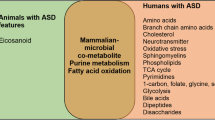
Abstract
Background
Diagnosis of autism spectrum disorder (ASD) is generally made phenotypically and the hunt for ASD-biomarkers continues. The purpose of this study was to compare urine organic acids profiles of ASD versus typically developing (TD) children to identify potential biomarkers for diagnosis and exploration of ASD etiology.
Methods
This case control study was performed in the Department of Pathology and Laboratory Medicine in collaboration with the Department of Pediatrics and Child Health, Aga Khan University, Pakistan. Midstream urine was collected in the first half of the day time before noon from the children with ASD diagnosed by a pediatric neurologist based on DSM-5 criteria and TD healthy controls from August 2019 to June 2021. The urine organic acids were analyzed by Gas Chromatography-Mass Spectrometry. To identify potential biomarkers for ASD canonical linear discriminant analysis was carried out for the organic acids, quantified in comparison to an internal standard.
Results
A total of 85 subjects were enrolled in the current study. The mean age of the ASD (n = 65) and TD groups (n = 20) was 4.5 ± 2.3 and 6.4 ± 2.2 years respectively with 72.3% males in the ASD group and 50% males in the TD group. Parental consanguinity was 47.7 and 30% in ASD and TD groups, respectively. The common clinical signs noted in children with ASD were developmental delay (70.8%), delayed language skills (66.2%), and inability to articulate sentences (56.9%). Discriminant analysis showed that 3-hydroxyisovalericc, homovanillic acid, adipic acid, suberic acid, and indole acetic were significantly different between ASD and TD groups. The biochemical classification results reveal that 88.2% of cases were classified correctly into ASD& TD groups based on the urine organic acid profiles.
Conclusion
3-hydroxy isovaleric acid, homovanillic acid, adipic acid, suberic acid, and indole acetic were good discriminators between the two groups. The discovered potential biomarkers could be valuable for future research in children with ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a complex neuro-developmental metabolic disorder involving multiple organ systems that can cause significant social, communication, and behavioral challenges in patients [1,2,3]. Many researchers report that there exist multiple factors involved in the pathogenesis of ASD including nutrients, infections, genetic disorders, and toxins [4,5,6,7]. ASD is highly genetically heterogeneous and both inheritable and de novo gene variations may contribute to its pathophysiology [8]. Since its discovery in 1943, ASD is still widely diagnosed through clinical and behavioral observation, due to a lack of reliable laboratory or radiological scans [1, 4, 9] In the past decade, numerous genes have been identified that contribute to the serious deficits in communication, social cognition, and behavior that individuals with ASD often experience [8]. ASD are categorized by impaired social interactions, difficulty in communications skills, repetitive behaviors, and additional stereotypical behavioral patterns [1]. Diagnosis is based on developmental history and examination of the patient using diagnostic criteria i.e. Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-5 criteria) [10]. Behavioral tests like DSM-5 are subjective and time-consuming, require professional staff to be administered, and can only be used from age 3 years once the child is old enough to communicate. The purpose of identification of symptoms earlier is to provide earlier interventions that might improve outcomes [1].
Even though there are no objective means to diagnose ASD, it is significantly increasing in prevalence [11,12,13]. The publications on the prevalence of ASD have also increased over the last decade [13,14,15,16]. Several reasons may contribute to this rise, such as the definition of ASD has become broader, changes in the use of screening tools and diagnostic criteria, greater awareness, and recognition of ASD [15, 17]. Initially, in the 1970s, ASD was considered a rare disorder, and its prevalence was estimated to be in around 2 of 10,000 children [18]. In comparison to the USA, the prevalence of ASD was 99–116.1 per 10,000 in UK versus 34 per 10,000 in the USA [19]. The estimated prevalence of ASDs in Asia varied from 1.1 to 21.8 per 10,000 [17, 20,21,22,23]. According to the Pakistan Autism Society (PAS), in Pakistan, there are more than 350,000 children with ASD [24,25,26]. There are no national registries for ASD in Pakistan and there is a dearth of reliable data to know the exact incidence of ASD in Pakistan.
