Abstract
Background
A fraction of patients with penetrating artery infarction (PAI) experience progressive motor deficit deterioration (PMD). We sought to investigate the role of high-sensitivity C-reactive protein (hs-CRP) at admission in predicting PMD.
Methods
From January 2015 to September 2018, consecutive patients with PAI from three centers were prospectively enrolled in this study. PMD was defined as worsening of motor function score by ≥1 point on the National Institutes of Health Stroke Scale during the first 5 days after admission. Multivariable logistic regression analyses were performed to explore the relationship between hs-CRP and PMD in patients with PAI. We also performed receiver operating characteristic curve analysis and constructed a nomogram to assess the overall discriminative ability of hs-CRP in predicting PMD.
Results
We ultimately included 544 patients (mean age, 65.4 ± 11.8 years). A total of 85 (15.6%) patients were identified to have PMD. Multivariate logistic regression analysis showed that hs-CRP was independently associated with PMD (P = 0.001). The optimal cutoff value for hs-CRP as a predictor for PMD was 3.48 mg/L, with a sensitivity of 73.64% and a specificity of 82.35% (area under curve, 0.792). Moreover, the nomogram we constructed indicated that higher level of hs-CRP was an indicator of PMD (c-index = 0.780, P < 0.001).
Conclusions
Our study suggested that hs-CRP might be a useful biomarker for predicting the risk of PMD in patients with PAI.
Similar content being viewed by others
Introduction
Ischemic stroke is one of the leading causes of mortality and disability worldwide [1,2,3]. Progressive motor deficit (PMD) is one of the most common neurological deterioration during the acute stage of penetrating artery infarction (PAI), which accounts for almost 25% of all ischemic stroke [4]. The incidence of PMD ranges from 13 to 38% in patients with PAI [5,6,7,8]. Several reports have shown that PMD is also associated with poor prognosis of PAI [9,10,11]. Although several biomarkers [4, 12] have been identified in previous studies, PMD remains insidious and largely unpredictable in clinical practice. Therefore, exploration of the potential mechanisms and measurable biomarkers of PMD among patients with PAI is important.
Neuroinflammatory processes play a fundamental role in the acute stage of ischemic stroke [13,14,15]. Several inflammatory biomarkers was reported to be correlated with neurological deterioration in patients with acute ischemic stroke, such as lipoprotein-associated phospholipase A2, [16]. neutrophil–lymphocyte ratio [17] and so on. Previous studies have revealed that high-sensitivity C-reactive protein (hs-CRP) may act as an inflammatory factor that responds to ischemic stroke [18, 19]. A high hs-CRP level has been found to show predictive value for poststroke depression [18] and poor outcome [19,20,21,22] in ischemic stroke patients. The levels of hs-CRP may be associated with the risk of excessive ischemic stroke independently [23]. However, there are few studies that focused on the clinical value of hs-CRP in patients with PAI. The association between hs-CRP and PMD in ischemic stroke remains unclear. Thus, the purpose of this tricenter observational study was to assess the association between hs-CRP levels at admission and PMD in patients with PAI.
Methods
Patient selection
Consecutive patients who presented with symptoms of a lacunar syndrome between January 2015 and September 2018 underwent a standard in-house procedure [24, 25] and prospectively recruited from three hospitals. All the patients were treated in the stroke units and received treatments, such as antiplatelet therapy statin therapy and risk factor management. Magnetic resonance (MR), computed tomography, electrocardiogram, echocardiography, carotid ultrasonography and transcranial Doppler, and were performed for assessing the stroke etiology. Eligible patients were included in the present analysis if they met the following criteria.
The inclusion criteria were as follows:
- (1)
admission within 24 h of onset with a lacunar syndrome;
- (2)
patients with penetrating artery infarctions;
- (3)
age more than 18 years.
The exclusion criteria were as follows:
- (1)
Patients who had a potential source of cardioembolism or >50% stenosis of the extracranial carotid artery;
- (2)
severe inflammatory diseases or infectious diseases;
- (3)
lack of motor deficits, such as patients with pure sensory syndrome;
- (4)
renal failure or hepatic failure;
- (5)
medical history of Parkinson’s disease or other dyskinesia;
- (6)
the neurological deficits of patients cannot be evaluated over the following 5 days after admission.
