Abstract
Background
In neuromyelitis optica (NMO), one of the underlying pathogenic mechanisms is the formation of antigen-antibody complexes which can trigger an inflammatory response by inducing the infiltration of neutrophils in lesions. Epithelial neutrophil-activating peptide 78 (ENA 78), known as Chemokine (C-X-C motif) ligand 5 (CXCL5), belongs to the ELR-CXCL family. It recruits and activates neutrophils. The aim of this study was to evaluate ENA 78, IL-1β and TNF-α plasma levels in multiple sclerosis (MS) and neuromyelitis optica (NMO) patients.
Methods
ENA 78, IL-1β and TNF-α plasma levels were detected in 20 healthy controls (HC), 25 MS and 25 NMO patients using MILLIPLEX® map Human High Sensitivity Cytokine/Chemokine Panels.
Results
Plasma levels of ENA 78 were significantly higher in NMO patients than in HC (P < 0.001) and MS patients (P < 0.05). The NMO patients showed higher plasma levels of IL-1β compared with HC (P < 0.01). Further, increased plasma levels of TNF-α were found in the MS (P < 0.05) and NMO patients (P < 0.001). In addition, NMO patients had higher Expanded Disability Status Scale (EDSS) scores compared with MS patients (P < 0.05). EDSS scores were correlated with plasma levels of ENA 78 in NMO patients (P < 0.05). There were no significant correlations between EDSS scores and plasma levels of ENA 78 in MS patients (P > 0.05).
Conclusions
The overproduction of pro-inflammatory cytokines such as IL-1β and TNF-α during the remission of NMO activates ENA 78, which in turn leads to neutrophil infiltration in lesions. ENA 78 plasma levels were correlated with EDSS scores in NMO patients. Elevated secretion of ENA 78 may be a critical step in neutrophil recruitment during the remission of NMO.
Similar content being viewed by others
Background
Neuromyelitis optica (NMO, Devic’s syndrome) and multiple sclerosis (MS) are autoimmune and degenerative diseases characterized by demyelination of central nervous system (CNS), potentially leading to paralysis and other clinical symptoms [1–4]. NMO and MS are two of the most common diseases causing neurological disability in young adults [1, 5, 6]. Accumulating evidence has shown that NMO pathogenesis differs from MS, including aquaporin 4 (AQP4)-IgG increase and infiltration of granulocytes and macrophages [7, 8].
A significant feature distinguishing NMO from MS is the relatively higher number of neutrophils, eosinophils, macrophages and fewer T cells in the lesions [2, 8, 9]. Abnormal neutrophil aggregation in the lesions and increased AQP4-IgG are the notable features distinguishing NMO from MS [8, 10]. Neutrophil protease inhibition reduces AQP4-IgG damage in the mouse brain, which suggests that neutrophils play an important role in NMO pathology [7]. Studies also suggest a tight regulation of neutrophils and immune cell recruitment in NMO [2]. The innate immune response is orchestrated by inflammatory cells as a cascade of events, and each stage is associated with inflammatory cell recruitment and infiltration. Neutrophil infiltration is triggered by epithelial neutrophil-activating peptide 78 (ENA 78), which plays a role in tissue repair, metabolism, microbial killing, and angiogenesis [11, 12]. ENA 78 is a member of CXC chemokines, which enhance leukocyte recruitment and activation in autoimmune disorders and inflammatory diseases [13–15]. Its aberrant expression has been detected in rheumatoid arthritis, psoriasis, autism, bacterial meningitis, etc. [16–20]. ENA 78 is categorized into sub-classes based on the sequence and function, and characterized by the ELR (glutamic acid-leucine-arginine) motif preceding the N-terminal Cys and activating C-X-C chemokine receptor type (CXCR) 2 selectively [21]. ENA 78 as a potent ELR+ CXC chemokine attracts and activates polymorphonuclear leukocytes (PMNLs) which are higher in patients with infections, inasmuch as the PMNLs are among the first cells to exist the peripheral blood and migrate to the inflammatory site [16, 22, 23].