Organic acids are important metabolites in major metabolic pathways of fatty acids, carbohydrates, proteins, neurotransmitters, vitamins, co-factors, pathways involved in energy production (Krebs cycle), oxidative damage, and intestinal dysbiosis [27]. Few studies have demonstrated the utility of urine organic acid in children with ASD [28, 29]. Recent researchers have reported that several known neurometabolic disorders, inherited metabolic disorders (IMDs) and chromosomal alterations are thought to contribute to the development of ASD. Some of these metabolic disorders include phenylketonuria, disorders of purine metabolism, biotinidase deficiency, disorders of neurotransmitters, methylmalonic acidemias, and dicarboxylic aciduria [28,29,30]. There is also increasing awareness that oxidative stress may be implicated in the pathophysiology of ASD with evidence of oxidative stress markers in urine [31]. A study by Emond et al. showed that levels of glycolate, citrate and succinate were increased in the urine sample of ASD children [32]. Another study showed significant differences of 2-oxoglutaric, isocitric, citric, 4-hydroxybenzoic, adipic, 4-hydroxyphenyl acetic, hippuric, and suberic metabolites between ASD children and the control group [33]. There is no national newborn screening program in Pakistan for screening of IMDs.
There is not sufficient literature on the metabolomic data from urine organic acid testing in ASD and none from this part of the world [28, 34, 35]. The main aim of this study was to identify the metabolites and organic acids in the urine of children with and without ASD using gas chromatography-mass spectrometry (GC-MS).
Material and methods
This case-control study was conducted in the Biochemical Genetics Laboratory (BGL) of Section of Chemical Pathology, Department of Pathology and Laboratory Medicine in collaboration with the Department of Pediatrics and Child Health, Aga Khan University Hospital (AKUH), Karachi Pakistan. This exploratory study was carried out from August 2019 to June 2021 after the approval from AKU’s ethical review committee (Reference number 2019–1213-4357). Our study is fully compliant with the Declaration of Helsinki [36]. The study cases (ASD group) were recruited from the Child Neurology Clinic and Child Development Program at the Department of Pediatrics and Child Health AKUH after the diagnosis of ASD was confirmed by a pediatric neurologist using the DSM-5 criteria [37] and after taking informed consent from parents or guardians of the children. Clinical history was obtained on a structured questionnaire including demographics, age at diagnosis of ASD, behavioral history, developmental history, and clinical history. None of them had any history of fever, vomiting, hypotonia, poor feeding, jaundice, and failure to thrive in the last 1 month before sampling. Children with known metabolic diseases, any recent hospital admission (< 1 month), presence of certain factors that would interfere with the detection of urine organic acids (compromised kidney function, hepatic insufficiency, dietary intervention therapy), diagnosis of other neuropsychiatric disorders, and children with any known inherited metabolic disorders for which treatment was received, were excluded from the study.
Healthy or typically developing (TD) children were selected randomly as controls, from children of laboratory employees who volunteered to participate in the study, without any history of learning and psychiatric abnormalities. For the control group, the same exclusion criteria were applied. Both cases and controls were selected through a convenient sampling method.