Vascular risk factors
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg/or diastolic blood pressure (DBP) ≥90 mmHg or use of antihypertensive medication within 2 weeks. SBP and DBP were measured and recorded soon after admission. Diabetes mellitus was defined as either a fasting blood glucose (FBG) level > 7.0 mmol/L on more than two occasions or the use of an antidiabetic medication. Dyslipidemia was defined as a total cholesterol level > 5.70 mmol/L and/or a triglyceride level > 5.18 mmol/L on more than two occasions or the use of lipid-lowering agents. Current smoking and drinking habits were defined as regular smoking and/or drinking at the time of stroke, respectively.
MR imaging
All participants underwent MR imaging (MRI) and MR angiography (MRA). MRI scans were performed with 3.0-T superconducting magnets. Intracranial artery vessels, including the middle cerebral arteries (MCAs) and vertebrobasilar arteries (VBAs), were assessed by MRA. The severity of stenosis in each intracranial artery was graded based on maximal luminal narrowing according to the following criteria: normal, mild stenosis (< 50%) and moderate or severe stenosis (50% or more).
The severity of carotid artery atheromatosis was graded based on the examination results of carotid ultrasonography, which was divided into the following three categories: absence, moderate (< 70%) and severe (70% or more).
Definition of penetrating artery infarction and progressive motor deficit
PAI was defined as a relevant deep, single hyperintensity in the territory of penetrating arteries 20 mm or less in diameter on axial slices of an MRI with diffusion-weighted imaging (DWI) that corresponded to one of the lacunar syndromes during a patient’s presentation in the acute phase.
The evaluation of neurological deficits was conducted using the National Institutes of Health Stroke Scale (NIHSS) score on admission and continued over the following 5 days 2–3 times every day after admission by two certified neurologists blind to clinical information.
PMD was defined as worsening of motor function by ≥1-point on the motor section of NIHSS during the first 5 days after admission [12, 26,27,28].
White matter lesions were defined as diffuse hyperintensities that were located in the subcortical and periventricular white matter on T2-weighted images and proton density images. Silent lacunar infarcts were defined as penetrating artery occlusions 3 to 15 mm in diameter in horizontal sections with high intensity on both T2-weighted images and DWI.
Masurement of hs-CRP
All the blood samples were collected at 7 AM the second day after admission. The levels of hs-CRP were measured with an immunoturbidimetry assay on an Architect c16000 chemistry analyzer (Abbott Diagnostics, Abbott Park, USA).
Statistical analysis
Statistical analyses were performed with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Continuous variables that followed a normal distribution were expressed as the mean ± standard deviation; other continuous variables that did not follow normal distributions were presented as the median and interquartile range (25th to 75th percentile). Categorical variables were expressed as constituent ratios. Differences in baseline characteristics among the hs-CRP quartiles were tested using analysis of variance or the Kruskal-Wallis test for continuous variables, and Pearson’s chi-square test for categorical variables. We also used binary logistic regression analysis to detect the risk factors for PMD. Multivariable analysis was adjusted for all potential confounders with a statistically significant association at P < 0.05 in univariate regression analysis. Receiver operating characteristic (ROC) curve analysis was performed to assess the overall discriminative ability of hs-CRP to predict PMD and to establish optimal cutoff points at which the sum of the specificity and sensitivity was the highest. A MedCalc 15.6.0 (MedCalc Software Acacialaan 22, B-8400 Ostend, Belgium) packet program was used to obtain the ROC curve and to analyze specify and sensitivity of hs-CRP for the exitus status. In addition, a nomogram based on the independent predictors was constructed by R software with the package rms. The predictive capacity of the nomogram was determined by Harrell’s c-index. A two-tailed value of P < 0.05 was considered significant.
Results
From January 2015 to September 2018, 642 patients with acute PAI who were admitted within 24 h of stroke onset were screened for 5 days in this study (Fig. 1). Thirty-one patients’ neurological deficits could not be evaluated over the following 5 days after admission. Sixty-eight patients were excluded for the following reasons: other causes of infarction (n = 24), severe inflammatory or infectious diseases (n = 11), lack of motor deficits, such as patients with pure sensory syndrome (n = 32), hepatic failure (n = 9), renal failure (n = 10), medical history of Parkinson’s disease or other dyskinesia (n = 12). A total of 544 subjects (387 men; mean age, 65.4 ± 11.8 years) were included in the final analysis (Fig. 1). PMD was observed in 85 patients (15.6%).
A comparison of the baseline characteristics of the groups with and without PMD are presented in Table 1. The PMD group had significantly higher levels of hs-CRP than the non-PMD group (5.9 [4.0, 19.8] versus 2.0 [1.3, 3.8], P < 0.001).