This study was the first to evaluate plasma levels of ENA 78 and its relation to Expanded Disability Status Scale (EDSS) scores in NMO patients.
Methods
Study populations
Written informed consent was obtained from all participants. The study protocol was approved by the local ethics committee (IRB of Beijing Tiantan Hospital Affiliated to Capital Medical University, No. KY2015-003-02). MS and NMO patients were recruited from Beijing Tiantan Hospital, Capital Medical University. This study was conducted between May and July 2015 on 20 healthy controls (HC), 25 patients with MS and 25 patients with NMO. Plasma samples were obtained from MS patients, NMO patients and 20 HC recruited from the general population without immune diseases. Infections were ruled out by full blood count in all subjects.
The MS diagnosis was determined according to the 2010 revised McDonald criteria [24] and the NMO diagnosis was based on the revised diagnostic criteria for NMO [25]. The interviews, neurological examinations and EDSS scores of the MS and NMO groups were conducted in an MS cohort study. All the NMO patients had optica neuritis and myelitis and met at least 2 of the 3 supporting criteria (Brain MRI-, AQP4-IgG+, negative for MS).
Plasma chemokine and cytokines levels
To exclude the effect of different time points and other factors on the level of chemokines and cytokines, all blood samples (2 mL each) were obtained at 9:00 a.m using disposable Ethylenediaminetetraacetic acid (EDTA) vacuum blood collection tubes (BD, USA) and tested over 8 h. After 2 h of standing at 4 °C, the supernatant was pipetted into EP tubes and stored at −80 °C. Plasma ENA 78, IL-1β and TNF-α were measured using MILLIPLEX® map Human High Sensitivity Cytokine/Chemokine Panels (Cat. No. HCYP2MAG-62 K; Cat. No. HCYTOMAG-60 K), according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software, Inc., California) and the data were reported as Means ± SEM. Mann–Whitney U and Kruskal-Wallis tests were used to compare 2 to 3 groups, respectively. Pearson’s test was used to perform correlations. A P value of < 0.05 was considered statistically significant.
Results
Clinical demographics
The subjects included 20 HC, 25 MS and 25 NMO patients. No significant age differences were found in the three groups. Age of onset, disease duration and annual relapse rate (ARR) of MS and NMO patients also showed no significant differences. All the subjects were aged between 10 and 60 years. There was a significant difference between the MS and NMO groups in EDSS scores (P < 0.05) (Table 1).
Increased plasma ENA 78, IL-1β and TNF-α levels in NMO patients
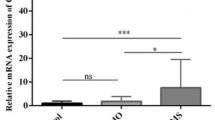
ENA 78 expression was higher in NMO plasma than in HC (P < 0.001) and MS (P < 0.05) (Fig. 1a). The cytokine IL-1β potentially induces ENA 78 secretion. As shown in (Fig. 1b), the IL-1β level in NMO was higher than in HC (P < 0.01) with no significant difference between MS and HC, MS and NMO. The cytokine TNF-α levels were higher in NMO than in HC (P < 0.001) (Fig. 1c).
Comparison of chemokine and cytokine levels among the HC, MS and NMO groups. HC = healthy controls; MS = multiple sclerosis; NMO = neuromyelitis optica. a Comparison of plasma ENA 78 levels among the HC, MS and NMO. b Comparison of plasma IL-1β level among the HC, MS and NMO. c Comparison of plasma TNF-α level among the HC, MS and NMO. *P < 0.05, **P < 0.01, ***P < 0.001
Correlation between ENA 78 gradients and EDSS scores in the MS and NMO groups
ENA 78 plasma levels were not correlated with EDSS scores in MS (Fig. 2a). Significant correlation existed between ENA 78 gradients and EDSS scores in NMO (P < 0.05) (Fig. 2b).