Midstream urine was collected in the first half of the day time before noon in sterile tubes without preservatives. The samples were placed on dry ice or in a freezer as soon as possible to avoid bacterial growth and transported to the Biochemical Genetics Laboratory (BGL), AKUH. The urine samples were frozen and stored at − 20 °C in aliquots until analysis. At the Biochemical Genetics Laboratory (BGL), before analyses, all urine sample concentrations were normalized with urine creatinine as a way of minimizing variability due to variability in urine concentration. Urine creatinine analysis (mmol/l) was performed for all samples on Siemens ADVIA 1800 by Jaffe’s kinetic method. One milliliter (ml) of undiluted urine was used for analysis in case of urine creatinine concentration < 1 mmol/l, while for samples with urine creatinine > 1 mmol/l, the volume of urine (patient/control) required for analysis was calculated by using eq. (1/ urine creatinine concentration of urine and 0.2 ml of deionized water was added to make up sample volume to 1 ml. One ml urine sample was taken in screw-capped glass tubes with a patient identification number. One hundred microliter of internal standard (3,3 dimethyl glutaric acid) was added followed by vortex and incubated at 60 °C in the water bath for 30 min and cooled to room temperature. The sample was then saturated with sodium chloride (NaCl) for oximation and mixed with a vortex mixer. Sample extraction was carried out with 50 μl of 6 M hydrochloric acid (HCL) and 5 ml of ethyl acetate. With help of the Pasteur pipette, upper ethyl acetate layered was transferred to a clean glass tube to which 10 μl of 25% ammonia was added. Vortexed briefly and dried down under nitrogen (oxygen-free) at room temperature for approximately 1 hour. For derivatization 80 μl of N, O-Bis-trimethylsilyl) trifluoroacetamide with 1% trimethyl chlorosilane and 20 μl of pyridine was added to dried extracts. Vortexed briefly and incubated at 75 °C for 30 min and cooled the tubes at room temperature. After cooling, 900 μl of iso-octane was added and mixed. The mixture was transferred to the micro-vial labeled with the patient’s identification numbers and screwed. Micro-vial containing the pre-treated sample was loaded into the auto sampler’s tray of GC-MS by Agilent Technologies United States. The Agilent Technology’s J&W Ultra Inert HP-5MS ultra inert capillary column was used with helium as carrier gas. Scan mode was created in the mass spectrometer acquisition software. The data generated from the mass detector was analyzed by Chem station software with the help of the following libraries: ORGASID, ACID97, and NIST. Semi quantitation of organic acids was done using the formula: area under curve of metabolite/area under curve of internal standard × concentration of internal standard. Organic acids were reported in pseudo units. Organic acid chromatograms of all study subjects were critically reviewed by credentialed chemical pathologists. During the period of study, the BGL successfully participated in multiple proficiency testing surveys from European Research Network for Evaluation and Improvement of Screening, Diagnosis, and Treatment of Inherited Disorders of Metabolism (ERNDRIM) and College of American Pathologists (CAP) for urine organic acids.
Statistical analysis was done using SPSS software (IBM SPSS, Statistics for Windows, USA) version 21.0. Mean and standard deviation were computed for quantitative data and frequencies were generated for qualitative data. The analytes for which we did not have pure standards were assigned a relative response factor that corresponded to the response factor for the internal standard. The height of each organic acid peak was interpreted as small, medium, and large with reference to internal standard peak height. The qualitative variables were compared with Chi-square statistics. To identify potential urinary biomarkers for ASD canonical linear discriminant analysis was carried out for the following organic acids that were quantified in comparison to internal standard: 3-hydroxyisovaleric acid, homovanillic acid, 2-ketoglutaric acid, 3- hydroxybutyric acid, adipic acid, suberic acid, 4-hydroxyphenyl acetic acid, indole acetic acid, lactic acid, and citric acid. This was an exploratory study and the level of statistical significance was defined as p-value < 0.05.
Results
A total of 85 subjects were enrolled in the current study. The mean age of the ASD (n = 65) and TD (n = 20) groups were 4.5 ± 2.3 years and 6.4 ± 2.2 years, respectively. There were 72.3% (n = 47) were male subjects in the ASD group while there was 50% (n = 10) male in the TD group. Parental consanguinity was 47.7% (n = 31) and 30% (n = 6) in ASD and TD groups, respectively. In ASD 73.8% (n = 48), 9.2% (n = 6) and 16.9% (n = 11) were fed with formula, breastfeeding and mixed feeding in infancy, respectively. In TD 90% (n = 18) and 10% (n = 2) were fed with formula and mixed feeding in infancy, respectively. In the ASD group mean age at the time of diagnosis of ASD was 2.3 ± 1.5 years and 21.5% (n = 14) suffered from epilepsy. In ASD the common clinical signs noted were developmental delay (70.8%), followed by delayed language skills (66.2%) and inability to articulate sentences (56.9%), shown in Fig. 1. Physical examination, to rule out any gross morphological abnormality, conducted in both groups was unremarkable.