The median hs-CRP was 6.46 mg/L, with quartile levels as follows: 0.18 mg/L to 1.28 mg/L (first quartile); 1.28 mg/L to 2.33 mg/L (second quartile); 2.35 mg/L to 5.19 mg/L (third quartile); 5.26 mg/L to 293.00 mg/L (fourth quartile). Baseline characteristics of the study population according to hs-CRP quartiles are provided in Table 2. The results showed that increased hs-CRP was significantly related to PMD in patients with acute PAI (P = 0.001).
Table 3 shows the results of logistic regression analysis for risk factors of PMD. Univariable logistic regression analysis was used to investigate the significance of variables on predicting PMD in patients with PAI. Univariate logistic regression analyses demonstrated that the third quartile of hs-CRP, the fourth quartile of hs-CRP, age, diabetes mellitus, and levels of FBG and glycated hemoglobin were associated with PMD (P < 0.05). Significant predictors in the univariable analysis were included in a multivariable regression model to determine independent predictors. After adjusting for all potential confounders, age, glycated hemoglobin level and the third quartile and fourth quartile of hs-CRP (first quartile used as the reference value) were identified as independent predictors for PMD.
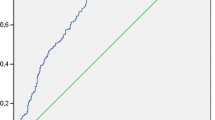
To further assess the clinical significance of hs-CRP in PMD, we performed a ROC curve analysis as depicted in Fig. 2. We observed that the area under curve (AUC) of hs-CRP was 0.792 (95% CI, 0.756–0.826) with the ability to discriminate PMD. The optimal cutoff value for hs-CRP as a predictor of PMD was determined to be 3.48 mg/L in the ROC curve analysis, yielding the largest Youden’s index value (a sensitivity of 73.64% and a specificity of 82.35%). The AUC was 0.792 (95% CI, 0.756–0.826).
The nomogram is shown in Fig. 3, and the concordance index of this model was 0.780 (P < 0.001). These findings were similar to those obtained previously in the multivariate logistic models.
Nomograms of patients with PAI for predicting PMD. Each factor was given a point on the basis of the nomograms. The final total points were obtained by adding the individual score of each of the 3 risk factors. The estimated probability of PMD of the individual patient with PAI can be easily obtained from the nomogram based on the total points
Discussion
Our observational study revealed that elevated plasma levels of hs-CRP remained an independent predictor for PMD in patients with PAI after adjusting for age, diabetes mellitus and other possible confounders. In general, a biomarker with 0.7 < area under the curve < 0.9 indicates a moderate diagnostic value. High hs-CRP levels (> 3.48 mg/L) have a moderate ability to diagnose PMD. Furthermore, our constructed nomogram indicated that higher hs-CRP was an indicator of PMD. Thus, the hs-CRP value at admission represented a readily available predictor for PMD in patients with PAI. The serum biomarker, hs-CRP at admission, is able to identify earlier than the standard clinical and imaging assessment. Furthermore, our study also showed that age and glycated hemoglobin were predictors of PMD, which was consistent with the findings of other studies [19, 28].
The influence of hs-CRP on ischemic stroke has been well established, and hs-CRP has been reported as a predictor of disease severity, prognosis and mortality in patients with ischemic stroke [19, 29]. Furthermore, high plasma hs-CRP levels are associated with clinical complications following acute ischemic stroke [18, 30] PMD, which may result in severe morbidity, commonly occurs in patients with PAI during the acute stage. This is the first study to explore the relationship between hs-CRP and PMD in patients with penetrating artery ischemic stroke. PMD was revealed to have an incidence of 15.6% in this trial, which was in accordance with a previous study [19, 28]. Moreover, in a previous case presentation, the patient with PMD was found to be complicated by depressive disorder and anxiety disorder [31]. Our observational study showed the predictive value of hs-CRP for the occurrence of PMD in patients with PAI. It provides a biomarker for early detection of PMD.
Hs-CRP, a systemic inflammatory marker, is produced in large amounts by hepatocytes in response to IL-1, IL-6 and TNF-α [32, 33]. Inflammatory responses play a vital role in ischemic stroke [13,14,15, 34, 35]. The ischemic tissues release inflammatory cytokines and chemokines, among which hs-CRP is one of the mediators of ischemic brain injury. Cytokines and inflammatory factors lead to neuronal necrosis, endothelial permeability of vessels and blood-brain barrier disruption, resulting in the mortality of neurons and induction of apoptosis [34, 35]. Hence, PMD is believed to result from biochemical abnormalities such as inflammation.
