Abstract
Background
Our previous randomized double-blind study showed that drinking hydrogen (H2) water for 48 weeks significantly improved the total Unified Parkinson’s Disease Rating Scale (UPDRS) score of Parkinson’s disease (PD) patients treated with levodopa. We aim to confirm this result using a randomized double-blind placebo-controlled multi-center trial.
Methods
Changes in the total UPDRS scores from baseline to the 8th, 24th, 48th, and 72nd weeks, and after the 8th week, will be evaluated. The primary endpoint of the efficacy of this treatment in PD is the change in the total UPDRS score from baseline to the 72nd week. The changes in UPDRS part II, UPDRS part III, each UPDRS score, PD Questionnaire-39 (PDQ-39), and the modified Hoehn and Yahr stage at these same time-points, as well as the duration until the protocol is finished because additional levodopa is required or until the disease progresses, will also be analyzed. Adverse events and screening laboratory studies will also be examined. Participants in the hydrogen water group will drink 1000 mL/day of H2 water, and those in the placebo water group will drink normal water. One-hundred-and-seventy-eight participants with PD (89 women, 89 men; mean age: 64.2 [SD 9.2] years, total UPDRS: 23.7 [11.8], with levodopa medication: 154 participants, without levodopa medication: 24 participants; daily levodopa dose: 344.1 [202.8] mg, total levodopa equivalent dose: 592.0 [317.6] mg) were enrolled in 14 hospitals and were randomized.
Discussion
This study will confirm whether H2 water can improve PD symptoms.
Trial registration
UMIN000010014 (February, 13, 2013)
Similar content being viewed by others
Background
In patients with Parkinson’s disease (PD), the pharmacologic replacement of dopamine and other antiparkinsonian drugs has been used for symptomatic therapy. However, none of these drugs stop or lessen the dopaminergic neuronal degeneration or the progression of the disease. Findings of increased iron and lipid peroxidation and decreased levels of reduced glutathione in the substantia nigra strongly suggest that enhanced oxidative stress is involved in the pathogenesis of PD [1, 2]. Thus, antioxidant therapies might slow the progression of PD. Molecular hydrogen (H2) has recently been highlighted as a therapeutic and preventive antioxidant. Since the first publication [3], more than 150 papers have confirmed the efficacy of H2 in various animal models [4]. H2-water reduced dopaminergic neuronal cell loss in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model [5] as well as 6-hydroxydopamine did [6]. Our previous randomized double-blind study has shown that drinking 1,000 mL of H2-water for 48 weeks significantly improved (p < 0.05) the total Unified Parkinson’s Disease Rating Scale (UPDRS) scores of patients with PD who were being treated with levodopa [7]. In the present study, we aimed to confirm these results by conducting a longer and more large-scale trial that also included patients who were not being treated with levodopa. Here, we present the design and the baseline characteristics of participants already enrolled in this study.
Methods
A placebo-controlled, randomized, double-blind, parallel-group (1:1) clinical multi-center trial was organized by the Department of Neurology of Juntendo University School of Medicine in accordance with Consolidated Standards of Reporting Trials (CONSORT) guideline. Fourteen hospitals are involved as trial centers. This trial was advertised in posters and on homepages of our clinic, and participants had to declare their intentions to participate voluntarily. The inclusion criteria required that the participants have a diagnosis of PD according to the United Kingdom Brain Bank criteria [8]. All the participants should have a modified Hoehn and Yahr staging (H & Y stage) in the on-phase between 1 and 4. For 8 weeks prior to establishing the baseline, the participants’ antiparkinsonian drugs were not changed. None of the participants have dementia (MMSE < 25) or dysphagia for water. Outpatients are preferred over admitted patients. The participants are to be older than 20 years. The exclusion criteria included the following: parkinsonism due to diseases other than PD, the presence of other serious diseases, malignant tumor(s), or adverse events caused by drugs.
The clinical study is registered at UMIN clinical trial registry (UMIN-CTR) UMIN000010014 (February 13, 2013). The Ethics Committee of the Juntendo University School of Medicine approved this study in February 2013, as did the ethics committees of other centers, and all participants provided signed informed consent forms.
Randomization and blinding
The enrolled participants have been assigned using a stratified randomization method according to their age and if they were receiving levodopa. The assignments were made by C.S. The participants and those assessing outcomes will remain blinded until all the participants have finished the protocol.
Procedures
On a daily basis, the participants will drink 1,000 mL of saturated H2-water containing 5 mM of dissolved H2 (using Hydrogen 7.0, supplied by Ecomo International Co., Ltd. [Fukuoka, Japan]; patent No: PCT/JP2011/063601) for 72 weeks. The placebo water is saturated with N2. The water is contained in a 500-mL plastic bottle, and the participants will drink 2 bottles per day. The participants will drink the water within 3 h of opening the cap, because H2 evaporates. The bottles of H2-water or placebo water will be sent to the participants’ home every week.
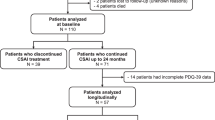
The schedule of the study is shown in Fig. 1. Changes in the total UPDRS scores from baseline to the 8th, 24th, 48th, and 72nd weeks, and after the 8th week, are evaluated. The primary endpoint of the efficacy of this treatment in PD is the change in the total UPDRS score from baseline to the 72nd week.