The mean urinary creatinine for ASD and TD groups were 5.2 ± 3.3 mmol/L and 6.2 ± 2.7 mmol/L, respectively. The urinary creatinine correlated with age in ASD (r = 0.31, p value 0.009) and not in TD group of children. Table 1 describes the organic acid peaks identified on chromatograms after analysis on GCMS. Comparison of organic acid peaks in ASD and TD groups separately using chi-square analysis showed a statistically significant difference in the following organic acids: aconitic acid, succinic acid, citric acid, indole acetic acid, palmitic acid, suberic acid, lactic acid, 2- ketoglutaric acid, adipic acid, hippuric acid, 5-hydroxymethyl-2-furoic acid, and pimelic acid. The urine organic acids that showed negative weak correlation with age were homovanillic acid (r = − 0.30, p value 0.004) and suberic acid (r = − 0.37; < 0.0001). The rest of the urine organic acids did not correlate with age. A sub-analysis was done to determine if gender differences affected any metabolite, no statistically significant effect of gender was seen on the urine organic acids.
Mean comparison (pseudo units) was statistically significant for the following organic acids in ASD versus TD group: 3-hydroxyisovaleric acid (0.2 ± 0.14 vs. 1.05 ± 1.1), homovanillic acid (10.2 ± 6.7vs. 6.6 ± 2.4), adipic acid (1.54 ± 2.4 vs. 0.015 ± 0.04), suberic acid (1.3 ± 2.3 vs. 0.005 ± 0.02) and indole acetic (0.6 ± 0.9 vs. 0.25 ± 0.04), respectively and these organic acids were good discriminators between ASD and TD groups, as the separations were large. The 3-hydroxyisovaleric acid was low in quantity in ASD as compared to TD children while homovanillic acid, adipic acid, suberic acid, and indole acetic were higher in ASD than the TD group. This comparison is further described in Table 2. Table 2 also presents the standardized canonical discriminant function coefficients. The standardized canonical discriminant function coefficients show all quantitative variables in the model on the same scale and provide an index of the importance of each predictor (urine organic acids). 3-hydroxy Isovaleric score was the strong predictor followed by homovanillic acid, indole acetic, adipic acid, and suberic acid. These five variables (urine organic acids) with large coefficients stand out as those that strongly predict allocation to the ASD and TD groups. 2-ketoglutaric acid, 3-hydroxybutyric acid, adipic acid, suberic acid, 4-hydroxyphenyl acetic acid, and citric acid scores were less successful as predictors. Canonical correlation of 0.707 suggests the model explains 49.98% of the variation in the grouping variable, i.e., whether ASD or TD, while 50% remains unexplained. Wilks’ lambda indicates the significance of the discriminant function, it shows a highly significant function (p < .0001) and provides the proportion of total variability not explained, i.e., it is the converse of the squared canonical correlation (R2).
The percent classification using discriminant function analysis results showed the sensitivity and specificity (95% CI) of urine organic acid to diagnose ASD were 100% (94.48 to 100) and 50% (27.2 to 72.8) respectively. According to the discriminant analysis 88.2% of cases were classified correctly into ‘ASD’ or ‘TD’ groups using urine organic acids (Table 3).
Discussion
In the omics era breakthrough advancements in the fields of genomics, proteomics, and metabolomics are adding to the data on ASD diagnostics. The profiling of small-molecule metabolites also known as metabolomics can provide means to identify disturbances in metabolic pathways in ASD [34, 38]. The metabolome reflects the interaction between genetic and environmental influences and can provide information to bridge the gap between genotype and phenotype in children suffering from ASD [28, 34, 35]. In this study the urinary creatinine correlated with age in ASD (r = 0.31, p value 0.009) which could be because of increasing muscle mass as age increases [39]. The analysis of the current study showed that children with ASD were more likely to have increased levels of homovanillic acid, indole acetic acid, adipic acid, and suberic acid. The modeling was performed using the discriminant analysis and canonical correlation for 10 urine organic acids to identify the potential biomarkers for ASD. The 3-hydroxy isovaleric acid (lower in ASD), homovanillic acid, adipic acid, suberic acid, and indole acetic were observed as good discriminators between the two groups. Our study findings showed that two dicarboxylic acids (adipic and suberic) were good discriminators between ASD and TD groups. The reason for the increase in these dicarboxylic acids in ASDs is unknown but from a biochemical point of view, the concentration of this acid might rise because of an increase in omega (ɷ) oxidation, which may be because of impaired β-oxidation of fatty acids in ASDs [40, 41]. Beta-oxidation occurs in the mitochondria and when mitochondrial