Discussion
The perivascular presence of neutrophils is one of the primary histological differences between MS and NMO, as reported in NMO patients as well as in mouse and rat models [2, 7, 26]. Neutrophils are elevated about 20 % in the CSF during remission of NMO patients [27]. In mouse models of NMO, tissue damage was ameliorated by eliminating neutrophils, whereas increased neutrophils exacerbated tissue damage [7, 28]. ENA 78 is one of the ELR+ chemokines specifically inducing neutrophil migration, with the ability to interact with chemokine receptors CXCR1 and CXCR2 [29, 30]. ENA 78 stimulates neutrophil directed chemotaxis by promoting the intracellular level of elastase and free calcium and inducing neutrophil pro-adhesive activity [31, 32]. In addition, inhibitors of neutrophil elastase, which are involved in neutrophil migration and neutrophil-mediated tissue damage, have been tested in experimental trials such as Sivelestat [7, 33–35]. Other studies also indicated that the increased ENA 78 amplified the pro-inflammatory cytokine response, which had a direct chemo-attracting effect on neutrophils [36–39]. Therefore, we studied ENA 78 and found that it was dramatically increased in the NMO patients (vs. HC, P < 0.001; vs. MS, P < 0.05). Studies proved that ENA 78 was detected in eosinophils, which also aggregated in the NMO lesions [2, 8, 40], suggesting that eosinophils recruit and activate CXCR2+ cells such as neutrophils by secreting ENA 78. In the present study, we found that the plasma ENA 78 gradient was correlated with EDSS in NMO patients rather than in MS (P < 0.05). ENA 78 causes neutrophil aggregation and hyperactivation around the lesions in NMO resulting in demyelination, which is different from the pathophysiological mechanisms of MS. The higher ENA 78 gradient in the blood leads to increased neutrophil aggregation around the lesions, causing severe clinical symptoms.
Although the precise mechanism involving ENA 78 upregulation is not fully understood, factors involved in modulating ENA 78 expression at the transcriptional level and signaling pathways are known in different types of cells [41–43]. Neutrophil chemoattractant chemokines belonging to ELR-CXCL family, especially ENA 78 binding with chemokine receptor CXCR2, mediate the IL-1β driven cell recruitment [43, 44]. ELR+ chemokines, including CXCL1, CXCL2 and ENA 78, are triggered by IL-1β [45–47]. IL-1β inducing ENA 78 expression by activating cAMP-response element binding protein (CREB) and NF-kB promoter of ENA 78 is part of the inflammatory response in vitro and in vivo [39, 43, 48–50]. IL-1β induces leukocyte rolling, adherence and emigration associated with an increase in kinin B1 receptor mRNA expression, which plays a role in neutrophil recruitment [44]. This current study results showed that increased IL-1β levels in NMO patients matched the higher ENA 78 levels in the periphery compared with the HC (P < 0.01). TNF-α, which induces neutrophil influx, exacerbates the lesions in ex vivo spinal cord and optical nerve of NMO [28, 51]. Our findings, herein, show that NMO and MS patients had higher plasma levels of TNF-α compared with HC (P < 0.001; P < 0.05, respectively). Further, TNF-α potentially increases the adhesion-molecule expression in the brain suggesting a role for anti-TNF therapies in NMO [8, 52, 53]. The overexpression of IL-1β and TNF-α might be one of the factors inducing severe lesions in NMO, exacerbating the damage mediated by higher ENA 78 levels.
Conclusions
In summary, the overproduction of pro-inflammatory cytokines such as IL-1β and TNF-α during remission of NMO might result in activation of ENA 78. High levels of ENA 78 may play a critical role in neutrophil infiltration during NMO inflammation. And these might lead to increased neutrophils aggregation around the lesion, causing severer clinical symptoms once NMO relapse. ENA 78 plasma levels were also correlated with EDSS scores in NMO remission. The current study enables the therapy of NMO patients.