However, several limitations should be considered. First, the sample size of our study was relatively small, and larger cohorts of subjects are needed. Second, we did not investigate dynamic changes in hs-CRP; the combination of baseline and dynamic hs-CRP may provide a more objective and comprehensive way to predict PMD in PAI patients. Third, we only performed digital subtraction angiography (DSA) for a limited number of patients. The severity of stenosis in each intracranial artery could only be assessed by by MRA instead of DSA,which may not be the most precise. Moreover, we did not perform the plaque imaging to evaluate carotid artery atheromatosis, which might be a factor that is related to inflammation process. Finally, many factors that might affect inflammatory markers were not taken into consideration.
Conclusion
In summary, based on the conclusion of our study, hs-CRP levels are able to serve as a useful noninvasive biomarker for the assessment of PMD. The association between hs-CR and PMD should be considered in the management of PAI.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under curve
- DWI:
-
Diffusion-weighted imaging
- FBG:
-
Fasting blood glucose
- hs-CRP:
-
High-sensitivity C-reactive protein
- MCA:
-
Middle cerebral artery
- MR:
-
Magnetic resonance
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- NIHSS:
-
National Institutes of Health Stroke Scale
- PAI:
-
Penetrating artery infarction
- PMD:
-
Progressive motor deficit
- ROC:
-
Receiver operating characteristic
- VBA:
-
Vertebrobasilar arteries
References
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–54.
Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–5.
Deng QW, Li S, Wang H, Lei L, Zhang HQ, Gu ZT, et al. The short-term prognostic value of the triglyceride-to-high-density lipoprotein cholesterol ratio in acute ischemic stroke. Aging Dis. 2018;9(3):498–506.
Kawano T, Miyashita K, Takeuchi M, Nagakane Y, Yamamoto Y, Kamiyama K, et al. Blood biomarkers associated with neurological deterioration in patients with acute penetrating artery territory infarction: a multicenter prospective observational study. Int J Stroke. 2018;13(2):207–16.
Yu YP, Tan L. The infarct shape predicts progressive motor deficits in patients with acute lacunae-sized infarctions in the perforating arterial territory. Intern Med. 2015;54:2999–3004.
Oh S, Bang OY, Chung CS, Lee KH, Chang WH, Kim GM. Topographic location of acute pontine infarction is associated with the development of progressive motor deficits. Stroke. 2012;43:708–13.
Kim YB, Moon HS, Suh BC, Park KY, Lee YT, Chung PW. Topographic patterns and stroke subtypes according to progressive motor deficits in lacunar syndrome. J Stroke Cerebrovasc Dis. 2011;20:352–6.
Serena J, Leira R, Castillo J, Pumar JM, Castellanos M, Dávalos A. Neurological deterioration in acute lacunar infarctions: the role of excitatory and inhibitory neurotransmitters. Stroke. 2001;32:1154–61.
Li JB, Cheng RD, Zhou L, Wen WS, Zhu GY, Tian L, et al. What drives progressive motor deficits in patients with acute pontine infarction? Neural Regen Res. 2015;10(3):501–4.
Dávalos A, Cendra E, Teruel J, Martinez M, Genís D. Deteriorating ischemic stroke: risk factors and prognosis. Neurology. 1990;40:1865–9.
Erro ME, Gállego J, Herrera M, Bermejo B. Isolated pontine infarcts: etiopathogenic mechanisms. Eur J Neurol. 2005;12:984–8.
Yamamoto Y, Ohara T, Hamanaka M, Hosomi A, Tamura A, Akiguchi I, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci. 2010;288:170–4.
Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. 2016;25:241–51.
Wu D, Zhi X, Duan Y, Zhang M, An H, Wei W, et al. Inflammatory cytokines are involved in dihydrocapsaicin (DHC) and regional cooling infusion (RCI)-induced neuroprotection in ischemic rat. Brain Res. 1710;2019:173–80.
Anrather J, Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13:661–70.
Wang Y, Hu S, Ren L, Lei Z, Lan T, Cai J, et al. Lp-PLA2 as a risk factor of early neurological deterioration in acute ischemic stroke with TOAST type of large arterial atherosclerosis. Neurol Res. 2019;41(1):1–8.
Gong P, Xie Y, Jiang T, Liu Y, Wang M, Sun H, et al. Neutrophil-lymphocyte ratio predicts post-thrombolysis early neurological deterioration in acute ischemic stroke patients. Brain Behav. 2019;9(10):e01426.