metabolism is impaired, β-oxidation can also be disturbed. A Polish group of researchers observed the levels of succinic acid, adipic acid, and suberic acid in the urine of children with ASD before and after vitamin B supplementation and the results showed that after supplementation the levels were reduced in children with ASD [33]. Excessive intake of adipic acid-containing foods such as gelatin-containing desserts including fruit gels, jelly, puddings, and no-bake cream pies may also be a contributor which was not evaluated in our study. Suberic acid, a dicarboxylic acid, is present in the urine of individuals with fatty acid oxidation disorders. Elevated levels are found in individuals with medium-chain acyl-CoA dehydrogenase deficiency [42]. It is also associated with carnitine-acylcarnitine translocase deficiency, malonyl-Coa decarboxylase deficiency. Moreover, adipic acid is also found to be associated with 3-hydroxy-3-methylglutaryl-CoA lyase deficiency, carnitine-acylcarnitine translocase deficiency, malonyl-Coa decarboxylase deficiency, and medium Chain acyl-CoA dehydrogenase deficiency [43]. Adipic acid is also a microbial metabolite found in Escherichia [44]. However, to date, it has not been possible to ascertain whether increased adipic and suberic acids may be involved in the pathogenesis or symptomatology of ASDs in children. Whether dicarboxylic acids cross the blood-brain barrier and disturb the central nervous system in ASD children remains to be established [40].
Indole acetic acid was also elevated in our ASD group, as in a previous study using hydrophilic interaction chromatography (HILIC)-UHPLC and mass spectrometry approach [35]. It is an intermediate metabolite of tryptophan metabolism, which in turn is a precursor of serotonin. Bacterial species (Clostridia species, Escherichia coli, Proteus Vulgaris, Paracolobactrum coliform, Achromobacter liquefaciens, and Bacteroides spp) express tryptophanase which is responsible for transforming tryptophan into indole derivatives (indolyl 3-acetic acid and indolyl lactate) [38, 45]. There is increasing evidence of a brain-gut–microbe connection, and their regular cross-communications [45]. Many children with ASD generally suffer from gastrointestinal (GI) symptoms that can be comparable to those of irritable bowel syndrome but the exact prevalence of GI symptoms in autism is not known, with estimates ranging widely from 9 to 70% [45,46,47]. Studies have also shown a strong correlation between GI dysfunction and autism severity, across all domains including speech, social, and behavior [45, 47]. In one study, children with regressive autism were treated with a 6-week course of oral vancomycin, an antibiotic active against clostridia. Significant improvement in neurobehavioral and GI symptoms was noted in eight of the ten children studied providing evidence in favor of a toxin-producing Clostridium as a potential cause of regressive autism [47]. Daneberger et al. reported that children with ASD showed changes in urinary organic acid spectra which could be useful as biomarkers for indicating specific changes in the gut microbiota [48].
Our study also showed significant differences in the level of homovanillic acid (an intermediate metabolite of dopamine metabolism) between the ASD and TD groups, consistent with studies done by Federica Gevi et.al [34]. This finding suggests potential dopaminergic abnormalities in the investigated ASD group, which can explain disturbances of functioning of the dopaminergic system (repeated behaviors, mood disorders, aggression attacks, and disorders of social relationships) noted in children with ASD [1, 34, 49]. Urinary homovanillic acid correlated with impairment in neurobehavioral function assessed by the World Health Organization Neurochemical Core Test battery [50]. In support of this finding, studies have shown that vitamin B6 supplementation reduced the concentrations of urinary homovanillic acid and significantly reduced neurological symptoms including improvements in learning skills, reduction in hyperactivity and seizures [51,52,53].
The current study has some limitations. This was a single centered study and the number of enrolled ASD, and TD children was small, which is not helpful in creating a definitive model for ASD prediction using urine organic acid.. Second gastrointestinal disorders and dietary habits were not evaluated by standardized specific tools in ASD children. In this study following confounding factors such as dietary intake and environmental factors in both children with ASD and TD children were not controlled. Subgroup analysis could not be done for the severity of ASD, symptomatology, ethnicity, cognitive level, to explain the pathogenesis of ASD more extensively. There is a need to conduct similar metabolomic studies on larger sample size focusing on the correlation of metabolomic profiles and ASD phenotypic changes and severity.