Abbreviations
AQP, aquaporin; CREB, cAMP-response element binding protein; CNS, central nervous system; CXCL, chemokine (C-X-C motif) ligand; NMO, Neuromyelitis optica; NMO, neuromyelitis optica, ENA, epithelial neutrophil-activating peptide, EDTA, Ethylenediaminetetraacetic acid, MS, multiple sclerosis; HC, healthy controls; EDSS, expanded disability status score; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor α; PMNLs, polymorphonuclear leukocytes; ELR, glutamic acid-leucine-arginine motif
References
Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53:1107–14.
Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–61.
Asgari N, Owens T, Frokiaer J, Stenager E, Lillevang ST, Kyvik KO. Neuromyelitis optica (NMO)--an autoimmune disease of the central nervous system (CNS). Acta Neurol Scand. 2011;123:369–84.
Shiee N, Bazin PL, Zackowski KM, Farrell SK, Harrison DM, Newsome SD, et al. Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One. 2012;7:e37049.
Confavreux C, Vukusic S. The clinical epidemiology of multiple sclerosis. Neuroimaging Clin N Am. 2008;18:589–622. ix-x.
Amato MP, Goretti B, Ghezzi A, Lori S, Zipoli V, Moiola L, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology. 2010;75:1134–40.
Saadoun S, Waters P, MacDonald C, Bell BA, Vincent A, Verkman AS, et al. Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Ann Neurol. 2012;71:323–33.
Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol. 2014;10:493–506.
Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21.
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–12.
Rowland KJ, Diaz-Miron J, Guo J, Erwin CR, Mei J, Worthen GS, et al. CXCL5 is required for angiogenesis, but not structural adaptation after small bowel resection. J Pediatr Surg. 2014;49:976–80. discussion 980.
Zheng J, Zhu X, Zhang J. CXCL5 knockdown expression inhibits human bladder cancer T24 cells proliferation and migration. Biochem Biophys Res Commun. 2014;446:18–24.
Sanz MJ, Kubes P. Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur J Immunol. 2012;42:278–83.
Lira SA, Furtado GC. The biology of chemokines and their receptors. Immunol Res. 2012;54:111–20.
Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533:277–88.
Zwijnenburg PJ, de Bie HM, Roord JJ, van der Poll T, van Furth AM. Chemotactic activity of CXCL5 in cerebrospinal fluid of children with bacterial meningitis. J Neuroimmunol. 2003;145:148–53.
Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244–51.
Sundaram K, Rao DS, Ries WL, Reddy SV. CXCL5 stimulation of RANK ligand expression in Paget’s disease of bone. Lab Invest. 2013;93:472–9.
Mostafa GA, Al-Ayadhi LY. The possible link between elevated serum levels of epithelial cell-derived neutrophil-activating peptide-78 (ENA-78/CXCL5) and autoimmunity in autistic children. Behav Brain Funct. 2015;11:11.
Gillitzer R, Ritter U, Spandau U, Goebeler M, Bröcker EB. Differential expression of GRO-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J Invest Dermatol. 1996;107:778–82.
Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–43.
Rocha BC, Marques PE, Leoratti FM, Junqueira C, Pereira DB, Antonelli LR, et al. Type I Interferon Transcriptional Signature in Neutrophils and Low-Density Granulocytes Are Associated with Tissue Damage in Malaria. Cell Rep. 2015;13:2829–41.
Deng M, Ma T, Yan Z, Zettel KR, Scott MJ, Liao H, et al. Toll-like Receptor 4 Signaling on Dendritic Cells Suppresses Polymorphonuclear Leukocyte CXCR2 Expression and Trafficking via Interleukin 10 During Intra-abdominal Sepsis. J Infect Dis. 2015;213(8):1280–8.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9.
Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–61.
Jarius S, Paul F, Franciotta D, Ruprecht K, Ringelstein M, Bergamaschi R, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306:82–90.
Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol. 2011;70:943–54.
Tsai HH, Frost E, To V, Robinson S, Ffrench-Constant C, Geertman R, et al. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–83.
Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–20.
Zineh I, Aquilante CL, Langaee TY, Beitelshees AL, Arant CB, Wessel TR, et al. CXCL5 gene polymorphisms are related to systemic concentrations and leukocyte production of epithelial neutrophil-activating peptide (ENA-78). Cytokine. 2006;33:258–63.