Schedule of enrolment, intervention, and assessment. Study period of enrolment, allocation, and baseline may coincide. It is approximately 1 week to 10 days from allocation to the first day to start shipping the water. *1: Unified Parkinson’s Disease Rating Scale. *2: Parkinson’s Disease Questionnaire-39
The changes in UPDRS part II, UPDRS part III, each UPDRS score, PD Questionnaire-39 (PDQ-39), and the H & Y stage at these same time-points, as well as the duration until the protocol is finished because additional levodopa is required or until the disease progresses, will also be analyzed. Adverse events and screening laboratory parameters (total protein, albumin, alkaline phosphatase, aspartate transaminase, alanine transaminase, serum urea nitrogen, calcium, chloride, creatinine, glucose, lactate dehydrogenase, potassium, sodium, creatinine kinase, uric acid, choline esterase, LDL-cholesterol, HDL-cholesterol, and triglyceride levels) will also be examined.
Statistical analysis
We calculated that a minimum of 95.4 participants need to be enrolled to detect a 5-point difference in the UPDRS scores between the 2 groups, with a standard deviation of the mean difference of 10, and a 2-sided alpha level of 0.05 and 80 %. Assuming a 35 % dropout, 96 participants will be required in total. A period of 72 weeks’ follow-up is a relatively long-term clinical trial in PD; hence, we assumed a 35 % dropout rate.
Variations in the endpoints between the baseline value and treatment assessment points will be compared between the groups, using a t-test or a Mann–Whitney U-test. The statistical tests are 2-sided, and the significance level is set at 0.05. The number of participants in the treatment and placebo groups who drop out due to disease progression will be analyzed using Kaplan − Meier curves and the log-rank test. Subgroup analysis (sex, and treatment with or without levodopa) will be performed.
Enrollment and data collection
One-hundred-and-seventy-eight Japanese participants with PD have already been enrolled in 14 hospitals and have been randomized, between April 1, 2013 and September 30, 2015, at their baseline visit. The participants will be followed-up for 80 weeks. The baseline characteristics are shown in Table 1. Despite extensive efforts to enroll more participants, the expected number of participants was not met, as it is difficult for some patients to consume 1000 mL water daily.
Discussion
Fujita et al. have indicated that the intake of H2-water, even after MPTP administration, reduces neurotoxic damage [5]. The findings of our previous study [7] on PD patients are in agreement with the previous results that were obtained in animal models.
Antioxidant supplements that are considered medicinal products should undergo sufficient evaluation before marketing, as they might be harmful at high doses [9]. H2 selectively reduces •OH radicals, but not O2−•, H2O2, or NO• [4]. It is expected that prolonged application of H2 will have no or little adverse effects in chronic diseases. The effects of H2 could be mediated by modulating activities and expression of various molecules, such as Lyn, ERK, p38, JNK, ASK1, Akt, GTP-Rac1, iNOS, Nox1, NF-κB, p65, Iκba, STST3, NFATc1, c-Fos, and ghrelin [10]. Iuchi et al. proposed a hypothetical model in which H2 is linked to the modulation of Ca2+ signal transduction and the nuclear factor of activated T cells (NFAT) pathway via oxidized phospholipid species [11].
Our previous trial was the first randomized double-blind study of H2-water in patients with PD [7]. H2-water exhibited no adverse effects at a dose of 1000 mL/day in PD subjects receiving levodopa treatment. The results of the previous study will be confirmed in this longer and larger-scale study that includes patients who are not medicated with levodopa. This study will confirm the safety and tolerability of H2-water and if H2-water can improve PD symptoms.
Ethics approval
A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease was approved by the Ethics Committee of the Juntendo University School of Medicine, and the ethics committees of other centers.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Abbreviations
- CONSORT:
-
Consolidated Standards of Reporting Trials
- H2:
-
hydrogen
- H & Y:
-
modified Hoehn and Yahr staging
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NFAT:
-
nuclear factor of activated T cells
- PD:
-
Parkinson’s disease
- PDQ-39:
-
Parkinson’s disease Questionnaire-39
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
Dexter DT, Wells FR, Agid F, Lees AJ, Jenner P, Marsden CD. Increased nigral iron content in postmortem Parkinsonian brain. Lancet. 1987;8569:1219–20.
Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696–701.
Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94.
Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 1820;2012:586–94.
Fujita K, Seike T, Yutsudo N, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS One. 2009;30:e7247.
Fu Y, Ito M, Fujita Y, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453:81–5.
Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–9.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;28:842–57.
Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen—comprehensive review of 321 original articles. Med Gas Res. 2015;5:12.
Iuchi K, Imoto A, Kamimura N, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–53.
Acknowledgements
We thank MiZ Co. Ltd. and Ecomointernational Co., Ltd. for supplying the Suisosui 7.0 (H2-water) and placebo water.
Funding
This work is supported by a grant from Japan Society for the Promotion of Science KAKENHI (Grant Number 15 K09360).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
N. Hattori has served as an advisory board member for Boehringer Ingelheim and FP Pharmaceutical Company (PC) and he has consulted for Ohtsuka PC, Kyowa Hakko Kirin PC, GlaxoSmithKline, Novartis, Abbot, Hisamitsu, and Schering-Plough (MSD); he also received personal compensation for attending these advisory board meetings. Other authors: None
Authors’ contributions
AY conceived and designed the study, acquired data, performed statistical analysis, and drafted and revised the manuscript. TA, CO, TM, HW, MH, SK, YO, HS, GO, JF, YS, TH, YM, HM, MT, MH, and HS acquired the data. CS performed randomization. AK helped in checking the data. TK acquired the data and revised the manuscript. NH conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yoritaka, A., Abe, T., Ohtsuka, C. et al. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: protocol and baseline characteristics. BMC Neurol 16, 66 (2016). https://doi.org/10.1186/s12883-016-0589-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-016-0589-0