Conclusion
The early diagnosis and therapeutic intervention improve long-term outcomes in ASD. Our findings open new perspectives for a better understanding of the correlation between the clinical phenotype of children with ASD and their urine metabolome. Concentration (as evident by height and number of peaks) of aconitic acid, succinic acid, citric acid, indole acetic acid, palmitic acid, suberic acid, lactic acid, 2-ketoglutaric acid, adipic acid, hippuric acid, 5-hydroxymethyl-2-furoic acid, and pimelic acid was significantly higher in ASD as compared to TD children. However, the five organic acids that were good discriminators between ASD and TD groups were 3-hydroxy isovaleric acid, homovanillic acid, adipic acid, suberic acid, and indole acetic. Further larger metabolomic studies are required to study the etiology of ASD and to find the accurate diagnostic biomarker for this complex disorder.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ASD:
-
Autism spectrum disorders
- TD:
-
Typically developing
- DSM-5 criteria:
-
Diagnostic and Statistical Manual of Mental Disorders 5th edition
- BGL:
-
Biochemical Genetics Laboratory
- CAP:
-
College of American Pathologists
- GC-MS:
-
Gas Chromatography-Mass Spectrometry
- ERNDRIM:
-
European Research Network for Evaluation and Improvement of Screening, Diagnosis, and Treatment of Inherited Disorders of Metabolism
References
Kałuzna-Czaplińska J, Socha E, Rynkowski J. Determination of homovanillic acid and vanillylmandelic acid in urine of autistic children by gas chromatography/mass spectrometry. Med Sci Monit. 2010;16(9):Cr445–50.
Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(Suppl 1):S55–65.
Kerub O, Haas E, Menashe I, Davidovitch N, Meiri G. Autism Spectrum disorder: evolution of disorder definition, risk factors and demographic characteristics in Israel. Isr Med Assoc J. 2018;20(9):576–81.
Kałużna-Czaplińska J, Żurawicz E, Struck W, Markuszewski M. Identification of organic acids as potential biomarkers in the urine of autistic children using gas chromatography/mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2014;966:70–6.
Baj J, Flieger W, Flieger M, Forma A, Sitarz E, Skorzynska-Dziduszko K, et al. Autism spectrum disorder: trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci Biobehav Rev. 2021;129:117–32.
Dolen G, Sahin M. Editorial: essential pathways and circuits of autism pathogenesis. Front Neurosci. 2016;10:182.
Dong D, Zielke HR, Yeh D, Yang P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. 2018;11(7):1076–90.
Rylaarsdam L, Guemez-Gamboa A. Genetic causes and modifiers of autism Spectrum disorder. Front Cell Neurosci. 2019;13:385.
Chang Y, Kim B, Youn M. Changes in children with autism Spectrum disorder after Theraplay application. Soa Chongsonyon Chongsin Uihak. 2021;32(3):112–7.
Kałużna-Czaplińska J, Zurawicz E, Jóźwik J. Chromatographic techniques coupled with mass spectrometry for the determination of organic acids in the study of autism. J Chromatogr B Anal Technol Biomed Life Sci. 2014;964:128–35.
Arinda A, Nakasujja N, Odokonyero R. Prevalence of autism spectrum disorder symptoms in a paediatric neurology clinic at a tertiary hospital in Uganda. S Afr J Psychiatry. 2021;27:1548.
Blaxill M, Rogers T, Nevison C. Autism tsunami: the impact of rising prevalence on the societal cost of autism in the United States. J Autism Dev Disord. 2021.
Brondino N, Bertoglio F, Forneris F, Faravelli S, Borghesi A, Damiani S, et al. A pilot study on Covid and autism: prevalence, clinical presentation and vaccine side effects. Brain Sci. 2021;11(7).
Sabbagh HJ, Al-Jabri BA, Alsulami MA, Hashem LA, Aljubour AA, Alamoudi RA. Prevalence and characteristics of autistic children attending autism centres in 2 major cities in Saudi Arabia: a cross-sectional study. Saudi Med J. 2021;42(4):419–27.