Tang CZ, Zhang YL, Wang WS, Li WG, Shi JP. Serum levels of high-sensitivity C-reactive protein at admission are more strongly associated with Poststroke depression in acute ischemic stroke than Homocysteine levels. Mol Neurobiol. 2016;53(4):2152–60.
Cai Z, He W, Zhuang FJ, Chen Y. The role of high high-sensitivity C-reactive protein levels at admission on poor prognosis after acute ischemic stroke. Int J Neurosci. 2019;129:423–9.
Wu X, Liu G, Zhou W, Ou A, Liu X, Wang Y, et al. Outcome prediction for patients with anterior circulation acute ischemic stroke following endovascular treatment: a single-center study. Exp Ther Med. 2019;18(5):3869–76.
Gao Y, Liu J, Wang W, Gao C, Yu C, Liu S, et al. An elevated high-sensitivity C-reactive protein level is associated with unfavorable functional outcomes of Small-artery occlusion in patients without diabetes. Eur Neurol. 2017;78(1–2):48–55.
Hou D, Liu J, Feng R, Gao Y, Wang Y, Wu J. The role of high-sensitivity C-reactive protein levels in functional outcomes in patients with large-artery atherosclerosis and small-artery occlusion. Neurol Res. 2017;39(11):981–7.
Zhou Y, Han W, Gong D, Man C, Fan Y. Hs-CRP in stroke: a meta-analysis. Clin Chim Acta. 2016;453:21–7.
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early Management of Patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–35.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early Management of Patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–99.
Kim SK, Song P, Hong JM, Pak CY, Chung CS, Lee KH, et al. Prediction of progressive motor deficits in patients with deep subcortical infarction. Cerebrovasc Dis. 2008;25:297–303.
Audebert HJ, Pellkofer TS, Wimmer ML, Haberl RL. Progression in lacunar stroke is related to elevated acute phase parameters. Eur Neurol. 2004;5:125–31.
Kim YS, Lee KY, Koh SH, Park CY, Kim HY, Lee YJ, et al. The role of matrix metalloproteinase 9 in early neurological worsening of acute lacunar infarction. Eur Neurol. 2006;55:11–5.
Yu H, Huang Y, Chen X, Nie W, Wang Y, Jiao Y, et al. High-sensitivity C-reactive protein in stroke patients - the importance in consideration of influence of multiple factors in the predictability for disease severity and death. J Clin Neurosci. 2017;36:12–9.
Martin AJ, Price CI. A Systematic Review and Meta-Analysis of Molecular Biomarkers Associated with Early Neurological Deterioration Following Acute Stroke. Cerebrovasc Dis. 2018;46:230–41.
Small G. Progressive motor deficits and psychosis after stroke: a case presentation. J Neurosci Nurs. 2016;48(2):68–70.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97.
Butterweck V, Prinz S, Schwaninger M. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav Brain Res. 2003;144:49–56.
Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in schaemic stroke. Nat Rev Neurol. 2016;12:594–604.
Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2009;147:S232–40.
Acknowledgements
Not applicable.
Funding
This work was supported by the Major Program of Nanjing Medical Science and Technology Development Foundation (ZDX16002), the Key Program of Nanjing Medical Science and Technology Development Foundation (ZKX16050) and the Ordinary Program of Nanjing Medical Science and Technology Development Foundation (YKK16142). The the role of the funding bodies is data collection and document retrieval.
Author information
Authors and Affiliations
Contributions
Data curation, TH, WC, YG and XZ; Formal analysis, XZ; Investigation, YL and MW; Methodology, TJ, ML and YZ; Writing original draft, PG; Writing review and editing, QD and JZ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Patients were prospectively recruited from Nanjing First Hospital, the Second Affiliated Hospital of Nanjing University of Chinese Medicine and Nantong Third People’s Hospital. Eligible patients were included in the present analysis if they met the following criteria. Protocols were approved by the Ethical Committee of Nanjing First Hospital, the Ethical Committee of the Second Affiliated Hospital of Nanjing University of Chinese Medicine and the Ethical Committee of Nantong Third People’s Hospital. Written Informed consent for this study was obtained from all patients or their family members.
Consent for publication
Not Applicable..
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gong, P., Liu, Y., Huang, T. et al. The association between high-sensitivity C-reactive protein at admission and progressive motor deficits in patients with penetrating artery infarctions. BMC Neurol 19, 346 (2019). https://doi.org/10.1186/s12883-019-1538-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-019-1538-5