Zineh I, Luo X, Welder GJ, Debella AE, Wessel TR, Arant CB, et al. Modulatory effects of atorvastatin on endothelial cell-derived chemokines, cytokines, and angiogenic factors. Pharmacotherapy. 2006;26:333–40.
Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, et al. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004;172:4493–502.
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91.
Iwata K, Doi A, Ohji G, Oka H, Oba Y, Takimoto K, et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med. 2010;49:2423–32.
Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81:786–92.
Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog. 2009;5:e1000648.
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–54.
Guha D, Klamar CR, Reinhart T, Ayyavoo V. Transcriptional Regulation of CXCL5 in HIV-1-Infected Macrophages and Its Functional Consequences on CNS Pathology. J Interferon Cytokine Res. 2015;35:373–84.
Persson T, Monsef N, Andersson P, Bjartell A, Malm J, Calafat J, et al. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003;33:531–7.
Vieira SM, Lemos HP, Grespan R, Napimoga MH, Dal-Secco D, Freitas A, et al. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009;158:779–89.
Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–46.
Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, et al. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem. 2003;278:4675–86.
Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al. A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5. J Immunol. 2007;179:4849–56.
Bersinger NA, Gunthert AR, McKinnon B, Johann S, Mueller MD. Dose–response effect of interleukin (IL)-1beta, tumour necrosis factor (TNF)-alpha, and interferon-gamma on the in vitro production of epithelial neutrophil activating peptide-78 (ENA-78), IL-8, and IL-6 by human endometrial stromal cells. Arch Gynecol Obstet. 2011;283:1291–6.
Carrero R, Cerrada I, Lledo E, Dopazo J, Garcia-Garcia F, Rubio MP, et al. IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev. 2012;8:905–16.
Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, Masuda T, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer. 2012;131:2234–41.
Sun H, Chung WC, Ryu SH, Ju Z, Tran HT, Kim E, et al. Cyclic AMP-responsive element binding protein- and nuclear factor-kappaB-regulated CXC chemokine gene expression in lung carcinogenesis. Cancer Prev Res (Phila). 2008;1:316–28.
Mamik MK, Banerjee S, Walseth TF, Hirte R, Tang L, Borgmann K, et al. HIV-1 and IL-1beta regulate astrocytic CD38 through mitogen-activated protein kinases and nuclear factor-kappaB signaling mechanisms. J Neuroinflammation. 2011;8:145.
Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, et al. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 2013;125:897–908.
Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–602.
Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–10.
Kim S, Steelman AJ, Koito H, Li J. Astrocytes promote TNF-mediated toxicity to oligodendrocyte precursors. J Neurochem. 2011;116:53–66.
Acknowledgements
We thank Hui Wang, Yuezhi Kang and Cuicui Cheng who collected blood from patients and health subjects and thereby made it possible to carry out the study.
Funding
Yongping Fan, Lei Wang were responsible for accessing research funds: National Natural Science Foundation of China (CN) (81072765, 81173237, 81473640, 81273742); Natural Science Foundation of Beijing Municipality (CN) (7142053).
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Authors’ contributions
TY, SW, LW carried out the MILLIPLEX® map Human High Sensitivity Cytokine/Chemokine Panels tests, participated in the collection of samples and drafted the manuscript. QZ, QL, MW and ZD performed the statistical analysis and valued EDSS scores of patients. TY, SW and YF participated in the design of the study and coordination. TY conceived of the study, and participated in its design. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the local ethics committee (IRB of Beijing Tiantan Hospital Affiliated to Capital Medical University, No. KY2015-003-02). Written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, T., Wang, S., Zheng, Q. et al. Increased plasma levels of epithelial neutrophil-activating peptide 78/CXCL5 during the remission of Neuromyelitis optica. BMC Neurol 16, 96 (2016). https://doi.org/10.1186/s12883-016-0622-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-016-0622-3