Sun X, Allison C, Matthews FE, Sharp SJ, Auyeung B, Baron-Cohen S, Brayne C. Prevalence of autism in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. Molecular autism. 2013;4(1):1-3.
Fuentes J, Basurko A, Isasa I, Galende I, Muguerza MD, Garcia-Primo P, et al. The ASDEU autism prevalence study in northern Spain. Eur Child Adolesc Psychiatry. 2021;30(4):579–89.
Wang F, Lu L, Wang SB, Zhang L, Ng CH, Ungvari GS, Cao XL, Lu JP, Hou CL, Jia FJ, Xiang YT. The prevalence of autism spectrum disorders in China: a comprehensive meta-analysis. International journal of biological sciences. 2018;14(7):717.
Khalid M, Raza H, M Driessen T, J Lee P, Tejwani L, Sami A, Nawaz M, Mehmood Baig S, Lim J, Kaukab Raja G. Genetic risk of autism spectrum disorder in a Pakistani population. Genes. 2020;11(10):1206.
Durkin MS, Wolfe BL. Trends in autism prevalence in the U.S.: a lagging economic Indicator? J Autism Dev Disord. 2020;50(3):1095–6.
Zhang ZC, Han J. The first National Prevalence of autism Spectrum disorder in China. Neurosci Bull. 2020;36(9):959–60.
Delobel-Ayoub M, Saemundsen E, Gissler M, Ego A, Moilanen I, Ebeling H, et al. Prevalence of autism Spectrum disorder in 7-9-year-old children in Denmark, Finland, France and Iceland: a population-based registries approach within the ASDEU project. J Autism Dev Disord. 2020;50(3):949–59.
Honda H, Shimizu Y, Misumi K, Niimi M, Ohashi Y. Cumulative incidence and prevalence of childhood autism in children in Japan. Br J Psychiatry. 1996;169(2):228–35.
Li J, You Y, Yue W, Jia M, Yu H, Lu T, et al. Genetic evidence for possible involvement of the Calcium Channel gene CACNA1A in autism pathogenesis in Chinese Han population. PLoS One. 2015;10(11):e0142887.
Furrukh J, Anjum G. Coping with Autism spectrum disorder (ASD) in Pakistan: A phenomenology of mothers who have children with ASD. Cogent Psychology. 2020;7(1):1728108.
Nadeem G, Bibi A, Suhaib B, Ahmed S, Ali S. Clinical and demographic features of 76 children with autism spectrum disorders at a centre in Pakistan. Journal of the College of Physicians and Surgeons Pakistan. 2019;29(4):390-1.
Minhas A, Vajaratkar V, Divan G, Hamdani SU, Leadbitter K, Taylor C, Aldred C, Tariq A, Tariq M, Cardoza P, Green J. Parents’ perspectives on care of children with autistic spectrum disorder in South Asia–Views from Pakistan and India. International Review of Psychiatry. 2015;27(3):247-56.
Jones PM, Bennett MJ. Urine organic acid analysis for inherited metabolic disease using gas chromatography-mass spectrometry. Methods Mol Biol. 2010;603:423–31.
Goldani AA, Downs SR, Widjaja F, Lawton B, Hendren RL. Biomarkers in autism. Frontiers in psychiatry. 2014;5:100.
Kumps A, Duez P, Mardens Y. Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: a comprehensive table. Clin Chem. 2002;48(5):708–17.
Page TJJoa, Disorders d. metabolic approaches to the treatment of autism spectrum disorders. 2000;30(5):463–469.
Manzi B, Loizzo AL, Giana G, Curatolo P. Autism and metabolic diseases. Journal of child neurology. 2008;23(3):307-14.
Noto A, Fanos V, Barberini L, Grapov D, Fattuoni C, Zaffanello M, et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J Matern Fetal Neonatal Med. 2014;27(Suppl 2):46–52.
Kałużna-Czaplińska J, Socha E, Rynkowski JJNr. B vitamin supplementation reduces excretion of urinary dicarboxylic acids in autistic children. 2011;31(7):497–502.
Gevi F, Belardo A, Zolla L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165859.
