgements
Not applicable.
Funding
This project was supported by the health talent special project in Jilin Provincial Department of Finance (JLSCZD2019–014), Health talent special project in Jilin Provincial Department of Finance (JLSWSRCZX2020–047) and Natural Science Foundation of Jilin Province (YDZJ202101ZYTS019).
Author information
Authors and Affiliations
Contributions
ZB contributed to the writing of the manuscript. WXX contributed significantly to the medical conception of the data collection. CG interpreted the medical results of the study. WJP accompanied the entire research process and contributed significantly to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Ethics Committees of the First Hospital of Jilin University (No. 2021–362). Verbal and written informed consent were obtained from all individual participants included in the study. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, B., Wang, X., Gang, C. et al. Acute percheron infarction: a precision learning. BMC Neurol 22, 207 (2022). https://doi.org/10.1186/s12883-022-02735-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02735-w