Gevi F, Zolla L, Gabriele S, Persico AM. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Molecular autism. 2016;7(1):1-1.
Padulo J, Oliva F, Frizziero A, Maffulli N. Basic principles and recommendations in clinical and field science research: 2018 update; 2018.
Coury DL. DSM-5 and autism spectrum disorders: implications for families and clinicians. J Dev Behav Pediatr. 2013;34(7):494–6.
Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9(6):2996–3004.
Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–54.
Puig-Alcaraz C, Fuentes-Albero M, Cauli O. Relationship between adipic acid concentration and the core symptoms of autism spectrum disorders. Psychiatry Res. 2016;242:39–45.
Vianey-Liaud C, Divry P, Gregersen N, Mathieu M. The inborn errors of mitochondrial fatty acid oxidation. J Inherit Metab Dis. 1987;10(Suppl 1):159–200.
Gong Z, Liang L, Qiu W, Zhang H, Ye J, Wang Y, et al. Clinical, biochemical, and molecular analyses of medium-chain acyl-CoA dehydrogenase deficiency in Chinese patients. Front Genet. 2021;12:577046.
Chapel-Crespo C, Gavrilov D, Sowa M, Myers J, Day-Salvatore DL, Lynn H, et al. Clinical, biochemical and molecular characteristics of malonyl-CoA decarboxylase deficiency and long-term follow-up of nine patients. Mol Genet Metab. 2019;128(1–2):113–21.
Yu JL, Xia XX, Zhong JJ, Qian ZG. Direct biosynthesis of adipic acid from a synthetic pathway in recombinant Escherichia coli. Biotechnol Bioeng. 2014;111(12):2580–6.
Ding HT, Taur Y. Walkup JTJJoa, disorders d. Gut Microbiota Autism Key Concepts Find. 2017;47(2):480–9.
de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–24.
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22.
Daneberga Z, Nakazawa-Miklasevica M, Berga-Svitina E, Isarova D, Cupane L, Masinska M, Nartisa I, Lazdane A, Miklasevics E. Urinary organic acids spectra in children with altered gut microbiota composition and autistic spectrum disorder. Nordic Journal of Psychiatry. 2021;1-7.
Kałużna-Czaplińska J. Noninvasive urinary organic acids test to assess biochemical and nutritional individuality in autistic children. Clin Biochem. 2011;44(8–9):686–91.
Pavăl D. A dopamine hypothesis of autism spectrum disorder. Developmental neuroscience. 2017;39(5):355-60.
Lelord G, Callaway E, Muh JP, Arlot JC, Sauvage D, Garreau B, et al. Modifications in urinary homovanillic acid after ingestion of vitamin B6; functional study in autistic children (author's transl). Rev Neurol. 1978;134(12):797–801.
Nye C, Brice AJCDoSR. Combined vitamin B6-magnesium treatment in autism spectrum disorder, vol. 4; 2005.
Khan F, Rahman MS, Akhter S, Momen AB, Raihan SG. Vitamin B6 and magnesium on neurobehavioral status of autism spectrum disorder: a randomized, double-blind, placebo controlled study. Bangladesh Journal of Medicine. 2021;32(1):12-8.
Acknowledgments
The authors express their gratitude to all enclosed subjects in this study.
Funding
The study was supported by a Resident Research Grant from the Department of Pathology & Laboratory Medicine, Aga Khan University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design, analysis, and writing of this manuscript in a significant and meaningful manner. LJ, HM and ZNK originated the hypotheses and study design, literature search, performed analyses and interpreted the result. PC have contributed in identifying children with ASD. KA, AW and SE aided in providing samples, ethical approval and statistical analysis and interpreted the data, AH and SA have contributed to discussion and have taken part in the drafting of the final document. All authors edited, revised, and approved the final version of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethical committee at Aga Khan University Hospital, Karachi. Written informed consent was signed by the parents or guardians of the children. The study is fully complaint with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khan, Z.U.N., Chand, P., Majid, H. et al. Urinary metabolomics using gas chromatography-mass spectrometry: potential biomarkers for autism spectrum disorder. BMC Neurol 22, 101 (2022). https://doi.org/10.1186/s12883-022-02630-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02630-4